Introduction
Cyclic peroxides such as the substituted 1,2,4,5-tetroxanes undergo thermolysis, both in the gas [
1] and solution phase [
2] by a stepwise mechanism, where the activation parameters values of the initial unimolecular homolysis fall within a limited range, although significant steric [
3] and solvent effects [
4] have also been observed. Moreover, the experimental activation energies for their thermolyses are usually in good agreement with calculated values based on a peroxidic bond homolysis of the corresponding molecular rings [
5]. The first step in the mechanism of thermolysis of
cis - fused 1,2,4-trioxanes seems to be the rupture of their molecular peroxidic bond [
6] with looser transition states. Because the
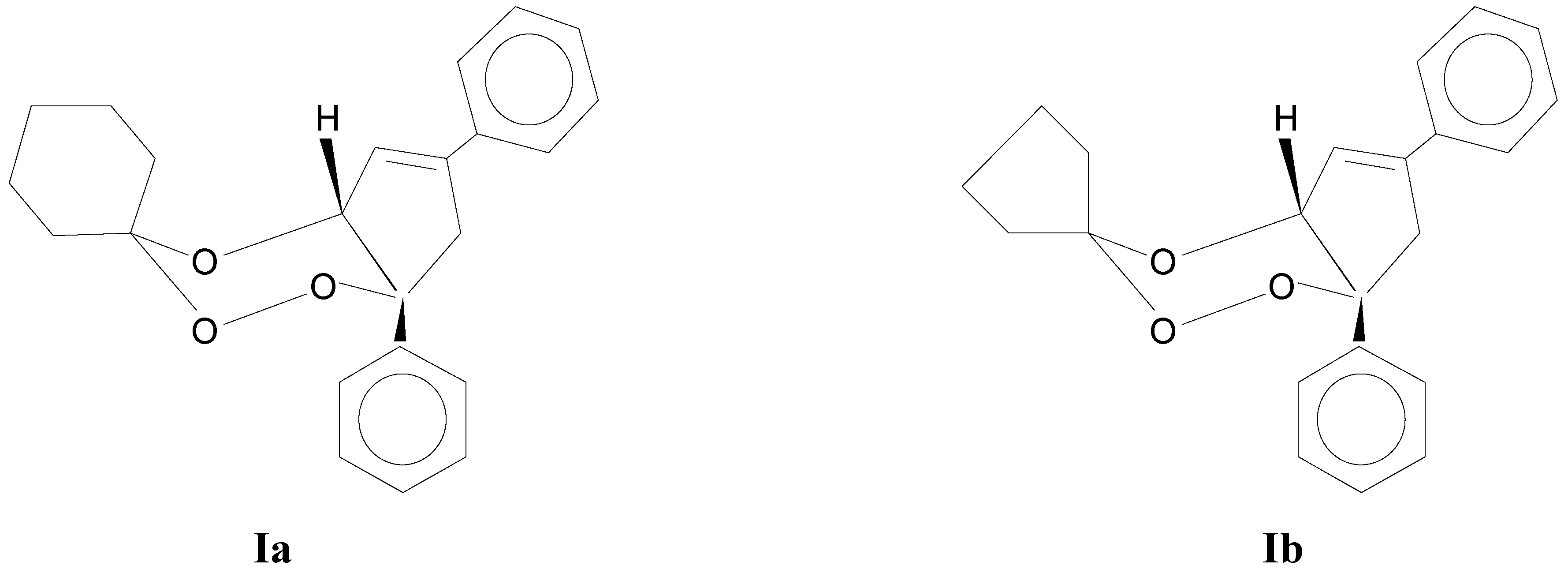
cis-6-phenyl-5,6-(2-phenylpropyliden)-3,3-pentamethylene-1,2,4-trioxacyclohexane (
Ia) and the
cis-6-phenyl-5,6-(2-phenylpropyliden)-3,3-tetramethylene-1,2,4-trioxacyclohexane (
Ib) molecules (
Scheme 1) have different cyclic atomic groups on the corresponding C-3 atoms it is relevant to report the activation parameters for both thermolyses and also to compare the effects of the reaction solvents.
Consequently the decomposition reactions of Ia in methanol and benzene solutions have now been investigated to learn about on the thermal stability in solution of this cyclic peroxide, which is related to the chemotherapy with Qinghaosu and other synthetic drugs of the same type.
Results and discussion
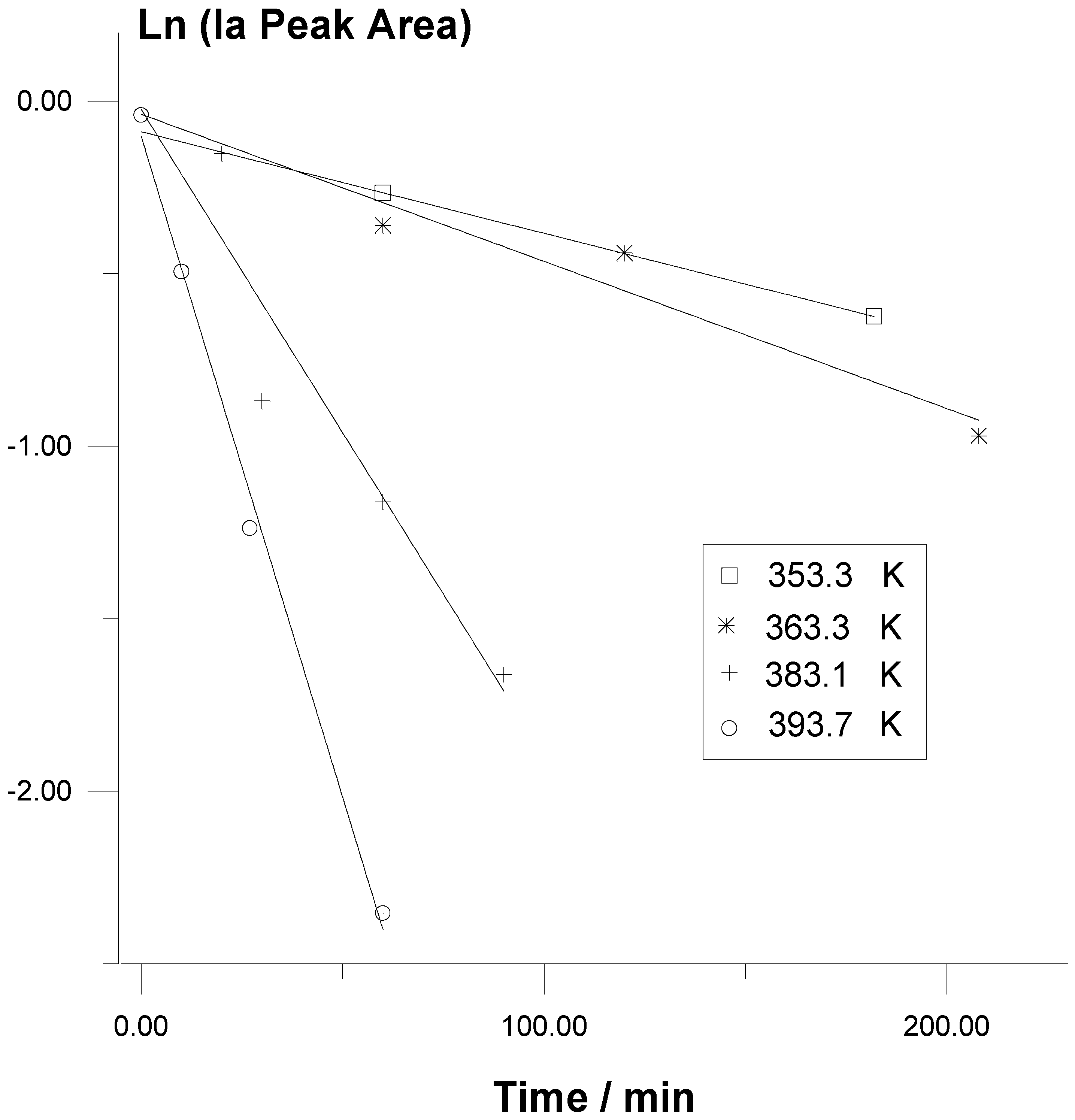
Rate measurements of the thermal decomposition reactions of
Ia in benzene and in methanol solutions indicate that at each temperature the thermolyses follow first-order kinetic laws up to at least ca.
20% conversions (
Figure1).
Figure 1.
Representation through First-order Kinetic Plots of the Data of Typical Runs of Ia Thermolysis in Methanol Solutions at Different Temperatures.
Figure 1.
Representation through First-order Kinetic Plots of the Data of Typical Runs of Ia Thermolysis in Methanol Solutions at Different Temperatures.
Moreover, many runs, including those without a previous Na
2EDTA treatment of the methanol solvent, showed that type of behaviour for higher conversions of the trioxane. In the thermolysis kinetics of substituted 1,2,4,5-tetroxanes the observed rate constant values show significant increases with the initial concentrations in the solutions [
2]. This seems to be a general behaviour in the thermolysis of cyclic peroxides because of bimolecular induced decomposition processes. However, this is not the case for reactions of
Ia, probably due to the rather low initial concentrations used (
Table I).
Table I.
First-Order Rate Constant Values for the Thermal Decomposition Reactions of Ia in Solution.
Table I.
First-Order Rate Constant Values for the Thermal Decomposition Reactions of Ia in Solution.
| Temp (K) | Reaction solvent | 103·[Trioxane]a (moles.dm-3) | 106·kobs s-1 |
| 353.3 | methanol | 1.1 | 4.5±0.2 |
| 353.3 | methanolb | 1.1 | 2.0±0.1 |
| 353.3 | methanolb | 1.1 | 2.5±0.1 |
| 353.3 | benzene | 13.1 | 4.6±0.3 |
| 363.3 | methanol | 4.8c | 10.4±0.5 |
| 363.3 | methanolb | 1.1 | 7.8±0.4 |
| 363.3 | benzene | 4.8c | 6.9±0.3 |
| 363.3 | benzeneb | 12.4 | 5.4±0.3 |
| 375.9 | benzene | 13.1 | 11.5±0.6 |
| 375.9 | benzeneb | 13.1 | 10.2±0.5 |
| 383.1 | methanol | 4.8c | 26.6±1.1 |
| 383.1 | methanolb | 4.8c | 24.1±1.0 |
| 393.7 | methanol | 1.1 | 44.8±2.0 |
| 393.7 | methanolb | 1.1 | 50.5±2.0 |
| 393.7 | benzene | 13.1 | 25.6±1.1 |
| 393.7 | benzene | 13.1 | 28.6±1.0 |
| 413.2 | methanol | 1.1 | 114±5.0 |
| 413.2 | benzene | 1.1 | 69.6±2.1 |
| 413.2 | benzeneb | 13.1 | 77.7±3.1 |
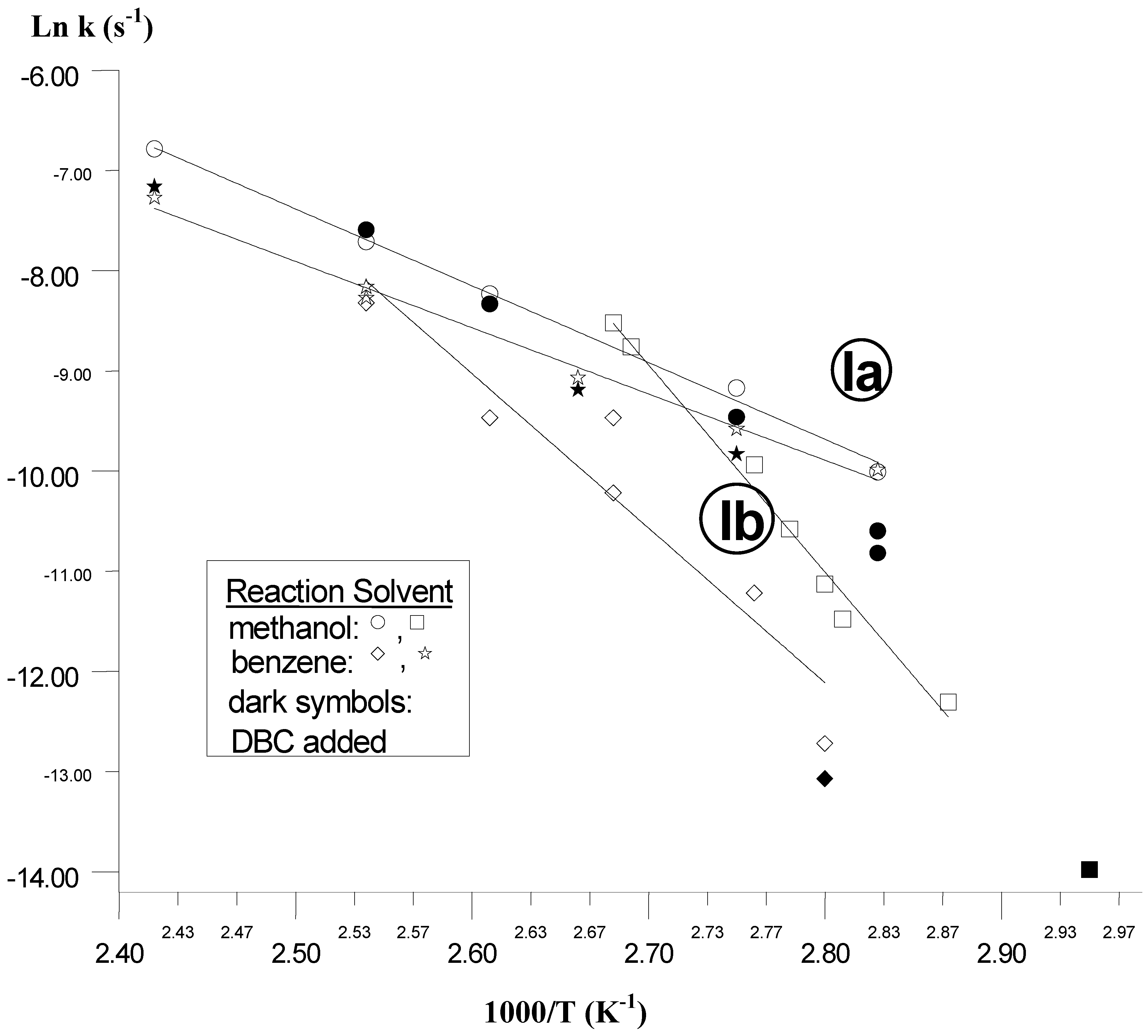
Nevertheless, a kinetic solvent effect on
Ia thermolysis is evident (
Table I) as the reaction rates are significantly faster in methanol than in benzene. Under comparable experimental conditions, the rate constant values turn to be ca. 1.6 times greater in methanol, except at the lowest temperature where they are very similar. Besides, the kinetics of
Ia thermolysis in the temperature range investigated and in the same solvent turn out to be faster (
Figure 2) compared with the corresponding
Ib reaction [
6]. Furthermore, in general the experiments performed in the solvents with added DBC show k
obs values similar to those found without that scavenger. However, it is apparent in both solvents at the lower experimental temperatures a kinetic lowering effect of the added cresol, probably related to the change of the physicochemical properties (e.g. polarity) of the reaction solvents.
Figure 2.
Arrhenius Equation Plots for the Thermolysis of Trioxanes Ia and Ib in Solution.
Figure 2.
Arrhenius Equation Plots for the Thermolysis of Trioxanes Ia and Ib in Solution.
The temperature effects on
Ia thermolyses in methanol and in benzene solutions, evaluated through the Arrhenius equation method, show plots (
Figure 2) which are linear (
Table II) over relatively large temperature intervals (> 55 K). This supports the fact that the corresponding activation parameters for
Ia (
Table II) and
Ib thermolyses belong to simple processes.
Table II.
Activation Parametersa for the Unimolecular Thermolysis in Solution of Substituted 1,2,4-trioxanes.
Table II.
Activation Parametersa for the Unimolecular Thermolysis in Solution of Substituted 1,2,4-trioxanes.
| Trioxane | Reaction Solvent | ΔH#
kcal mol-1 | ΔS#
cal mol-1 K-1 | ΔG#
kcal mol1 | r b | Reference |
| Ia | methanol | 20.2 ± 0.6 | 0.1 ± 1.6 | 20.2 ± 0.6 | 0.9902 | this work |
| Ia | benzene | 15.4 ± 0.2 | -13.2 ± 0.5 | 20.5 ± 0.2 | 0.9992 | this work |
| Ib | methanol | 39.6 ± 0.6 | 30.2 ± 1.6 | 28.8 ± 0.7 | 0.9961 | [6] |
| Ib | benzene | 30.9 ± 1.7 | 3.8 ± 4.3 | 29.5 ± 1.7 | 0.9479 | [6] |
It is not likely that both the unimolecular homolytic reaction and the alternative concerted process (in that case corresponding to a pericyclic reaction) for the thermal decomposition of
Ia have identical activation parameters. Moreover, if the thermolyses were of the latter type, where the bond breaking at the transition state is partly compensated by bond making, the experimentally found activation entropies would turn to very negative values. Hence, the experimental activation parameters for the decomposition reactions of
Ia in methanol and benzene solutions (
Table II) correspond to their unimolecular homolysis because alternative reaction pathways can not be reasonably postulated.
The very similar values of ΔG
# for both trioxane’s reactions (
Table II) support an analogous type of interactions between substrate and the solvent molecules in each unimolecular thermolysis. This means that the corresponding activation enthalpies of the reactions are almost compensated by the entropies of activation, yielding practically constant free energies of activation for each trioxane. The passage of the
Ia initial stage in solution to the corresponding diradical transition state would engender many degrees of freedom. Moreover, the reaction transition state in methanol would have relatively more degree of freedom reflected in the observed ΔS
# value (
Table II) than those achieved in the hydrocarbon solvent. Although it is accepted that activation enthalpies near 33 kcal mol
-1 correspond to peroxidic ruptures in most organic molecules [
8] the rather low values found (
Table II) for
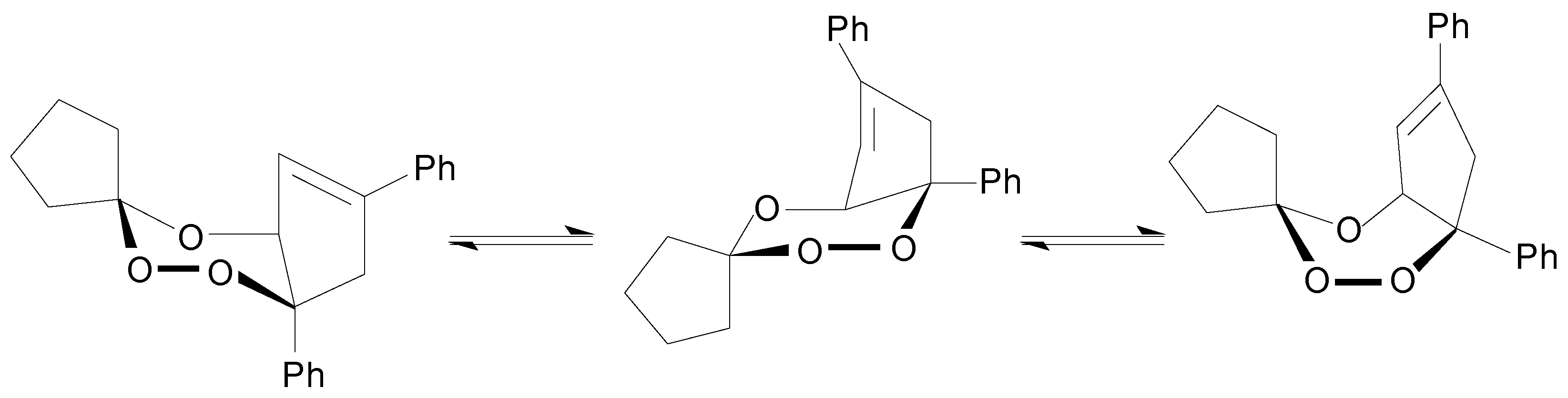
Ia thermolysis can be ascribed to its particular kinetic behaviour in the solvents investigated. Thus, the first step in the rupture of the peroxidic ring in the
Ia thermolysis would be the unimolecular homolysis to give the corresponding diradical which undergoes subsequently further decompositions to yield the observed reaction products (Equation 1).
The quantitatively different solvent effects noted for
Ia and
Ib homolysis (
Figure 2) may be ascribed to the structures, conformations and reaction environments of those cyclic peroxide molecules. Although in the
cis-fused bicyclic trioxanes considered both the α and β faces of the corresponding molecules should be equally accessible, the solvation of the transítion state arising from
Ia seems to be less effective due to the more bulky substituent on C-3. Referring to that it is worth noting that the
cis-fused bicyclic rings of the analogous
Ib molecule (
Scheme 2) would invert rapidly between chair and non-chair conformations allowing relatively more easy its interaction with the reaction solvent.
Scheme 2.
Inversion of Chair and Boat-like Conformers of the Ib Molecule
Scheme 2.
Inversion of Chair and Boat-like Conformers of the Ib Molecule
Similar solvent effects have been shown to operate for other cyclic peroxides of the substituted 1,2,4,5-tetroxane family [
4]. The decomposition of
Ia differs from the reaction of
Ib in some aspects. Not only are the solvent effects quantitatively different but the main products are dìfferent too (
Table III).
Table III.
Products Arising from the Thermolysesa of Ia and Ib in Different Solvents
Table III.
Products Arising from the Thermolysesa of Ia and Ib in Different Solvents
| Trioxaneb | Solvent | Reaction Productsc | References |
| Ia (0.013) | benzene | cyclohexanone (68 %), 1,4-diphenyl-3,4-cyclopentenediol (3 %), cyclohexanone diperoxide (10 %) d. | This work |
| Ia (0.001) | methanol | cyclohexanone (63 %), 1,4-diphenyl-3,4-cyclopentenediol (6 %), 1,4-diphenyl-3,4-dimethoxycyclopentene (0.5%), 1,4-diphenyl-3-methoxy-cyclopentene-4-ol (1 %), cyclohexanone diperoxide (2 %) d. | This work |
| Ib (0.003) | benzene | cyclopentanone (70 %), 1,4-diphenyl-3,4-cyclopentenediol (8 %), cyclopentanone diperoxide (9 %)d. | [9] |
| Ib (0.002) | methanol | cyclopentanone (60 %), 1,4-diphenyl-3,4-cyclopentenediol (7 %), cyclopentanone diperoxide (9 %)d. | [9] |
However, in methanol or benzene solutions both trioxanes furnish the corresponding ketones in nearly the same molar yields (
Table III), providing good evidence for the scission of the initial diradical (Equation 1). Then, the second step of
Ia and
Ib thermolysis is sìmilarly governed by the extrusion of stable fragments. Loss of ketone is also the main event in the thermal decomposition of related
cis-fused bicyclic 1,2,4-trioxanes in the solid state [
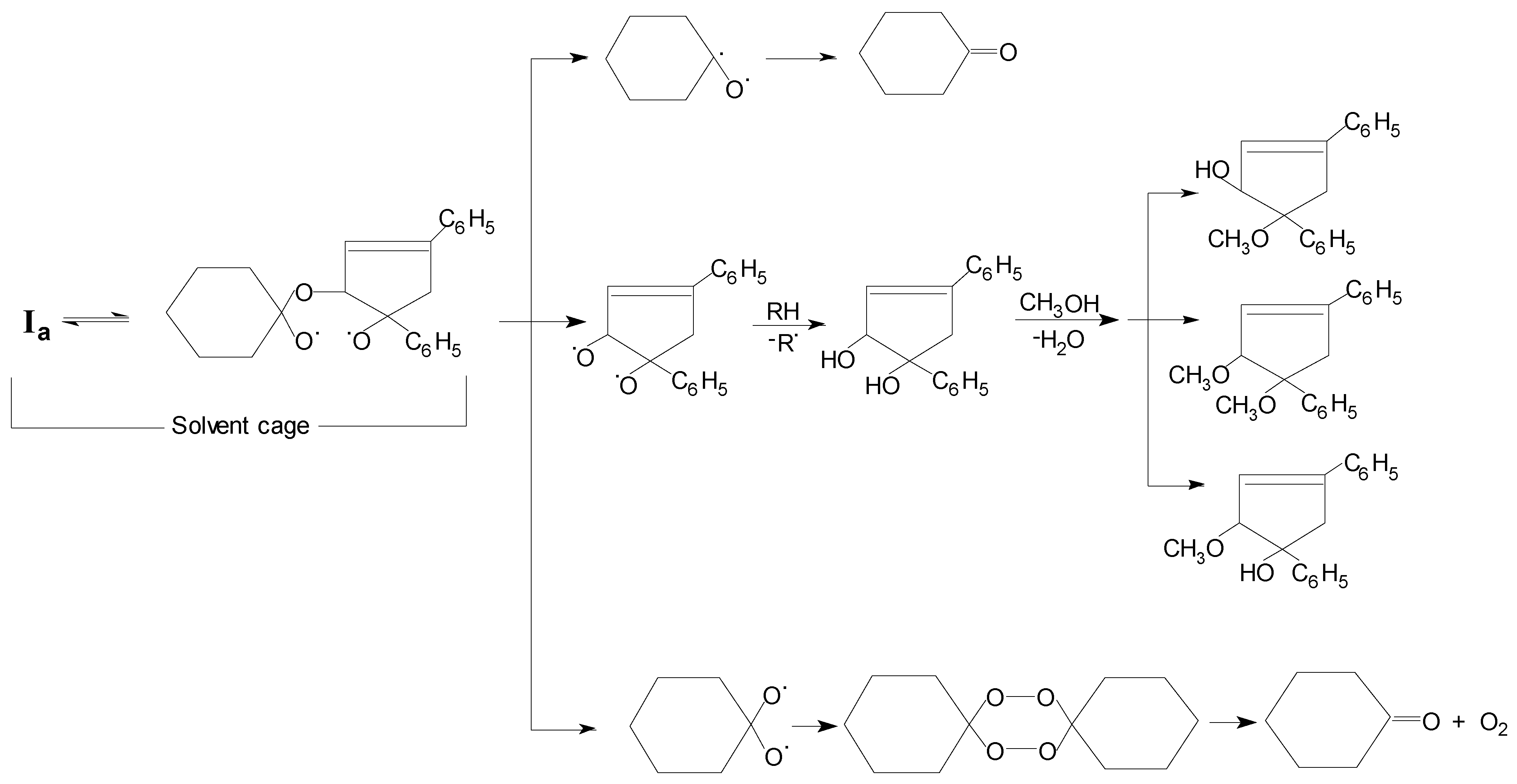
10]. Yet another similarity is seen for the thermolysis of both trioxanes: the formation of the diperoxide of the cyclic ketone. That diperoxide formation might be rationalised by recombination in the solvent cage of the corresponding 1,3-dioxy-diradical (
Scheme 3). In the
Ia thermal decomposition in methanol solution the cyclohexanone, the methoxy-substituted 1,4-diphenyl-cyclopentenyl derivatives and the 3,6-bis-pentamethylene-1,2,4,5-tetroxacyclohexane (cyclohexanone diperoxide) observed support the following reaction pathways (
Scheme 3).
Scheme 3.
Reaction Pathways for the Initial Diradical Decomposition of Ia Thermolysis at 413.2 K of in Methanol Solution.
Scheme 3.
Reaction Pathways for the Initial Diradical Decomposition of Ia Thermolysis at 413.2 K of in Methanol Solution.
Clearly an alternative avenue in both trioxanes reactions is competing with a non-radical elimination of the corresponding ketone, but this can be discarded considering the kinetic parameter values and the other products of their decompositions (
Table III). Then, in both solvents the initial diradical (Equation 1) would break down into a 1,3-dioxy-diradical and cyclopentenyl-diphenyl-substituted diradicals. The former may originate the corresponding ketones by hydrogen abstraction from the methanol solvent molecules or other sources of hydrogen atoms, through a thermally labile gem-diol intermediate. On the other hand, the 3,4-dihydroxy-diphenyl substituted derivative must necessarily arise from a competitive C-O bond rupture in the initially formed diradical (Equation 1). That hydroxylated product, evidently coming from the remainder of the trioxane molecules, would react further with methanol molecules yielding the secondary methoxy-substituted compounds only observed in this solvent. It is also evident that during the thermolyses of each trioxane the cyclohexanone or cyclopentanone diperoxides [
9] gradually decompose, most probably yielding additional ketone. For both trioxane reactions the overall mass balance (
Table III) may indicate further thermal decompositions of the final reaction products with probably some loss of volatile substances during the analytical procedures employed.