Results and Discussion
The 4-substituted 3-phenyl-4
H-1,2,4-triazoles and 5-methyl-3-phenyl-4
H-1,2,4-triazoles were best prepared from the corresponding 1,3,4-oxadiazoles by reflux with the appropriate amine [
3].
Thermolysis of 50 mg samples in evacuated, sealed glass tubes was carried out at 320 °C for exactly 20 min. This was accomplished by inserting the sample tubes into a closely fitting hole in a large metal block, functioning as a heat reservoir, placed in an oven kept at the desired temperature. In addition, a temperature probe was inserted in the hear reservoir. The compositions of the reaction mixtures were determined by GLC analyses and the identity of the products were determined by comparison to authentic samples or by their characteristic spectroscopic properties.
Table 1.
Products formed by thermolysis at 320 °C of the neat allyl substituted triazoles.
Table 1.
Products formed by thermolysis at 320 °C of the neat allyl substituted triazoles.
| Triazole | Products (yield, %) |
|---|
| 1 | 2 (4%) + 3 (2%) + 4 (48%) + 5 (34%) + 6 (4%) |
| 7 | 7 (40%) + 8 (24%) + 9 (20%) + 10 (5%) + 11(4%) + 6 (2%) |
| 12 | 13 (56%) + 14 (41%) + 6 (1%) |
| 15 | 16 (51%) + 17 (37%) + 18 (4%) + 19 (4%) + 20(2%) |
| 23 | 18 (22-25%) + 24 (14-9%) + 25 (8-6%) + 26(5%)a) + 27 (4%) + 28 (5%) + 29(28-37%) |
| 30 | 31 (75%) + 32 (22%) + 18 (1%) |
The results from these thermolyses are compiled in
Table 1. A seen in this table, rather complex mixtures with a number of products were generally formed. The triazoles that were thermolyzed and the reaction products formed are listed in
Table 2 and
Table 3.
Table 2.
The structures of the 3-Phenyl-5-methyl-1H-1,2,4-triazoles thermolyzed at 320 °C and the products formed.
Thermolysis of the neat 4-allyl-5-methyl-3-phenyl-4
H-1,2,4-triazole,
1, gave a 58:42 mixture of the two regioisomers, the 1- and 2-allyl substituted triazoles
4 and
5, together with small amounts of the
cis and
trans-4-(1-propenyl)-substituted triazoles
2 and
3 and the elimination product
6. Thermolysis of pure
2 and
3 clearly showed that these triazoles remained unchanged under the reaction conditions. The corresponding 4-(2-butenyl) substituted triazole
7 was found to react somewhat slower, as under the standard conditions the degree of conversion was merely 60 %. The products formed was the two 1- and 2- substituted triazoles
8 and
9 in an approximately 1:1 ratio. These products may well be formed by the previously proposed S
N2 type mechanism. Two other products,
10 and
11 were formed in a 5:4 ratio and may be formed by the corresponding S
N2'-type mechanism. These results clearly indicated that the pathways by which the 4-allyl-substituted triazoles rearranged presumably resemble the mechanism previously observed for the corresponding 4-alkyl-3,5-diphenyl-4
H-1,2,4-triazoles [
1].
Table 3.
The structures of the 3-Phenyl-1H-1,2,4-triazoles thermolyzed at 320 °C and the products formed.
Support for this mechanism was also found in the alkylation reactions with triazole 6 under basic conditions in DMF with the appropriate alkenyl halide. The only products formed in these reactions were the 1- or 2-substituted triazoles.
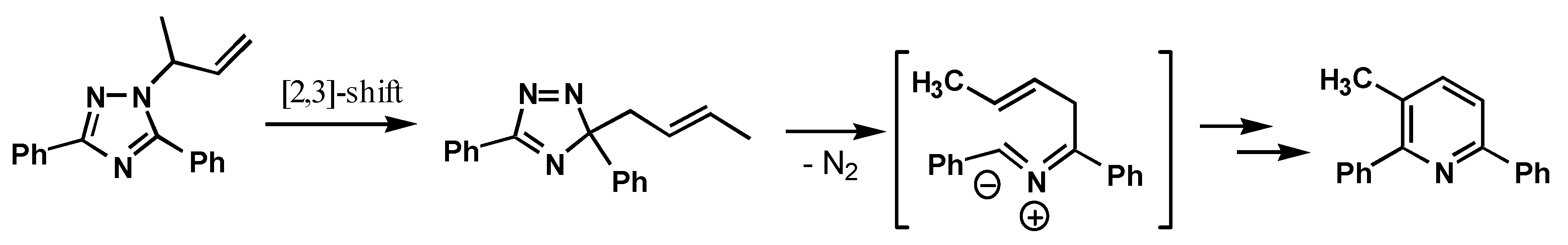
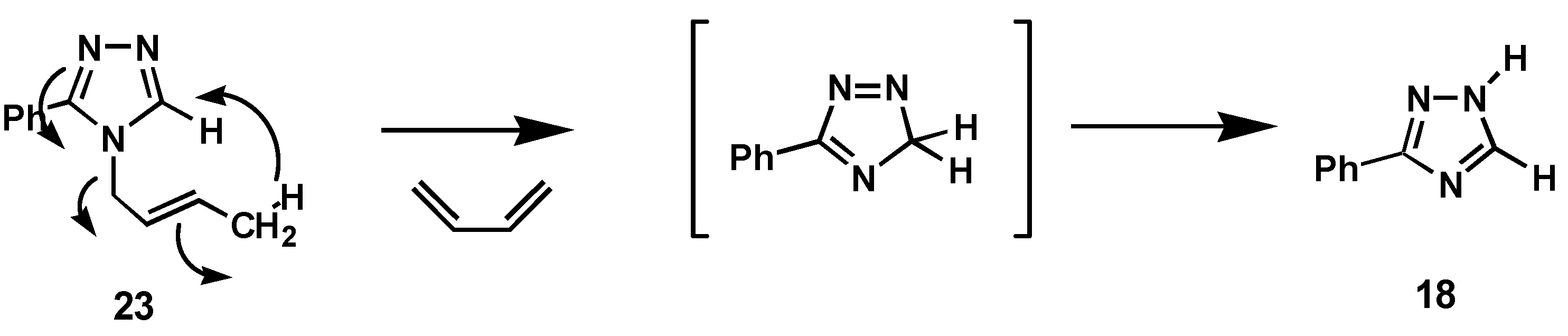
The 4-allyl- and 4-(2-butenyl)-substituted 3-phenyl-4H-1,2,4-triazoles, 15 and 23 exhibited a different reactivity under the standard reaction conditions. Thus, 15 did not yield the expected rearrangement product but gave as the main products the allyl isomerization products, cis- and trans-4- (1-propenyl)-4H-1,2,4-triazoles, 16 and 17, which were merely the results of double bond migrations in the allyl group. Only 6 % of the 1-substituted was formed and then also here as the corresponding vinylic products 19 and 20. No traces of the allylic rearrangement products 21 and 22 were detected. The corresponding 4-(2-butenyl)-substituted triazole 23 exhibited an unexpected behavior, and gave a rather complex product mixture with the elimination product 18 as a major product (25 %) together with minor amounts of SN2-type products 24 and 24 (20 %) and the SN2'-products 26 and 27 (9 %). In addition was isolated small amounts of a product 28 (5 %) which structure was not identified but appeared to be an isomer of the major product, 29 (28-37 %), which based on spectroscopic properties was assigned the structure 1,3-di(3-phenyl-1H-1,2,4-triazol-1-yl)butane.
That the 4-(2-butenyl)- substituted triazole,
23, so readily undergo an elimination reaction may be ascribed to reduced steric hindrance compared to triazole,
7, facilitating an elimination pathway which may be rationalized as shown in
Scheme 2.
Scheme 2.
Mechanism for the elimination of butadiene group upon thermolysis of triazole, 23
Scheme 2.
Mechanism for the elimination of butadiene group upon thermolysis of triazole, 23
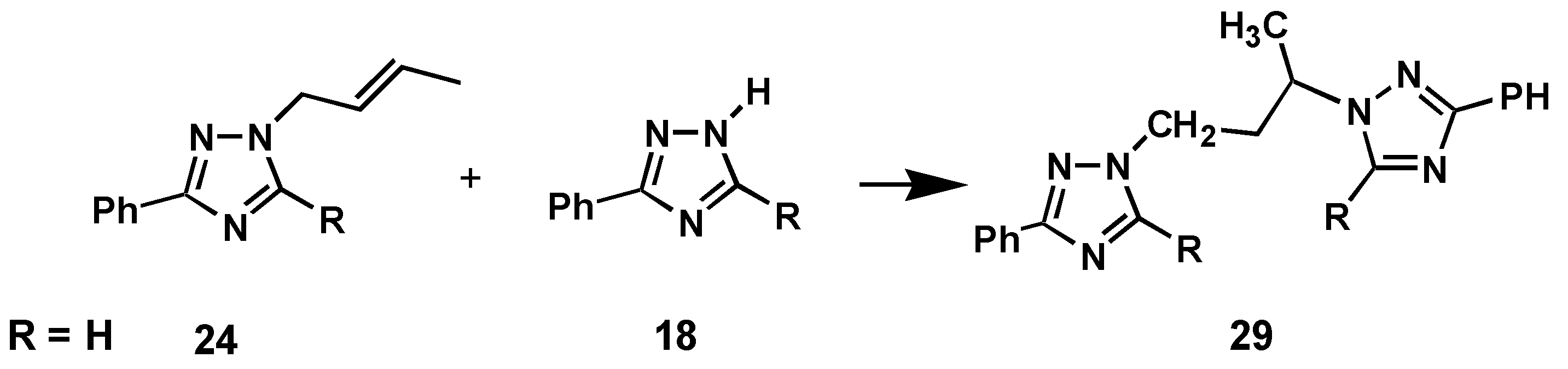
The build-up of high concentrations of
18 may also be a prerequisite for the formation of product
29, which may be formed by a simple addition of triazole
18 over the double bond in the unsaturated side chain of the rearranged triazole
24 (or
25) as indicated in
Scheme 3.
Scheme 3.
Formation of product 29
Scheme 3.
Formation of product 29
Support for this mechanism was established by a control experiment, where thermolysis under the standard conditions of a mixture of the authentic triazoles 18 and 24 gave rise to formation of the same product 29.
Thermolysis of the 4-benzyl substituted triazoles was straightforward as well, yielding exclusively the corresponding 1- and 2-benzyl substituted products (
Table 1). Contrary to what was observed for 3,5-diphenyl triazoles, formation of substituted pyridines was never observed.
Triazoles
1,
7 and
12 showed a preference for group migration to the triazole
N1-atom. In the context of the proposed S
N2-type mechanism, this was in reasonable agreement with the selectivities observed for alkylation of triazoles
6 and
18 (
Table 4). Uda
et al. [
4] in a study showed that the selectivity in the
N-alkylation of 1,2,4-triazoles was mainly controlled by steric factors. In our study thermolysis of the benzyl substituted triazole
12 gave a 58 : 42 mixture of the 1- and 2-benzyl triazoles.
Table 4.
Regioselectivities for thermolysis of the neat allyl substituted triazoles at 320 °C and for alkylation of the 1
H-triazoles.
Table 4.
Regioselectivities for thermolysis of the neat allyl substituted triazoles at 320 °C and for alkylation of the 1H-triazoles.
| Compound | Thermolysis a : b | Alkylation a : b |
| 1 | 58:42 | 93:7 |
| 7 | 55:45 | 80:20 |
| 12 | 58:42 | 90:10 |
| 15 | 100:0 | 89:11 |
| 23 | 100:0 | 96:4 |
| 30 | 77:33 | 64:36 |
The 3-phenyl group may stabilize a developing negative charge on the
N-1 atom, in agreement with reports by Gautun
et al. [
2] that electronic effects may control the regioselectivity. The regioselectivity in the rearrangement was further compared to the selectivity observed for the alkylation reactions with the anion of triazoles
6 and
18 in DMF. Conveniently, this study also supplied necessary reference compounds.
Triazole
6 was obtained as described by Francis
et al.[
5] Synthesis of
18, using a procedure described by Jacobs
et al. [
6] failed to give a satisfactory product. A viable alternative was deamination of 4-amino-3-phenyl-4
H-1,2,4-triazole using a modification of the procedure reported by Hoggarth [
7]. In this case the crude product consisted of a 2:3 mixture of the desired 3-phenyl-1
H-
1,2,4-triazole,
18, although obtained in merely 17 % after sublimation, together with 3,5-diphenyl-
1H-1,2,4-triazole! The mechanism for the formation of this product is not yet understood.
Allylations of
6 and
18 were carried out in DMF with the appropriate bromo-alkenes in the presence of sodium hydride. The reactions with
6 readily gave the desired products in good yields. However, alkylations of
18 were less successful, as all products were isolated in low yields only, 3-10 % after preparative chromatography. An important competing reaction appeared to be decomposition of
18 under the reaction conditions. The nature of this decomposition is so far not clear. Triazoles
6 and
18 yielded mixtures of the 1- and 2- substituted products. Typically, benzylation of
6 yielded products
13 and
14 in a 90:10 ratio. The regioisomers were all isolated by preparative TLC or flash chromatography. The identities of the regioisomers were established by NMR and NOE-measurements.
E.g., the assignment of structure
13 was based on the increased of intensity of the CH
3-NMR signal upon irradiation of the benzylic CH
2-signal. In addition, the
1H-NMR spectra of all the
N-1-alkylation products, the
orto-protons of the 3-phenyl group were shifted 0.4-0.5 ppm downfield to approx. 8.0 ppm, relative to triazole substituted at the
N-2 nitrogen. The regioselectivities were then determined by GLC analysis. The results are summarized in
Table 4.
The tendency in regioselectivity was the same for thermolysis at 320 °C as for alkylation at room temperature. Ratios were in better agreement for the less hindered 5-hydrogen-substituted systems than for the more hindered 5-methyl substituted triazoles. These results therefore constitute an additional support for the proposed nucleophilic displacement mechanism.
Other groups have studied thermal rearrangement reactions with triazoles. Thus Gilchrist and co- workers[
13] showed that 3,4,5-triphenyl-triazoles undergo a 1,5-shift reaction under vacuum flash conditions. Similar observations were done by Habraken
et al. [
8] for 1-nitro-1,2,4-triazoles. In light of these results, it may be surprising that in our study, no sign of products were isolated or could be detected, that corresponded to migration of the allyl moiety from 4- to the 3- or 5-positions of the triazoles. The possibility of intramolecular [2,3]-allyl shifts taking place was therefore ruled out.
Experimental
General
The 1H- and 13C-NMR spectra were recorded on a JEOL JNM-EX 400 FT NMR system using tetramethylsilane (TMS) as internal standard. DEPT information is shown in the listings of the individual 13C-NMR spectra. IR spectra were recorded in the gas phase at 270 °C (GC-FTIR) with a Nicolet 10-SXC FT-IR spectrometer equipped with a Carlo Erba HRGC 5160 Mega Series gas chromatograph equipped with a CP-Sil 5 CB capillary column (25 m). GLC analyses were performed on a Perkin-Elmer Autosystem gas chromatograph equipped with a CP-Sil 5 CB capillary column (25 m).
Methyl-3-phenyl-1H-1,2,4-triazole (6)
This compound was prepared according to the procedure described by Francis et al. [
5] The MS [
9] and
1H-NMR [
10] spectroscopic properties were in agreement with those described in the literature.
13C-NMR (100 MHz, CDCl
3): δ 12.3, 127.6, 130.1, 131.1, 157.3, 161.3 ppm; GC-FTIR: 3499, 3071, 2946, 1549, 1507, 1448, 1407, 1351, 1257, 1145, 1061, 1025, 1002, 775, 725 cm
-1.
4-Amino-3-phenyl-1H-1,2,4-triazole (36).
2-Phenyl-1,3,4-oxatriazole [
11], (96.71 g, 0.66 mol) was mixed with 260 mL of hydrazine hydrate (99%) and the mixture heated at 140 °C in a sealed tube over night. The precipitate was formed was isolated by filtration to yield 63.3 g (79%) of
36 as white needle shaped crystals, (m.p. 86-88 °C).
3-Phenyl-1H-1,2,4-triazole (18).
To a solution of 4-Amino-3-phenyl-1
H-1,2,4-triazole,
36, (30.0 g, 0.19 mol) in concentrated hydrochloric acid (650 mL) was slowly added sodium nitrite (90 g) dissolved in 1.5 L of water. The mixture was stirred over night, filtered and extracted with dichloromethane (2x750 mL). The aqueous phase was concentrated under reduced pressure and the residue dissolved in ethanol and filtered in order to remove sodium chloride. The crude product was a 3:2 mixture of 3,5-diphenyl-1,2,4-triazole and 3-phenyl-1,2,4-triazole (
18), which was obtained after repeated sublimation (140 °C, 5 mmHg) in 4.71 g (17 %) yield as a white powder with melting point 115-117 °C (Lit. 119 °C [
8]). The product was unstable.
1H-NMR (400 MHz, CDCl
3): δ 7.38-7.51 (m, 3H), 7.74-7.91 (m, 2H), 8.13 (s, 1H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 126.9, 128.6, 131.9, 132.7, 168.2 ppm; FT-IR: 3505, 3076, 3043, 1498, 1442, 1349, 1272, 1189, 1138, 1079, 1018, 978, 706 cm
-1; MS [
m/z (% rel. int.)]: 160 (100,
M+), 159(12), 145(8), 136(6), 105(38), 104(80), 103(14), 84(85), 77(48).
4-Allyl-5-methyl-3-phenyl-4H-1,2,4—triazole (
1),
4-benzyl-5-methyl-3-phenyl-1,2,4-4H-triazole (
12),
4-allyl-3-phenyl-4H-1,2,4-triazole (
15), and
4-benzyl-3-phenyl-4H-1,2,4-triazole (
30) were prepared as described for similar systems in the literature [
3] .
4-(Trans-2-butenyl)-5-methyl-3-phenyl-4H-1,2,4-triazole (7).
A solution containing 2-methyl-5-phenyl-1,3,5-oxadiazole (1.53 g, 9.58 mmol) and crotylamine (1.02 g, 14.4 mmol) in toluene (4 mL) was refluxed for 7 days. The crude product (2.05 g), gave after crystallization from toluene gave 0.98 g (48%) of pure 7 as colorless crystals. Mp. 103-104 °C. 1H-NMR (400 MHz, CDCl3): δ 1.71-1.73 (m, 3H), 2.47 (s, 3H), 4.44-4.47 (m, 2H), 5.41-5.56 (m, 2H), 7.46-7.49 (m, 3H), 7.59-7.62 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 11.1 (CH3), 17.6 (CH3), 45.7 (CH2), 124.5 (CH), 127.6 (C), 128.7 (CH), 128.8 (CH), 129.5 (CH), 129.9 (CH), 152.3 (C), 154.8 (C) ppm; GC-FTIR: 3072, 3035, 2935, 2874, 1525, 1476, 1411, 1352, 1292, 1236, 964, 766 cm-1; MS [m/z (% rel. int.)]: 213(78, M+), 212(4), 198(14), 160(7), 159(59), 131(4), 130(3), 118(36), 104(30), 103(10), 77(24). Found M+: 213.1263 Calc. for C13H15N3: 213.1266; Analysis: Calc. for C13H15N3 ; C, 72.21; H, 7.09; N, 19.70 Found: C, 72.42; H, 7.35; N, 19.66.
4-(Trans-2-butenyl)-3-phenyl-4H-1,2,4-triazole (23).
Refluxing a solution containing 2-phenyl-1,3,4-oxadiazole (2.26 g, 15.5 mmol) and crotylamine (1.70 g, 23.9 mmol) in toluene (4 mL) for 3 days, gave a crude product which after flash column chromatography (silica gel/acetone), yielded 1.50 g (49%) of the pure product as a colorless oil; 1H- NMR (400 MHz, CDCl3): δ 1.72-1.74(m, 3H), 4.55-4.57(m, 2H), 5.53-5.70(m, 2H), 7.48-7.50(m, 3H), 7.61-7.64(m, 2H), 8.21(s, 1H) ppm; 13C-NMR(100 MHz, CDCl3): δ 17.6 (CH3), 46.9 (CH2), 124.6 (CH), 126.8 (C), 128.7 (CH), 128.8 (CH), 130.0 (CH), 131.2 (CH), 144.3 (CH), 153.8 (C) ppm; GC-FTIR: 3073, 3037, 2933, 1494, 1471, 1377, 1361, 1188, 1066, 1023, 962, 815, 766 cm-1; MS [m/z (% rel. int.)]: 199(86, M+), 198(6), 184(19), 170(3), 145(69), 118(15), 104(30), 89(14), 77(10). Found M+: 199.1106 Calc. for C12H13N3: 199.1110; Analysis: Calc. for C12H13N3 ; C, 72.34; H, 6.58; N, 21.09 Found: C, 72.55; H, 6.69; N, 20.78.
Base catalyzed isomerization of allylic substituted triazoles. General procedure.
To solutions containing the appropriate triazole (0.15-0.20 g) in dry THF (5 mL) was added catalytic amounts of potassium tert-butoxide (0.02 g). The reaction mixtures were stirred at room temperature for 6 days, then quenched by addition of water and the solvent evaporated under reduced pressure. The residues were dissolved in dichloromethane (20 mL) and the solutions dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The pure products were obtained after flash chromatography or recrystallization.
5-Methyl-3-phenyl-4-(cis-vinyl)-4H-1,2,4-triazole (2).
Prepared from 1 (0.15 g) yielding an oil containing 95 % of 2 (together with 5 % of the trans-product). 1H-NMR (400 MHz, CDCl3): δ 1.35 (dd, J=1.9, 7.0 Hz, 3H), 2.32 (s, 3H), 5.92 (dq, J=7.5, 7.1 Hz, 1H), 6.40 (dq, J= 7.9, 1.9 Hz, 1H), 7.33-7.35 (m, 3H), 7.65-7.68 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 11.0, 12.3, 121.9, 127.5, 127.9, 128.6 (two peaks), 129.7, 130.9, 152.4, 153.6 ppm; GC-FTIR: 3071, 3050, 2938, 1660, 1522, 1473, 1447, 1407, 1373, 1334, 979, 928, 762 cm-1; MS [m/z (% rel. int.)]: 199(100, M+), 198(13), 185(8), 184(38), 131(15), 130(11), 104(19), 103(12), 82(17), 77(16), 76(5).
5-Methyl-3-phenyl-4-(trans-vinyl)-4H-1,2,4-triazole (3).
This compound was not be isolated from the mixture with 2. The yield in the isomerization reaction was low. The identification of 3 was based on the GC-IR data. GC-FTIR: 3071, 2939, 1673, 1524, 1474, 1409, 1331, 1027, 980, 934, 765 cm-1.
3-Phenyl-4-(cis-1-propenyl)-4H-1,2,4-triazole (16).
Prepared by isomerization of 4-allyl-3-phenyl-4H-1,2,4-triazole (15). The crystalline product contained 96 % of 16 together with 4 % of the trans-compound 17; 1H-NMR (400 MHz, CDCl3): δ 1.73 (dd, J=7.1, 1.9 Hz, 3H), 5.88 (dq, J=8.4, 7.1 Hz, 1H), 6.55 (dq, J=8.4, 1.9 Hz, 1H), 7.45-7.48 (m, 3H), 7.78- 7.80 (m, 2H), 8.22 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3): δ 12.3, 121.8, 126.2, 126.7, 128.4, 128.7, 130.1, 144.1, 153.1 ppm; GC-FTIR: 3070, 2932, 1662, 1484, 1404, 1379, 1187, 937, 819, 768, 741 cm-1; MS [m/z (% rel. int.)]: 185(100, M+), 184(24), 171(8), 170(63), 104(20), 103(11), 77(14); Analysis: Calc. for C11H11N3 ; C, 71.33; H, 5.99; N, 22.69. Found: C, 71.21; H, 6.18; N, 22.88.
Phenyl-4-(trans-1-propenyl)-1H-1,2,4-triazole (17).
1H-NMR (400 MHz, CDCl3): δ 1.87 (dd, J=6.8, 1.5 Hz, 3H), 6.06 (dq, J=14.3, 6.8 Hz, 1H), 6.59 (dq, J=14.3, 1.5 Hz, 1H), 7.49-7.51 (m, 3H), 7.70-7.72 (m, 2H), 8.35(s, 1H) ppm; 13C-NMR (100 MHz, CDCl3): δ 15.1, 121.9, 122.7, 126.6, 128.8, 128.9, 130.2 ppm; GC-FTIR: 3073, 2933, 1487, 1385, 1308, 1198, 1068, 1025, 940, 817, 764 cm-1; GC-MS [m/z (% rel. int.)]: 185(100, M+), 184(37), 171(11), 170(87), 158(4), 143(5), 104(31), 103(16), 90(10), 89(11), 77(31).
Alkylation of 1,2,4-triazoles. General procedure.
To a solution of the appropriate triazole (6 or 18) in DMF (20 mL/g of triazole) under a nitrogen atmosphere was added sodium hydride (1-2.5 eq). The mixture was stirred for 1 h at room temperature and then added the alkenyl bromide (1.5 eq). The resulting reaction mixture was stirred overnight, and then added water. The solution was concentrated under reduced pressure and dissolved in dichloromethane. The solution was washed with 1 M HCl, 1M NaOH, water and brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Reaction mixtures from the alkylation of 18 were dissolved in ethanol and filtered before the solvent was evaporated under reduced pressure. The products were separated by preparative TLC (silica / multi elution with chloroform).
1-Allyl-5-methyl-3-phenyl-1H-1,2,4-triazole (4) and 1-allyl-3-methyl-5-phenyl-1H-1,2,4-triazole (5).
Prepared from 6 (1.31 g, 8.26 mmol), sodium hydride (0.20 g, 8.26 mmol) and allyl bromide (1.50 g, >12.4 mmol) and gave a 93:7 mixture of 4 and 5. The components were separated by preparative thin layer chromatography. Compound 4: 1H-NMR (400 MHz, CD3OD): δ 2.43 (s, 3H), 4.65 (dt, J=5.3, 0.6 Hz, 2H), 5.06 (dd, J=17.3, 0.6 Hz, 1H), 5.20 (dd, J=10.3, 0.6 Hz, 1H), 5.90 (ddt, J=15.6, 10.3, 5.2 Hz, 1H), 7.32-7.41 (m, 3H), 8.04-8.06 (m, 2H) ppm; 13C-NMR (100 MHz, CD3OD): δ 11.9, 50.9, 116.8, 126.1, 129.0, 131.0, 131.9, 132.3, 152.9, 160.7 ppm; GC-FTIR: 3072, 2996, 2943, 1521, 1475, 1445, 1348, 1298, 1128, 991, 927, 787, 725 cm-1; MS [m/z (% rel. int.)]: 199(100, M+), 198(31), 184(6), 129(12), 104(21), 103(15), 77(10), 76(5); Found M+: 199.1108. Calc. for C12H13N3: 199.1110; Analysis: Calc. for C12H13N3; C, 72.34; H, 6.58; N, 21.09 Found: C, 72.18; H, 6.44; N, 21.35; Compound 5: 1H-NMR (400 MHz, CD3OD): δ 2.44 (s, 3H), 4.76 (dt, J=6.7, 1.5 Hz, 2H), 5.16 (dd, J=18.1, 0.9 Hz, 1H), 5.32 (dd, J=11.1, 0.8 Hz, 1H), 6.05 (ddt, J= 15.5, 10.3, 5.2 Hz, 1H), 7.47-7.49 (m, 3H), 7.63-7.64 (m, 2H) ppm; 13C-NMR (100 MHz, CD3OD): δ 13.9, 51.3, 118.3, 128.0, 128.6, 128.8, 130.1, 132.4, 160.3 ppm; GC-FTIR: 3075, 3040, 2995, 2945, 1506, 1456, 1413, 1381, 1338, 1288, 1248, 1182, 1016, 925, 805, 768 cm-1; MS [m/z (% rel. int.)]: 199(100, M+), 198(38), 184(6), 174(4), 159(4), 104(19), 77(10). Found M+: 199.1108 Calc. for C12H13N3: 199.1110
Trans 1-(2-Butenyl)-5-methyl-3-phenyl-1H-1,2,4-triazole (8) and trans-1-(2-butenyl)-3-methyl-5-phenyl-1H-1,2,4-triazole (9).
Prepared from 6 (1.18 g, 7.40 mmol), sodium hydride (0.18 g, 7.40 mmol) and crotyl bromide (80 % trans) (1.50 g, 11.10 mmol) in DMF (12 mL) yielding a 82:18 mixture of 8 and 9. Compound 8: 1H-NMR (400 MHz, CDCl3): δ 1.70 (dd, J=6.4, 1.5 Hz, 3H), 2.46 (s, 3H), 4.66 (dd, J= 4.1, 1.5 Hz, 2H), 5.61-5.68 (m, 2H), 7.33-7.49 (m, 3H), 8.05-8.07 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 12.0, 13.1, 17.6, 45.6, 50.5, 123.9, 124.6, 126.1, 128.5, 128.6, 128.9, 129.9, 131.2, 152.4, 152.6, 160.6 ppm; GC-FTIR: 3071, 3039, 2937, 2873, 1521, 1475, 1445, 1349, 1297, 1127, 965, 808, 782, 725 cm-1; MS [m/z (% rel. int.)]: 213(100, M+), 212(4), 199(7), 198(36), 160(12), 159(100), 118(19), 104(27), 103(12), 77(12). Found M+: 213.1269 Calc. for C13H15N3: 213.1266; Analysis: Calc. for C13H15N3 ; C, 72.34; H, 6.58; N, 21.09 Found: C, 72.66; H, 6.87; N, 20.78. Compound 9: 1H-NMR (400 MHz, CDCl3): δ 1.74 (d, J=4.9 Hz, 3H), 2.45 (s, 3H), 4.70 (d, J=3.4 Hz, 2H), 5.60-5.73 (m, 2H), 7.46-7.49 (m, 3H), 7.62-7.65 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 13.9, 17.8, 46.3, 50.8, 124.7, 125.2, 128.1, 128.5, 128.7, 130.0, 154.9, 160.1 ppm; GC-FTIR: 3073, 2945, 2872, 1504, 1458, 1412, 1380, 1340, 1242, 1017, 964, 811, 771, 725 cm-1; MS [m/z (% rel. int.)]: 213(73, M+), 212(9), 199(7), 198(54), 197(5), 172(5), 160(10), 159(100), 188(35), 104(26), 77(12). Found M+: 213.1269 Calc. for C13H15N3: 213.1266; Analysis: Calc. for C13H15N3 ; C, 73.21; H, 7.09; N, 19.70. Found: C, 73.44; H, 6.78; N, 19.95.
1-Benzyl-5-methyl-3-phenyl-1H-1,2,4-triazole (13), and 1-benzyl-3-methyl-5-phenyl-1H-1,2,4-triazole, (14).
Prepared from 6 (0.50 g, 3.14 mmol), sodium hydride (0.19 g, 7.85 mmol) and benzyl bromide (0.81 g, 3.14 mmol) in DMF (8 mL) yielding a 90:10 mixture of 13 and 14. Compound 13: 1H-NMR (400 MHz, CD3OD): δ 2.45 (s, 3H), 5.40 (s, 2H), 7.24-7.27 (m, 3H), 7.31-7.44 (m, 5H), 7.98-8.00 (m, 2H) ppm; 13C-NMR (100 MHz, CD3OD): δ 11.8, 53.1, 127.2, 128.3, 129.2, 129.7, 130.0, 130.4, 131.9, 137.0, 155.0, 161.6 ppm; GC-FTIR: 3073, 3038, 2945, 1521, 1475, 1445, 1348, 1301, 1175, 1108, 1028, 804, 725 cm-1; MS [m/z (% rel. int.)]: 249(92, M+), 248(22), 234(9), 172(5), 146(3), 104(13), 91(100), 77(8). Found M+: 249.1264 Calc. for C16H15N3: 249.1266; Analysis: Calc. for C16H15N3 ; C, 77.08; H, 6.06; N, 16.85. Found: C, 77.25; H, 6.17; N, 16.56; Compound 14: 1H-NMR (400 MHz, CD3OD): δ 2.40 (s, 3H), 5.40 (s, 2H), 7.09-7.11 (m, 2H), 7.28-7.32 (m, 3H), 7.51-7.58 (m, 5H) ppm; 13C-NMR (100 MHz, CD3OD): δ 11.0, 52.0, 125.7, 126.3, 127.5, 128.3, 128.5, 128.7, 129.7, 135.6, 155.2, 160.0 ppm; GC-FTIR: 3073, 3038, 2946, 1503, 1455, 1412, 1379, 1338, 1243, 1019, 915, 815, 765, 727 cm-1; MS [m/z (% rel. int.)]: 249(58, M+), 248(24), 179(3), 177(5), 149(12), 137(8), 125(13), 123(14), 113(10), 111(19), 109(14), 105(13), 104(11), 99(13), 97(35), 96(13), 95(17), 91(85), 85(38), 84(13), 83(31), 82(11), 81(19), 77(13). Found M+: 249.1264 Calc. for C16H15N3: 249.1266
1-Allyl-3-phenyl-1H-1,2,4-triazole (21).
Prepared from 18 (0.50 g, 5.29 mmol), sodium hydride (0.10 g, 3.45 mmol) and allyl bromide (0.64 g, 5.29 mmol) in DMF (10 mL) yielding a 89:11 mixture of 21 with 1-allyl-5-phenyl-1H-1,2,4-triazole (22). Compound 21: 1H-NMR (500 MHz, CDCl3): δ 4.83 (d, J= 5.8 Hz, 2H), 5.32-5.37 (m, 2H), 6.03- 6.08 (ddt, J= 17.5, 10.0, 5.0 Hz, 1H), 7.38-7.45 (m, 3H), 8.07-8.11 (m, 3H) ppm; 13C-NMR (100 MHz, CDCl3): δ 50.9, 120.0, 126.3, 128.6, 128.8, 129.2, 131.3, 143.6 ppm; GC-FTIR: 3070, 3036, 2981, 2944, 1522, 1496, 1442, 1330, 1292, 1202, 979, 930, 775, 726 cm-1; MS [m/z (% rel. int.)]: 185(70, M+), 184(23), 179(5), 159(19), 158(9), 145(18), 138(12), 118(10), 105(10), 104(47), 103(13), 91(18), 89(11), 84(12), 82(22), 81(12), 77(16). Found M+:185.0951 Calc. for C11H11N3: 185.0953; Analysis: Calc. for C11H11N3 ; C, 71.33; H, 5.99; N, 22.69. Found: C, 71.54; H, 6.29; N, 22.48.
1-(Trans-2-butenyl)-3-phenyl-1H-1,2,4-triazole (24), and 1-(cis-2-butenyl)-3-phenyl-1H-1,2,4-triazole, (25).
Prepared from 18 (0.50 g, 3.45 mmol), sodium hydride (0.10 g, 4.17 mmol) and crotyl bromide (0.70 g, 5.18 mmol) in DMF (10 mL) yielding a 66:28:6 mixture of 24, 25 and an unidentified product, probably the 1-(2-butenyl)-5-phenyl-1H-1,2,4-triazole. The products were inseparable by thin layer chromatography. Compound 24: 1H-NMR (500 MHz, CDCl3): δ 1.78 (dd, J=6.3, 1.5 Hz, 3H), 4.76 (d, J=6.3 Hz, 2H), 5.68-5.78 (m, 1H), 5.80-5.87 (m, 1H), 7.39-7.68 (m, 3H), 8.07-8.11 (m, 3H) ppm. Compound 25: 1H-NMR (500 MHz, CDCl3): δ 1.81 (dd, J= 1.0, 7.0 Hz, 3H), 4.86 (d, J=6.8 Hz, 2H), 5.68-5.78 (m, 1H), 5.87-5.94 (m, 1H), 7.39-7.68 (m, 3H), 8.07-8.11(m, 3H) ppm. The mixture of 24 and 25 exhibited the following spectroscopic properties: 13C-NMR (100 MHz, CDCl3): δ 17.8, 29.7, 46.5, 51.9, 122.8, 124.0, 126.3, 126.4, 128.6, 128.9, 129.2, 130.0, 130.9, 132.1, 143.1, 143.3 ppm; GC-FTIR: 3069, 3040, 2936, 1521, 1496, 1443, 1324, 1293, 1200, 1026, 966, 842, 790, 725, 694 cm-1; MS [m/z (% rel. int.)]: 199(49, M+), 184(6), 146(10), 145(100), 118(15), 104(32), 77(9). Found M+: 199,1108 Calc. for C12H13H3: 199.1110
1-Benzyl-3-phenyl-1H-1,2,4-triazole (31), and 1-benzyl-5-phenyl-1H-1,2,4-triazole (32).
Prepared from 18 (0.50 g, 3.44 mmol), sodium hydride (0.10 g, 4.17 mmol) and benzyl bromide (0.88 g, 5.15 mmol) in DMF (10 mL) yielding a 64:36 mixture of 31 and 32 together with large amounts of starting material. The products were unstable, and after work up only 7% of the products were isolated. Compound 31: 1H-NMR (400 MHz, CDCl3): δ 5.38 (s, 2H), 7.30-7.35 (m, 3H), 7.37-7.46 (m, 5H), 8.04 (s, 1H), 8.10-8.12 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 53.7, 126.2, 126.4, 127.9, 128.5, 129.1, 129.2, 130.9, 143.7, 143.9 ppm; GC-FTIR: 3072, 3035, 2946, 1497, 1472, 1445, 1349, 1300, 1176, 1106, 1028, 721 cm -1; MS [m/z (% rel. int.)]: 235(73, M+), 234(11), 132(15), 91(100). Found M+: 235.1108 Calc. for C15H13N3: 235.1110; Analysis: Calc. for C15H13N3 ; C, 76.57; H, 5.57; N, 17.86. Found: C, 76.22; H, 5.78; N, 17.59. Compound 32: 1H-NMR (400 MHz, CDCl3): δ 5.44 (s, 2H), 7.14-7.17 (m, 2H), 7.30-7.36 (m, 3H), 7.44-7.49 (m, 3H), 7.57-7.60 (m, 2H), 8.03 (s, 1H) ppm; 13C-NMR (100 MHz, CDCl3): δ 52.7, 126.9, 127.9, 128.1, 128.8, 128.9, 129.0, 130.2, 135.9, 151.3 ppm; GC-FTIR: 3073, 3037, 2951, 1483, 1457, 1378, 1278, 1239, 1177, 1121, 1015, 881, 767, 724 cm-1; MS [m/z (% rel. int.)]: 235(57, M+), 234(20), 220(3), 158(4), 132(12), 104(12), 91(100). Found M+: 235.1106 Calc. for C15H13N3: 235.1110
1-(1-Methyl-2-propenyl)-5-methyl-3-phenyl-1H-1,2,4-triazole (10) and 1-(1-Methyl-2-propenyl)-3- methyl-5-phenyl-1H-1,2,4-triazole (11).
Isolated from the thermolysis mixture of 7 and isolated by thin layer chromatography of the crude reaction. The products could not be completely separated from 8. Compound 10: 1H-NMR (400 MHz, CDCl3): δ 1.69 (d, J=6.8 Hz, 3H), 2.48 (s, 3H), 4.85-4.92 (m, 1H), 5.08 (d, J=17.3 Hz, 1H), 5.19 (d, J=10.7 Hz, 1H), 6.06 (ddd, J=17.3, 10.7, 7.1 Hz), 7.34-7.40 (m, 3H), 8.07-8.09 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 12.2(CH3), 20.0(CH3), 56.6(CH), 116.1(CH2). Further signals overlapped with those of the main product 8. GC-FTIR: 3072, 2990, 2946, 1519, 1470, 1445, 1413, 1354, 1299, 1256, 1177, 1226, 1063, 1030, 988, 927, 784, 725 cm-1; GC-MS [m/z (% rel. int.)]: 213(57, M+), 199(12), 198(79), 160(12), 159(100), 157(11), 130(15), 128(11), 118(63), 105(10), 104(85), 103(34), 91(14), 89(15), 77(48). Compound 11: 1H-NMR (400 MHz, CDCl3): δ 1.61 (d, J=6.8 Hz, 3H), 2.45 (s, 3H), 4.97-5.05 (m, 1H), 5.07 (d, J=17.5 Hz, 1H), 5.22 (d, J=10.3 Hz, 1H), 6.11 (ddd, J=17.5, 10.3, 5.7 Hz, 1H), 7.46-7.50 (m, 3H), 7.57-7.61 (m, 2H) ppm; 13C-NMR (100 MHz, CDCl3): δ 14.6(CH 3), 21.2(CH3), 56.9(CH), 116.8(CH2), Further signals coincide with those of the main product 9. GC- FTIR: 3075, 2987, 2945, 1504, 1451, 1407, 1381, 1358, 1321, 1181, 1013, 926, 773, 729 cm-1; GC-MS [m/z (% rel. int.)]: 213(28, M+), 212(6), 199(9), 198(66), 160(13), 159(100), 131(10), 130(11), 118(62), 104(56), 103(24), 95(22), 91(13), 89(11), 77(31).
Thermolysis of 15 gave two additional products which could not be separated by the available chromatographic methods. 1H-NMR data, GLC-MS and GLC-FTIR spectra indicated the product to be a mixture of 1-(cis-1-propenyl)-3-phenyl-1,2,4-1H-triazole (19) and 1-(trans-1-propenyl)-3-phenyl- 1,2,4-1H-triazole (20); Compound 19: GC-FTIR: 3070, 2936, 2874, 1676, 1523, 1495, 1446, 1321, 1294, 1233, 1206, 1105, 1023, 934, 842, 794, 725, 693 cm-1; GC-MS[m/z (% rel. int.)]: 186(10), 185(70, M+), 184(11), 158(33), 132(5), 105(22), 104(100), 103(17), 89(4), 82(22), 81(19), 77(30), 76(17); Compound 20,: GC-FTIR: 3069, 2934, 1670, 1523, 1495, 1445, 1317, 1294, 1196, 1103, 1020, 783, 726 cm-1; GC-MS [m/z (% rel. int.)]: 185(79, M+), 184(11), 158(33), 132(5), 105(22), 104(100), 103(19), 82(21), 81(19), 77(27).
The main product after thermolysis of 23 was assigned the structure1,3-di(3-phenyl-1H-1,2,4-triazol-1- yl)-butane (29): 1H-NMR (400 MHz, CDCl3): δ 1.62 (H-4, d, J= 6.8 Hz, 3H), 2.44-2.62 (H-2, m, 2H), 3.97-4.04 (H-1, m, 1H), 4.17-4.24 (H-1, m, 1H), 4.47-4.52 (H-3, m, 1H), 7.38-7.48 (m-, p- Ph, m, 6H), 8.08 (triazole-H, s, 1H), 8.09-8.15 (o-Ph, m, 4H), 8.20 (triazole-H, s, 1H) ppm; HH-COSY: The methyl doublet at 1.62 ppm coupled with the H-3-proton at 4,47- 4.52 ppm. The H-2-protons at 2.44-2.62 ppm coupled with each other as well as with the H-3-proton and the H-1-proton at 3.97-4.02 and the H-1 proton at 4.17- 4.24 ppm respectively. The H-1 and the H-1?-protons also coupled. The meta and para protons of the phenyl groups appeared between 7.4-7.5 ppm. The ortho protons appeared at 8.09-8.15 ppm. The 5-H-triazole protons were observed as singlets at 8.08 and 8.20 ppm, respectively; 13C-NMR (100 MHz, CDCl3): δ 21.2 (CH3), 36.3 (CH2), 46.0 (CH2), 53.2 (CH), 126.3 (CH, two peeks), 128.5 (CH), 128.8 (CH), 129.3 (CH), 129.4 (CH), 130.8 (C), 131.0 (C), 143.3 (CH), 144.2 (CH), 162.9 (C), 163.0 (C) ppm; GC-FTIR: 3070, 2987, 2950, 1521, 1493, 1440, 1334, 1291, 1199, 1107, 1023, 968, 844, 726 cm-1; MS [m/z (% rel. int.)]: 344(57, M+), 200(4), 199(11), 198(4), 187(14), 186(92), 184(6), 174(11), 173(100), 172(85), 159(23), 158(61), 146(21), 145(36), 132(4), 131(9), 118(5), 105(14), 104(82), 89(10), 77(17). Found M+: 344.1751, Calc for C20H20N6: 345.1749; Analysis: Calc. for C20H20N6; C, 69.75; H, 5.85; N, 24.40. Found: C, 69.51; H, 6.09; N, 24.18.
Minor amounts of a compound with very similar properties, which was assumed to be an isomer of 29, was isolated together with minor impurities and exhibited the following spectroscopic properties: GC- FTIR: 3128, 3069, 2985, 2947, 1522, 1494, 1439, 1279, 1202, 1026, 1010, 726, 696, 668 cm-1; GC-MS [m/z (% rel. int.)]: 344(4, M+), 199(7), 198(4), 186(9), 184(8), 174(11), 173(99), 172(78), 159(13), 158(100), 146(14), 145(23), 132(4), 131(11), 118(4), 105(9), 104(81), 103(11), 89(13), 77(22), 76(5). Found M+344.1751, Calc. for C20H20N6: 344.1742