Facile and efficient synthesis of 3β-hydroxy-eupholanost-8-en-24-one
Abstract
:Introduction
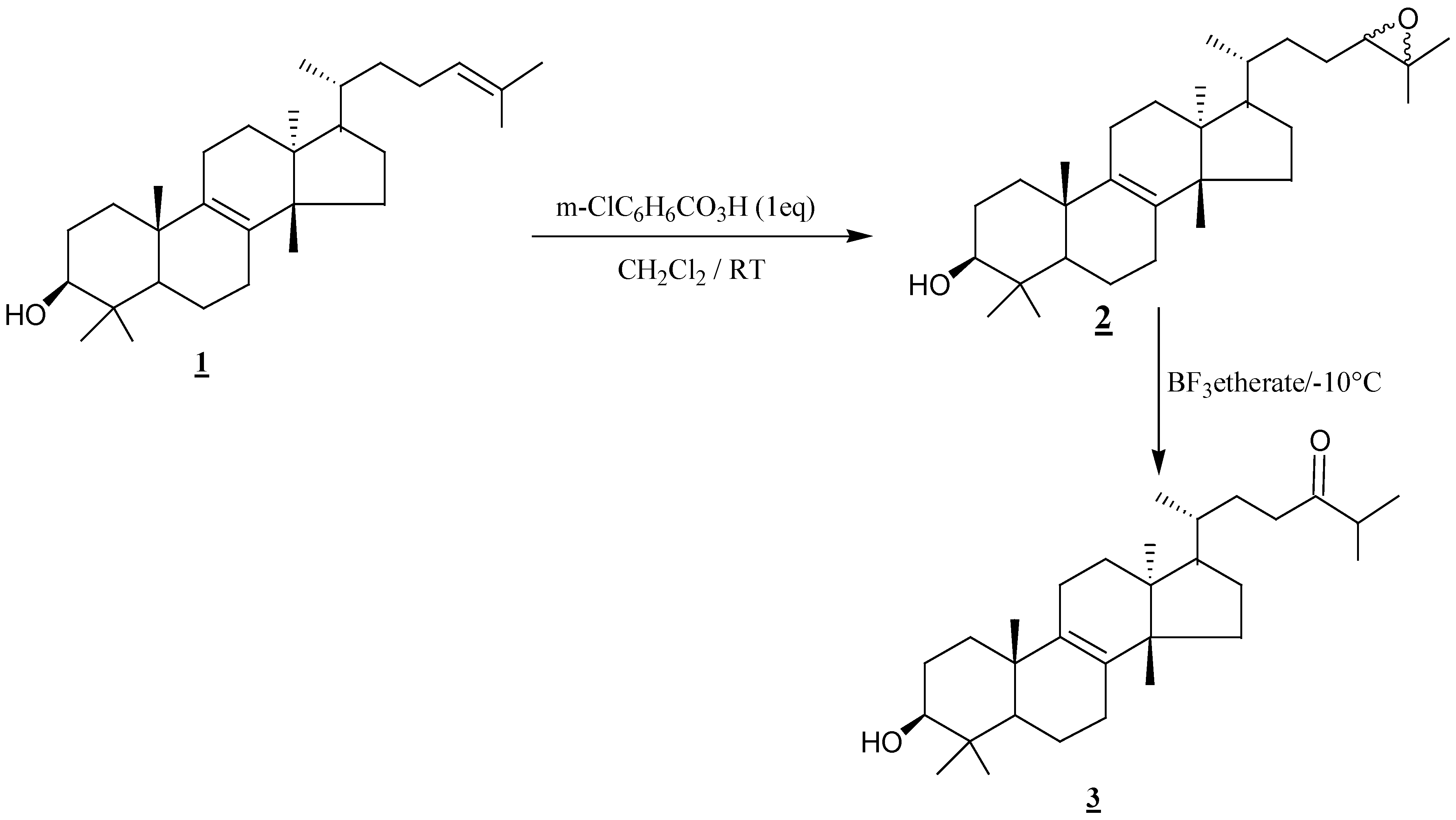
Results and discussion
Conclusions
Experimental
General
Epoxidation of α-euphol
Rearrangement of euphol monoepoxide (2) with boron trifluoride etherate in benzene
References
- Smith, J. G. Synthesis 1984, 629.
- Rao, A.S; Pakinkar, S.K.; Kirtane, J. G. Tetrahedron 1983, 39, 2323.
- Rickborn, B. Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: New York, 1991; vol.3, pp. 733–775. [Google Scholar]
- Barton, D. H. R.; Harrison, D.M.; Moss, G.P. Chem. Commun. 1996, 595.
- Bloch, K.; Urech, J. The Purification of Lanosterol. Biochem. Prep. 1958, 96, 32. [Google Scholar]
- Briggs, L. H.; Bartelly, J. P.; Rutledge, P. S. J. Chem. Soc., Perkin 1 1973, 806.
- Raab, K. H.; De Souza, N. J.; Nes, W .R. Biochem. Biophys. Acta 1968, 152, 742. [PubMed]
- Parish, E. J.; Kizito, S. A; Sun, H. J. Chem. Research (S). 1997, 64.
- Zdzislaw, P.; Martynow, J. J. Chem. Soc., Perkin Trans. 1. 1995, 201.
- Benharref, A.; Lavergne, J.-P. Bull. Soc. Chim. Fr. 1985, 965.
- Kaneshiro, E. S.; Amit, Z.; Swonger, M. M.; Kreishman, G. P.; Brooks, E. E.; Kreishman, M.; Jayasimhulu, K.; Parish, E. J.; Sun, H.; Kizito, S. A.; Beach, D. H. Biochemistry. 1999, 96, 97.
- Sample Availability: Samples of compounds 2 and 3 are available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes
Share and Cite
El Aouad, N.; Benharref, A.; Gree, R. Facile and efficient synthesis of 3β-hydroxy-eupholanost-8-en-24-one. Molecules 2001, 6, 468-471. https://doi.org/10.3390/60500468
El Aouad N, Benharref A, Gree R. Facile and efficient synthesis of 3β-hydroxy-eupholanost-8-en-24-one. Molecules. 2001; 6(5):468-471. https://doi.org/10.3390/60500468
Chicago/Turabian StyleEl Aouad, N., A. Benharref, and R. Gree. 2001. "Facile and efficient synthesis of 3β-hydroxy-eupholanost-8-en-24-one" Molecules 6, no. 5: 468-471. https://doi.org/10.3390/60500468



