Introduction
o-Quinodimethanes (o-xylylenes) are reactive intermediates widely used for the synthesis of polycyclic compounds via inter- or intramolecular Diels-Alder reactions [
1]. Because of their high reactivity and thermal instability they must be generated
in situ by various methods: dehalogenation of α,α'-dihalo-o-xylenes, thermal or photochemical extrusion of stable molecules or ring opening of benzocyclobutenes. The unsubstituted parent compound, generated at low temperature, has been proven to exist in a singlet ground state [
2]. To our knowledge, all synthetic or mechanistic work in this field has been performed in organic solvents under anhydrous conditions. Recent progress in aqueous Diels-Alder cycloadditions and organometallic reactions [
3], especially the successful Wurtz coupling of benzylic halides [
4] and their Barbier addition to carbonyl compounds [
5], prompted us to study the behaviour of α,α'-dihalo-o-xylenes (
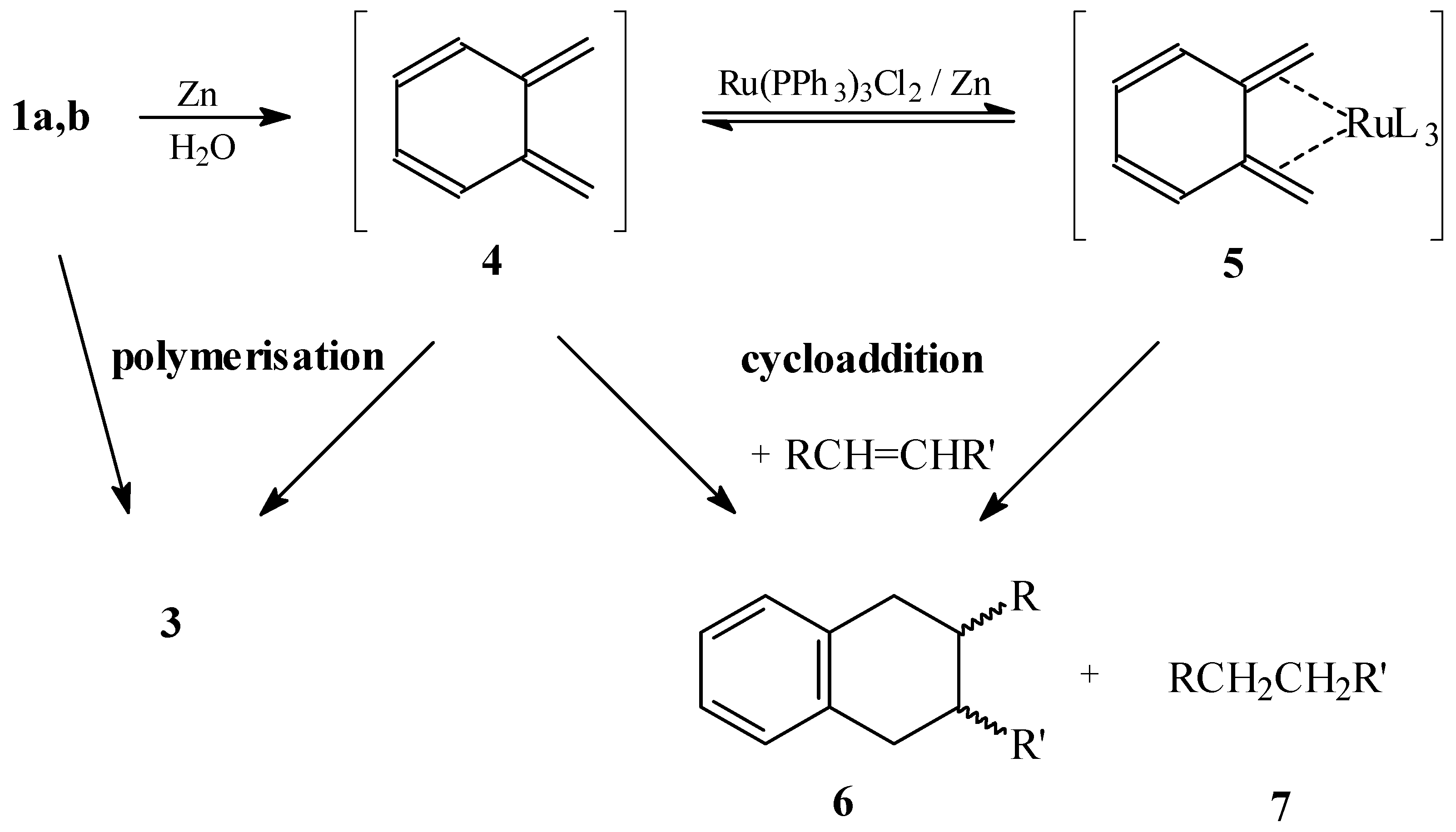
1) under similar conditions in the hope to obtain benzocyclobutene (
2) or, in the presence of dienophiles, cycloadducts of o-quinodimethane.
Results and Discussion
The reaction of the dibromo compound
1a with zinc dust in saturated aqueous ammonium chloride was complete in a few minutes at r.t. and produced a polymeric material
3 containing 67 % of C-C coupled methylene groups and 28 % of methyl groups as a result of reduction; only trace amounts of o-xylene and dimeric or trimeric coupling products, but no
2 at all could be detected by GC/MS (
Table 1, entry 1). These results agree rather well with those obtained by Alder under anhydrous conditions in DMF [
6]. The reaction of the dichloride
1b was much slower (1 h), but gave a very similar product composition (entry 2). Use of metal catalysts such as CuCl
2, CuI, AgNO
3 and Pb(OAc)
2, which had an important influence on the reactivity of benzylic monohalides [
4], brought no significant change.
More promising results were obtained in the presence of dimethyl fumarate (solubilized with CH3CN): 22 % of the trans-cycloadduct 6 was produced with the dibromide and 53 % with the dichloride. In both cases, polymerisation to 3 and reduction of the dienophile to dimethyl succinate were important side reactions (entries 3 and 4). In an attempt to obtain a cleaner reaction and better yield, different temperatures, acidic, neutral and basic salt solutions and several water soluble or unsoluble cosolvents were tried without positive results. Other metals were also tested in different solvent systems: Mg, Al, Mn and Ti gave no cycloadduct at all and In, Sn, Fe and Bi produced only modest yields (15 -30 %). Similarly, 15 different transition metal ions and the triphenylphosphine complexes of Ni, Pd and Ru were examined as catalysts in the Zn-promoted reaction. Cu and Fe were found to increase the amount of the polymer 3 and Co, Ni, Pd and Au catalyzed the reduction of 1b. The only improvement of the cycloaddition reaction was observed with tris-triphenylphosphine ruthenium(II) dichloride; using 5 mg of this catalyst, the yield of 6 raised to 40 % for the dibromide and 84 % for the dichloride (entries 5 and 6); in the latter case, all side reactions were reduced to a few percent. The use of the preformed catalyst was essential, because addition of ruthenium(III) chloride and triphenylphosphine had no effect (entry 7).
Table 1.
Reactions of α,α'-dihalo-o-xylenes (1) and zinc in aqueous mediuma
Table 1.
Reactions of α,α'-dihalo-o-xylenes (1) and zinc in aqueous mediuma
| | Yields (%)b |
|---|
| Entry | X | Salt | Dienophile | Cosolvent | Catalyst | Coupling | Reduction | 6 | 7 |
|---|
| 1 | Br | NH4Cl | - | - | - | 65 | 28 | - | - |
| 2 | Cl | NH4Cl | - | - | - | 62 | 25 | - | - |
| 3 | Br | NH4Cl | Dimethyl fumarate | MeCN | - | 70 | 4 | 22 | 22 |
| 4 | Cl | NH4Cl | Dimethyl fumarate | MeCN | - | 10 | 36 | 53 | 15 |
| 5 | Br | NH4Cl | Dimethyl fumarate | MeCN | Ru(PPh3)3Cl2 | 52 | 8 | 40 | 8 |
| 6 | Cl | NH4Cl | Dimethyl fumarate | MeCN | Ru(PPh3)3Cl2 | 5 | 10 | 84 | 7 |
| 7 | Cl | NH4Cl | Dimethyl fumarate | MeCN | RuCl3 + PPh3 c | 15 | 12 | 48 | 10 |
| 8 | Cl | K3PO4 | Methyl acrylate | MeCN | Ru(PPh3)3Cl2 | 3 | 12 | 85 | - |
| 9 | Cl | K3PO4 | Acrylonitrile | MeCN | Ru(PPh3)3Cl2 | 4 | 13 | 86 | - |
| 10 | Cl | K3PO4 | Methyl vinyl ketone | MeCN | Ru(PPh3)3Cl2 | 3 | 5 | 92 | 1 |
| 11 | Cl | NH4Cl | Dimethyl maleate | MeCN | Ru(PPh3)3Cl2 | 46 | 16 | 37 | 5 |
| 12 | Cl | NH4F | Methyl crotonate | - | Ru(PPh3)3Cl2 | 60 | 5 | 33 | - |
In the following experiments, the dichloride was reacted under standard conditions with other types of dienophiles. Electron deficient terminal olefins such as methyl acrylate, acrylonitrile and methyl vinyl ketone gave even higher yields of cycloaddition when basic conditions were employed to prevent polymerisation (entries 8-10). Somewhat surprisingly, dimethyl maleate produced only 37% of pure
cis-substituted cycloadduct (entry 11), probably due to a less favorable overlap in the endo transition state; in a competion experiment using a mixture of dimethyl fumarate and maleate (1 eq. of each), only the former reacted and produced
trans-cycloadduct
6. Methyl crotonate was also less reactive, giving the same 33 % yield in the presence or absence of catalyst, and unactivated olefins like 1-heptene, styrene and cyclohexene did not react at all. The observed differences in the reactivity agree with those found in previously reported demetalating reactions [
6,
7,
8]. However, the aqueous procedure gives much better yields than the electrochemical generation [
7], uses unexpensive starting materials
, is experimentally simple and avoids the use of DMF, a solvent which is toxic and difficult to dry.
From the mechanistic point of view, the effect of the ruthenium complex is remarkable but not surprising. Indeed, stable o-xylylene complexes of type
5 bearing aryldialkylphosphine ligands have been synthesized and characterized by x-ray analysis [
9]. Furthermore, oxidative decomposition of the
exo-complex in the presence of dimethyl acetylenedicarboxylate has produced a cycloadduct in very low yield [
10]. This cycloaddition under oxidative conditions and the inexistence of a stable, isolated complex with triphenylphosphine ligands suggest a similar, but less stable intermediate in our reaction, probably in equilibrium with the free o-quinodimethane
4 (
Scheme 1). The complexation may prevent the polymerisation of
4, increasing thus its lifetime. Highly reactive dienophiles probably react rapidly with the complex
5 or with free
4. Less reactive olefins need free
4 and have to compete with the polymerisation reaction; for this reason no improvement is observed in the presence of the catalyst. Benzocyclobutene (
2) can be excluded as a possible intermediate because it was recovered unchanged in control experiments in the presence or absence of catalyst.