Three-Component Halo Aldol Condensation of Thioacrylates with Aldehydes Mediated by Titanium (IV) Halide
Abstract
:Introduction
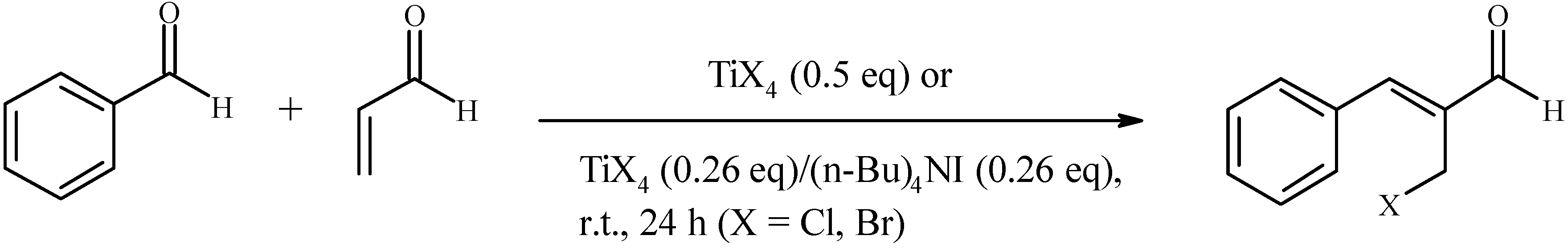
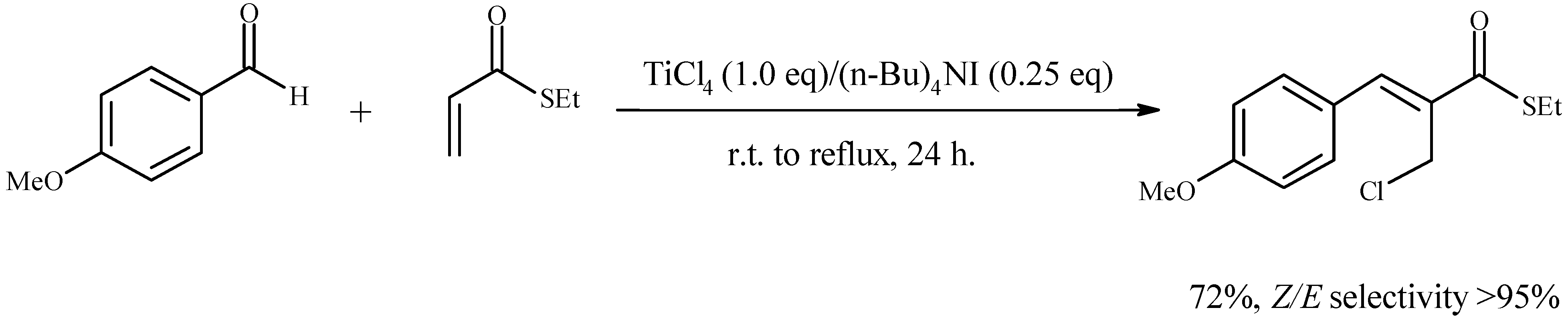
Results and Discussion
 |
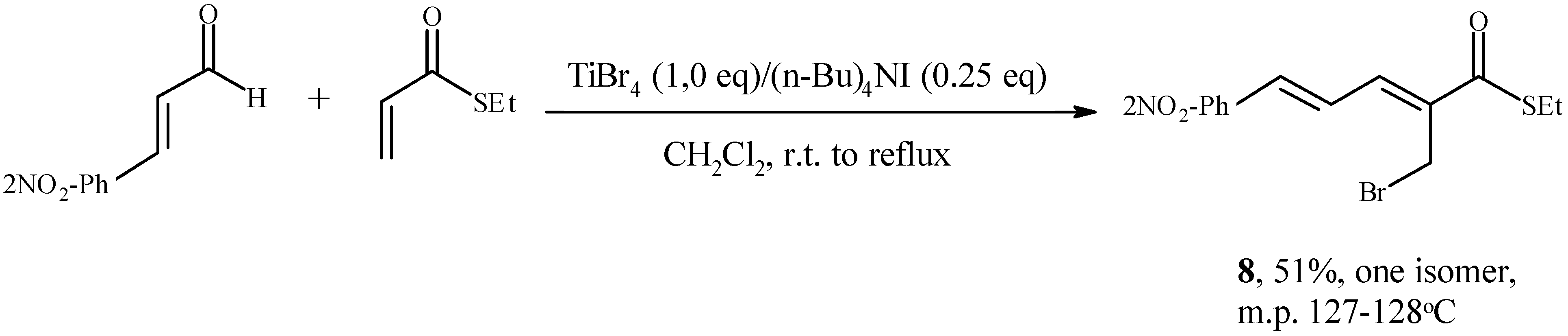
Conclusions
Experimental
General
Typical Experimental Procedure
Spectral data
- 1:
- 1H-NMR: δ 7.80 (s, 1H), 7.57-7.55 (m, 2H), 7.46-7.43 (m, 3H), 4.49 (s, 2H), 3.04 (q, J =7.42Hz, 2H), 1.34 (t, J =7.42Hz, 3H); 13C-NMR: δ 192.0, 141.3, 136.1, 133.8, 129.8, 129.7, 128.9, 38.4, 23.8, 14.5.
- 2:
- 1H-NMR: δ 7.77 (s, 1H), 7.59-7.56 (m, 2H), 7.00-6.97 (m, 2H), 4.53 (s, 2H), 3.86 (s, 3H), 3.03 (q, J=7.42Hz, 2H), 1.33 (t, J =7.42Hz, 3H); 13C-NMR: δ 192.0, 141.6, 138.7, 133.9, 132.0, 128.3, 114.4, 55.4, 38.9, 23.7, 14.6.
- 3:
- 1H-NMR: δ 7.73 (s, 1H), 7.52-7.41 (m, 4H), 4.45 (s, 2H), 3.04 (q, J =7.41Hz, 2H), 1.33 (t, J =7.41Hz, 3H); 13C-NMR: δ 191.7, 139.7, 136.5,135.9, 132.1, 130.9, 129.1, 38.1, 23.8, 14.5.
- 4:
- 1H-NMR: δ 7.71 (s, 1H), 7.61-7.58 (m, 2H), 7.45-7.42 (m, 2H), 4.45 (s, 2H), 3.05 (q, J =7.42 Hz, 2H), 1.34 (t, J = 7.42 Hz, 3H); 13C-NMR: δ 191.9, 139.9, 136.7, 132.7, 132.2, 131.2,131.1, 124.4, 38.2, 23.9, 14.5.
- 5:
- 1H-NMR: δ 6.94 (t, J = 7.56Hz, 1H), 4.33 (s, 2H), 2.97 (q, J=7.42Hz, 2H), 2.35 (q, J=7.46Hz, 2H), 1.55-150 (m, 2H), 1.37-1.21 (m, 5H), 0.88 (m, 3H); 13C-NMR: δ 191.5, 146.7, 137.0, 36.5, 31.8, 29.4, 29.3 (2), 29.2, 28.9, 28.4, 23.4, 22.6, 14.6, 14.1.
- 6:
- 1H-NMR: δ 7.56-7.46 (m, 3H, 7.40-7.35 (m, 3H), 7.20-7.04 (m, 2H), 4.54 (s, 2H), 3.02 (q, J=7.43Hz, 2H), 1.31 (t, J=7.43Hz, 3H); 13C-NMR: δ 190.9, 143.6, 141.2, 135.7, 134.4, 129.8, 128.9, 127.7, 122.3, 36.8, 23.6, 14.7.
- 7:
- 1H-NMR: δ 8.03 (dd, J=1.28, 8.16Hz, 1H), 7.76 (dd, J= 1.37, 7.86 Hz, 1H), 7.67 (m, 1H), 7.59-7.47 (m, 3H), 7.11 (dd, J = 11.3, 15.2Hz, 1H), 4.53 (s, 2H), 3.03 (q, J=7.41Hz, 2H), 1.33 (t, J=7.41Hz, 3H); 13C-NMR: δ 191.1, 148.1, 139.8, 137.4, 136.6, 133.4, 131.5, 129.7, 128.7, 126.9, 125.0, 36.5, 23.7, 14.6.
- 8:
- 1H-NMR: δ 8.03 (dd, J=1.28, 8.6Hz, 1H), 7.77 (dd, J=1.28, 7.9Hz, 1H), 7.67 (m, 1H), 7.61-7.45 (m, 3H), 7.10 (dd, J=11.3, 15.2Hz, 1H), 4.43 (s, 2H), 3.04 (q, J= 7.41Hz, 2H), 1.33 (t, J =7.41Hz, 3H); 13C-NMR: δ 190.9, 148.1, 139.2, 137.1, 137.0, 133.5, 131.6, 129.7, 128.7, 127.1, 125.1, 23.8, 23.1, 14.6.
Acknowledgments
References
- For reviews regarding the Baylis-Hillman reaction see: Ciganek, E. Org. React. 1997, 51, 201. Basavaiah, D.; Rao, P. D.; Hyma, R. S. Tetrahedron 1996, 52, 8001. Zhang, A. M.; Wang, W.; Lin, G. Q. Chinese J. Org. Chem. 2001, 21, 134. Li, G.; Hook, J.; Wei, H.-X. “Recent Research Developments in Organic & Bioorganic Chemistry”; Transworld Research Network, 2001; Volume 4, p. 49. [Google Scholar]
- Brzezinski, L. J.; Rafel, S.; Leahy, J. M. J. Am. Chem. Soc. 1997, 119, 4317. Iwabuchi, Y.; Nakatani, M.; Yokoyama, N.; Hatakeyama, S. J. Am. Chem. Soc. 1999, 121, 10219. Barrett, A. G. C.; Cook, A. S.; Kamimura, A. Chem. Commun. 1998, 2553. Kawamura, M.; Kobayashi, S. Tetrahedron Lett 1999, 40, 1539.
- Li, G.; Wei, H.-X.; Caputo, T. D. Tetrahedron Lett. 2000, 41, 1. Li, G.; Wei, H.-X.; Phelps, B. S.; Purkiss, D. W.; Kim, S. H. Organic Letters 2001, 3, 823. Ramachandran, P. V.; Reddy, M. V. R.; Rudd, M. T. Chem Commun. 1999, 19, 1979. [Google Scholar] Rosa, J. N.; Afonso, C. A. M.; Santos, A.G. Tetrahedron 2001, 57, 4189. Alcaide, B.; Almendros, P.; Aragoncillo, C. J. Org. Chem. 2001, 66, 1612. Shi, M.; Jiang, J.K.; Cui, S.C.; Feng, Y. S. J. Chem. Soc. Perkin Trans 1 2001, 390. Wei, H. X.; Caputo, T. D.; Purkiss, D. W.; Li, G. Tetrahedron 2000, 56, 2397. Kataoka, T.; Kinoshita, H.; Kinoshita, S.; Iwamura, T.; Watanabe, S. Angew. Chem. Int. Ed. 2000, 39, 2358.
- Buchholz, R.; Hoffmann, H. M. R. Helv. Chim. Acta 1994, 77, 1480. Xu, L.-X.; Kundig, E. P. Helv. Chim. Acta 1994, 77, 1480. Basavaiah, D.; Hyma, R. S.; Padmaja, K.; Krishnamacharyulu, M. Tetrahedron 1999, 55, 6971, and references cited therein.
- Li, G.; Gao, J.; Wei, H.-X.; Enright, M. Organic Letters 2000, 2, 617.
- Taniguchi, M.; Hino, T.; Kishi, Y. Tetrahedron Lett. 1986, 39, 4767. Yachi, K.; Maeda, K.; Shinokubo, H.; Oshima, K. Tetrahedron Lett. 1997, 38, 5161. Uehira, S.; Han, Z.; Shinokubo, H.; Oshima, K. Organic Lett. 1999, 1, 1383.
- Wei, H.-X.; Karur, S.; Li, G. Molecules 2000, 5, 1408. Kataoka, T.; Iwama, T.; Iwamura, T.; Kinoshita, S.; Tsujiyama, Y; Iwamura, S; Watanabe, S. Synlett 1999, 2, 197.
- A comprehensive review about Lewis acid carbonyl complexation including TiCl4 see: Shambayati, S.; Schreiber, S. L. Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; vol. 1, Pergamon, Oxford, 1991; pp. 283–321. [Google Scholar]
- Sample Availability: Available from the authors
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kim, S.H.; Wei, H.-X.; Gao, J.J.; Li, G. Three-Component Halo Aldol Condensation of Thioacrylates with Aldehydes Mediated by Titanium (IV) Halide. Molecules 2002, 7, 89-95. https://doi.org/10.3390/70100089
Kim SH, Wei H-X, Gao JJ, Li G. Three-Component Halo Aldol Condensation of Thioacrylates with Aldehydes Mediated by Titanium (IV) Halide. Molecules. 2002; 7(1):89-95. https://doi.org/10.3390/70100089
Chicago/Turabian StyleKim, Sun Hee, Han-Xun Wei, Joe J. Gao, and Guigen Li. 2002. "Three-Component Halo Aldol Condensation of Thioacrylates with Aldehydes Mediated by Titanium (IV) Halide" Molecules 7, no. 1: 89-95. https://doi.org/10.3390/70100089





