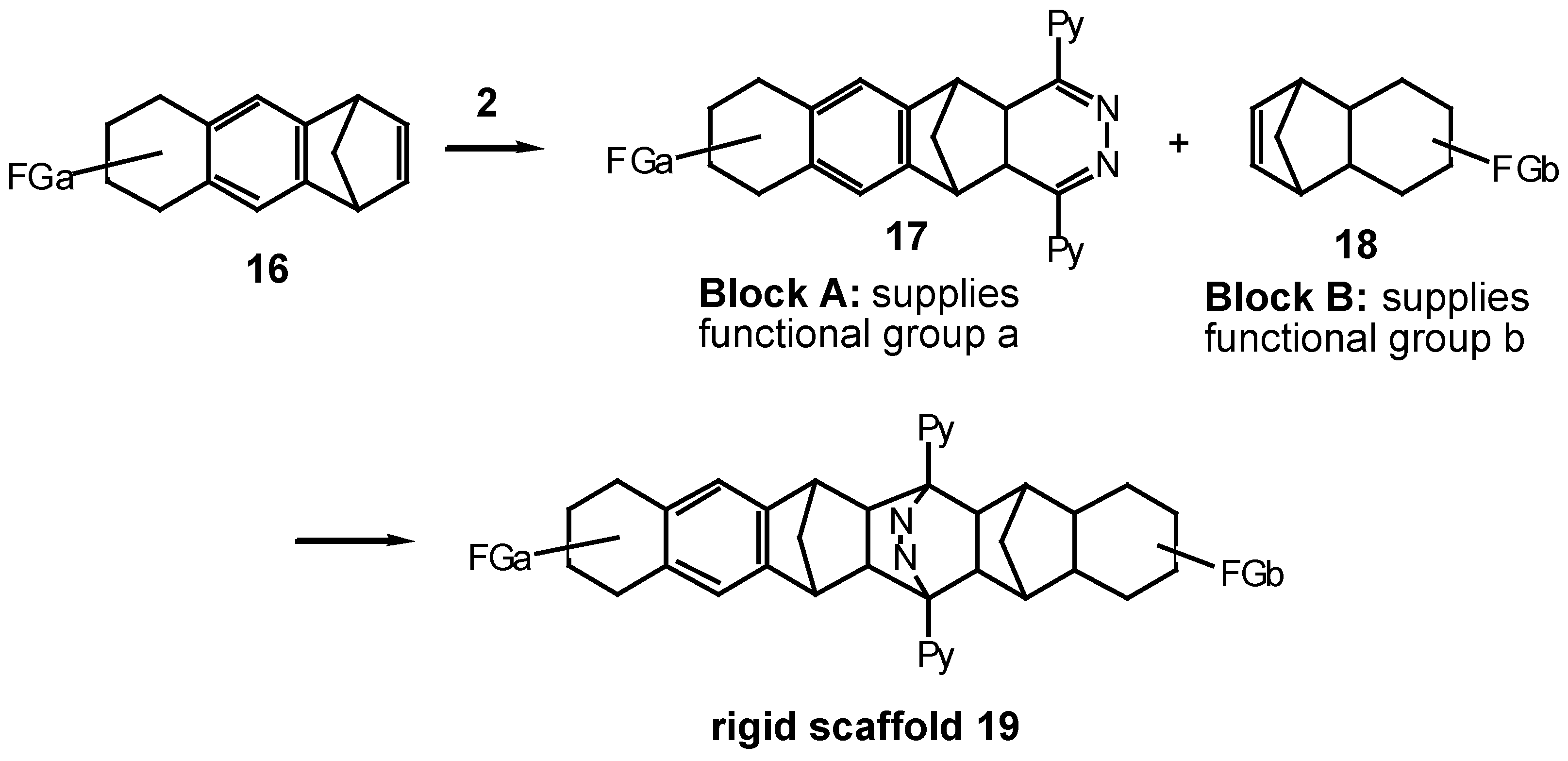
Results and Discussion
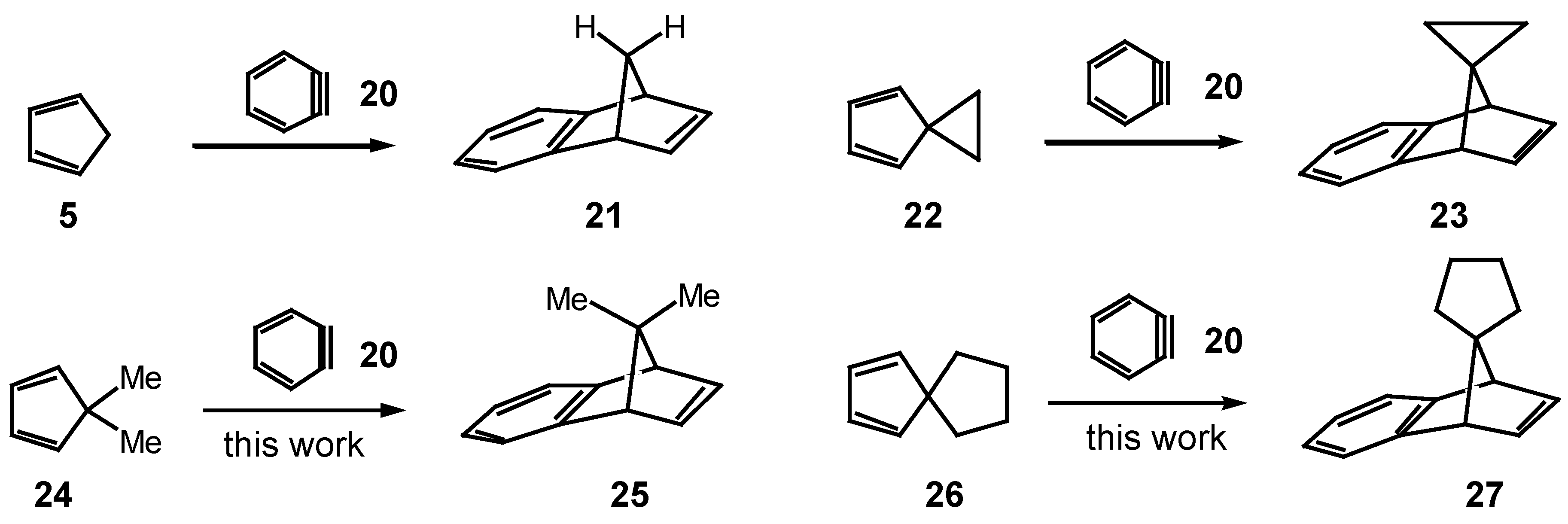
In this report, we describe the reaction of s-tetrazine 2 with benzonorbornadiene 21 and some of its derivatives 23, 25 and 27, where the 7-methano bridge of 21 has been modified by alkyl or ring substituents. The other feature of this study was to establish the effect that modification of the 7- substituent would have on the facial selectivity of attack at the norbornene π-bond and to assess this as a geometry-controlling element in the 'lego' block building program.
At the commencement of this program, the synthesis of benzonorbornadiene
21 and the spirocyclopropyl derivative
23 had been reported (
Scheme 4). The 7,7-dimethyl-derivative
25 was prepared using similar methodology by reacting 5,5-dimethylcyclopentadiene
24 [
9] with benzyne and isolated as a liquid. The
1H-NMR spectrum was very simple reflecting the expected Cs-symmetry of
25: singlets at δ 0.79, 1.25 for the methyl protons, with the higher-field resonance being ascribed to the methyl group under the shielding influence of the aromatic ring; coupled resonances between the bridgehead protons (δ 3.34) and the vinyl protons (δ 6.65) and two sets of finger-like quartets for the aromatic protons at δ 6.8-7.2 are entirely in congruence with the assigned structure.
The spirocyclopentyl derivative
27 was prepared from spiro[4,4]nona-1,3-diene
26 [
10] and benzyne in a similar fashion and it also was a liquid. The
1H-NMR spectrum was essentially identical with
25 except that the singlet methyl protons were replaced with a broad multiplet at δ 1.1-1.9 for the cyclopentyl methylene protons.
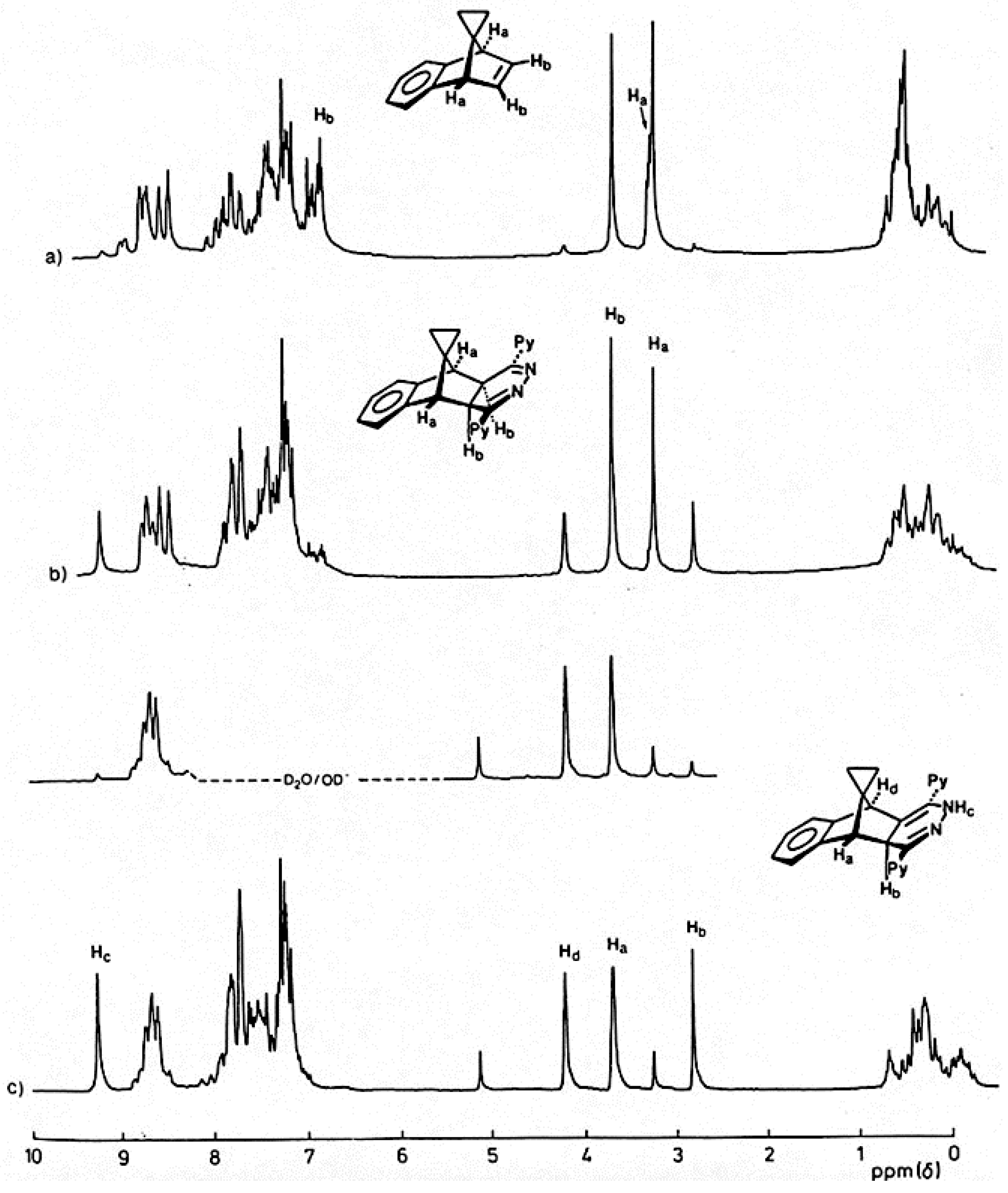
The reaction of the
s-tetrazine
2 with benzonorbornadiene
21 (
Scheme 5) was conducted in CHCl
3 at 30 °C and monitored by
1H-NMR spectroscopy; three traces are shown in
Figure 1. This indicated that significant reaction has occurred after 10 min (Trace 1a) to produce a single compound characterised by having singlet resonances at δ 3.58 and 3.79 assigned to the methine protons Ha, Hb of the 4,5-dihydropyridazine
28. While conversion of
21 to
28 was complete after 30 min, evidence for the formation of a second product had emerged (Trace 1b) and become the almost exclusive product after 2 hours (Trace 1c). This new product has lost the Cs symmetry of its precursor
28 and was assigned the rearranged 1,4-dihydropyridazine structure
29; there is good precedent for this rearrangement [
11]. It was characterised by the presence of four singlets δ 9.27, 4.36, 4.67, 2.67 where the low-field resonance is assigned to the NH proton, the presence of which was already confirmed by IR spectroscopy (ν
max 3350 cm
-1), and this assignment is supported by exchange with DO
- in D
2O. The resonance at δ 2.76 is attributed to the Hb proton of the heterocyclic ring which might be expected to resonate at much lower field as it is doubly allylic, so clearly an upfield component is also operative.
Figure 1.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s-tetrazine 2 and benzonorbornadiene 21 after (a) 10 min at 30 °C; (b) 30 min at 30 °C; (c) 120 min at 30 °C
Figure 1.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s-tetrazine 2 and benzonorbornadiene 21 after (a) 10 min at 30 °C; (b) 30 min at 30 °C; (c) 120 min at 30 °C
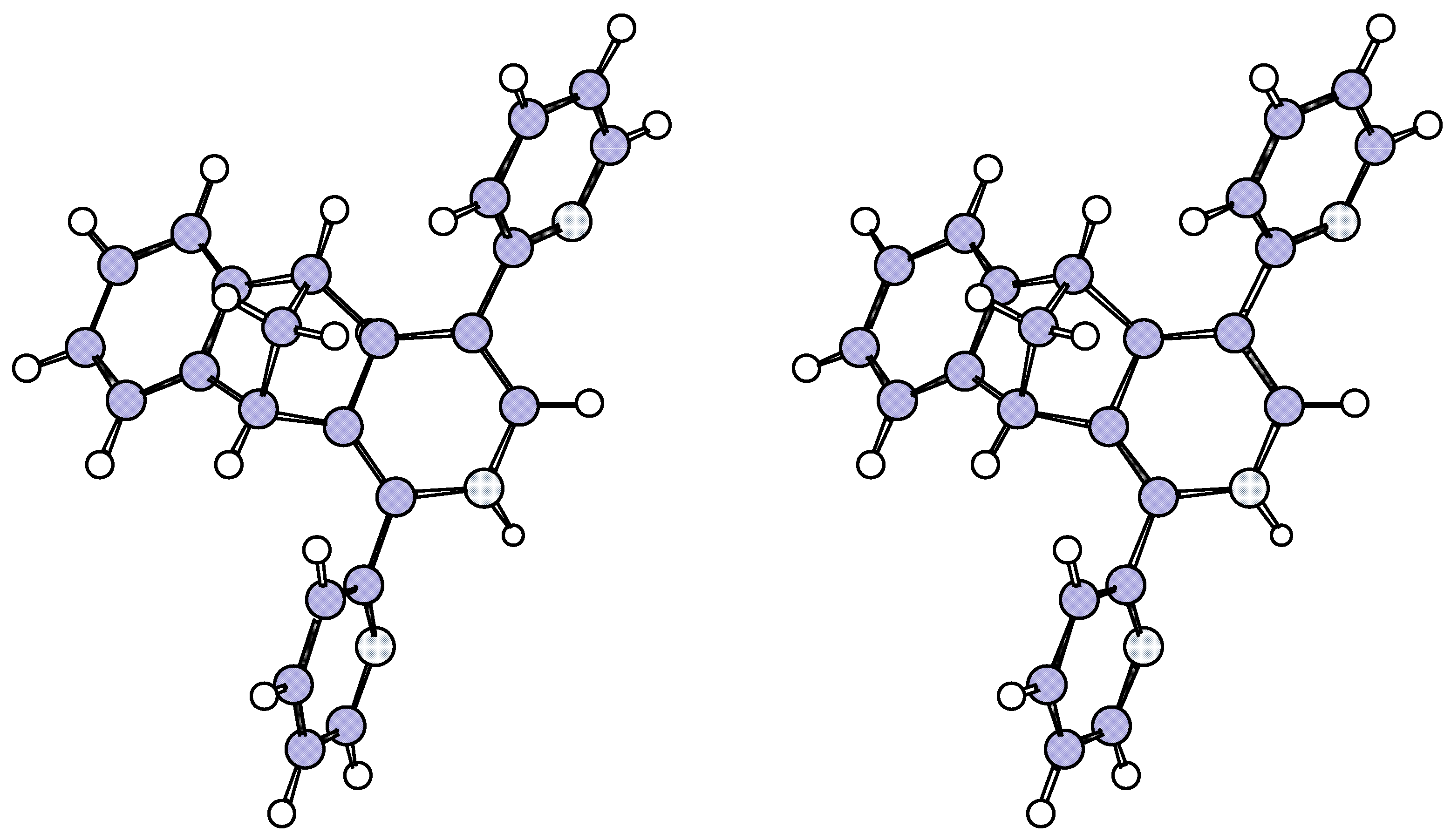
Figure 2.
Stereoview of preferred conformation of 29 determined by the AM1 method.
Figure 2.
Stereoview of preferred conformation of 29 determined by the AM1 method.
Molecular modelling (AM1,
Figure 2) of
29 indicated that the pyridyl ring vicinal to Hb and the phenylene ring each offer shielding to Hb which accounted for its upfield shift. This result also offered indirect confirmation of the
exo fusion in
29 and this was supported by the lack of coupling between Hb and the vicinal bridgehead proton Ha.
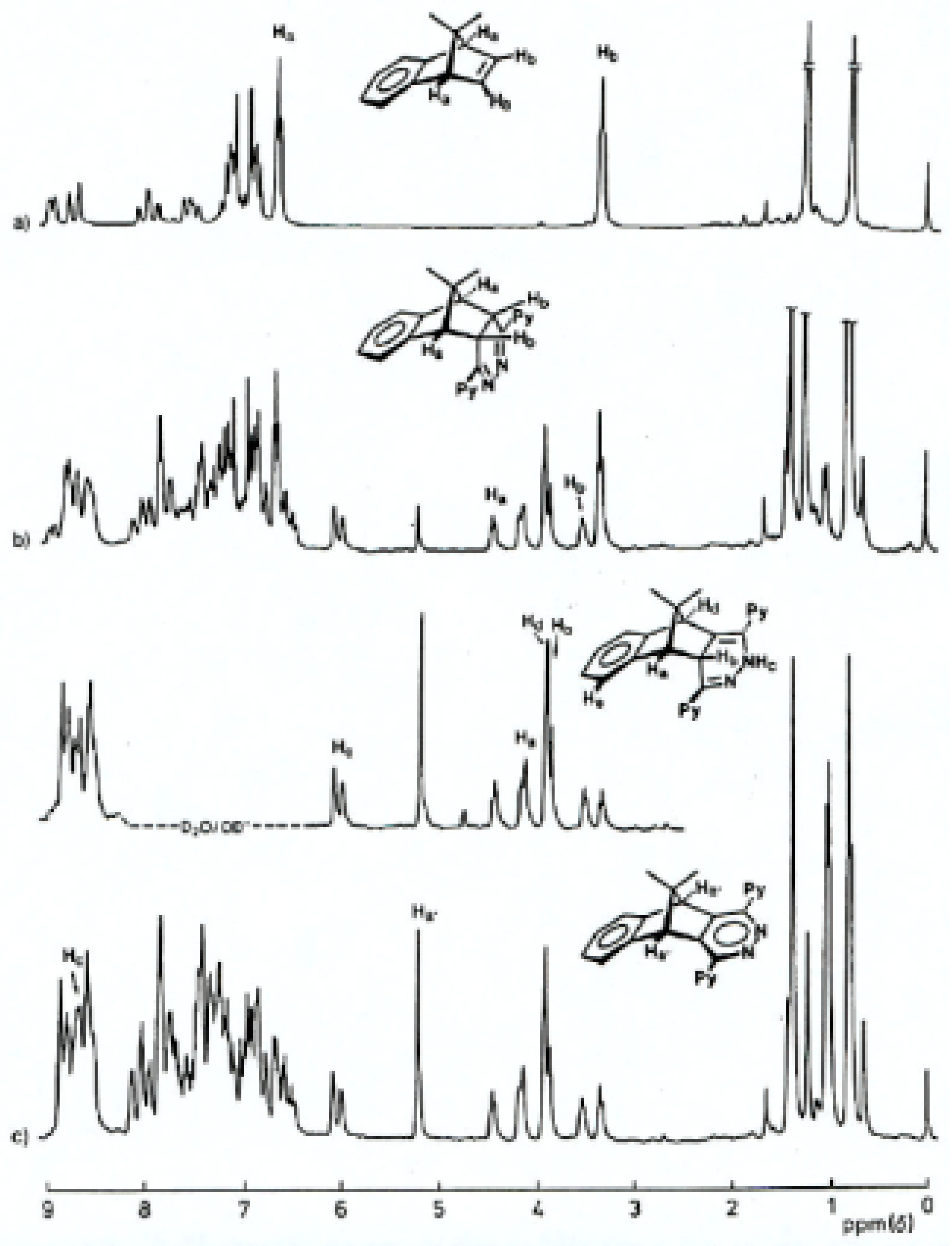
Figure 3.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s- tetrazine 2 and 7-spirocyclo- propylbenzonorbornadiene 23 after (a) 30 min at 30 °C ; (b) 120 min at 30 °C; (c) 24 hours at 60 °C
Figure 3.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s- tetrazine 2 and 7-spirocyclo- propylbenzonorbornadiene 23 after (a) 30 min at 30 °C ; (b) 120 min at 30 °C; (c) 24 hours at 60 °C
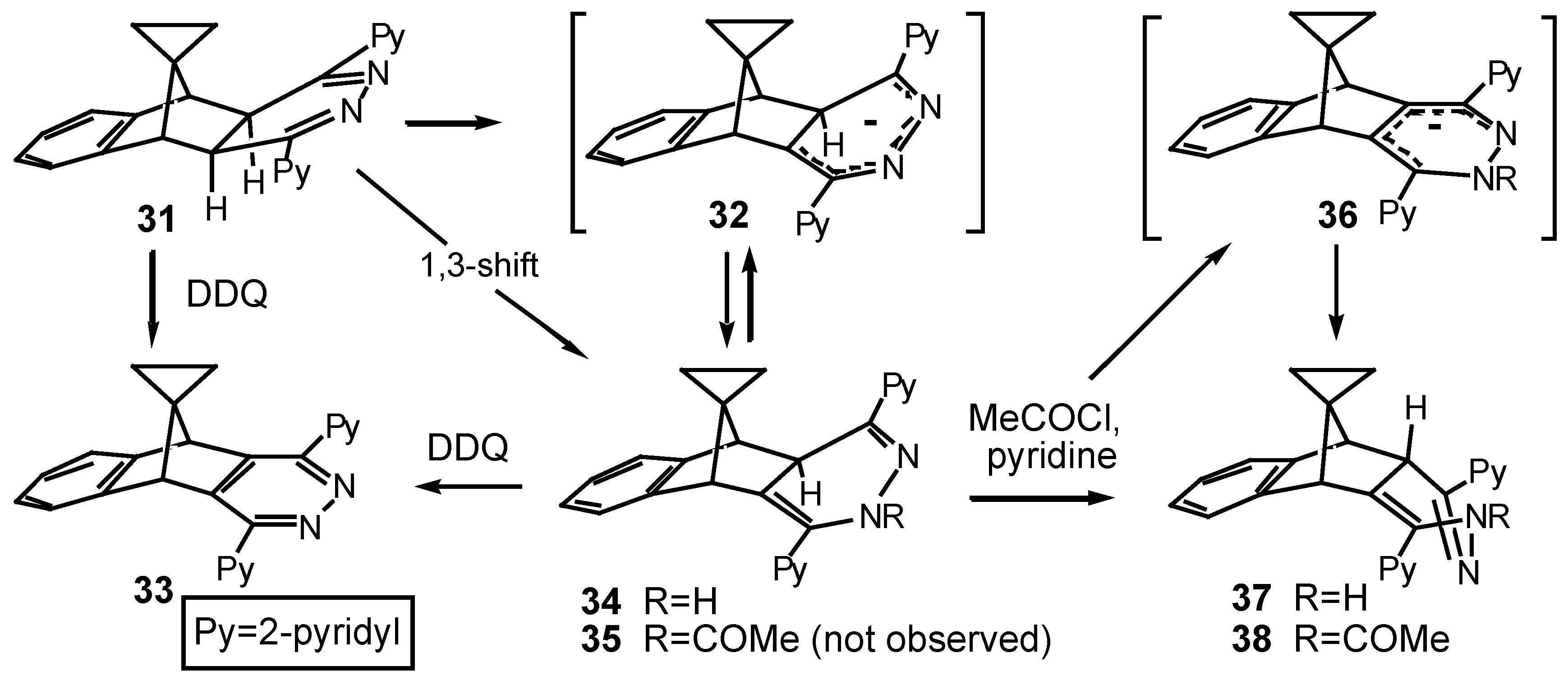
The commonality of basic ring-structures in
28 and
29 was confirmed by oxidation of mixtures of these products with dichlorodicyanoquinone (DDQ) to the same Cs-symmetric pyridazine
30. When the spirocyclopropylbenzonorbornadiene
23 was treated with
s-tetrazine
2 (
Scheme 6), only slow evolution of nitrogen was observed. The 4,5-dihydropyridazine intermediate
31 was none-the-less the first-formed product as deduced from the time-dependent
1H-NMR study shown in
Figure 3. The structure of the fused 4,5-dihydropyridazine
31 was again indicated by the presence of two singlet methine resonances at δ 3.21 and 3.67 which confirmed both the Cs symmetry of the product and its
exo stereochemistry (no
3J-coupling). Furthermore, under normal reaction conditions (56 °C), this intermediate did not fragment further to yield the respective isoindene, but preferentially underwent rearrangement.
The fact that rearrangement product
34 retained the benzonorbornanyl ring-structure present in its precursor
31, was established by oxidation with DDQ [
12], where the same pyridazine
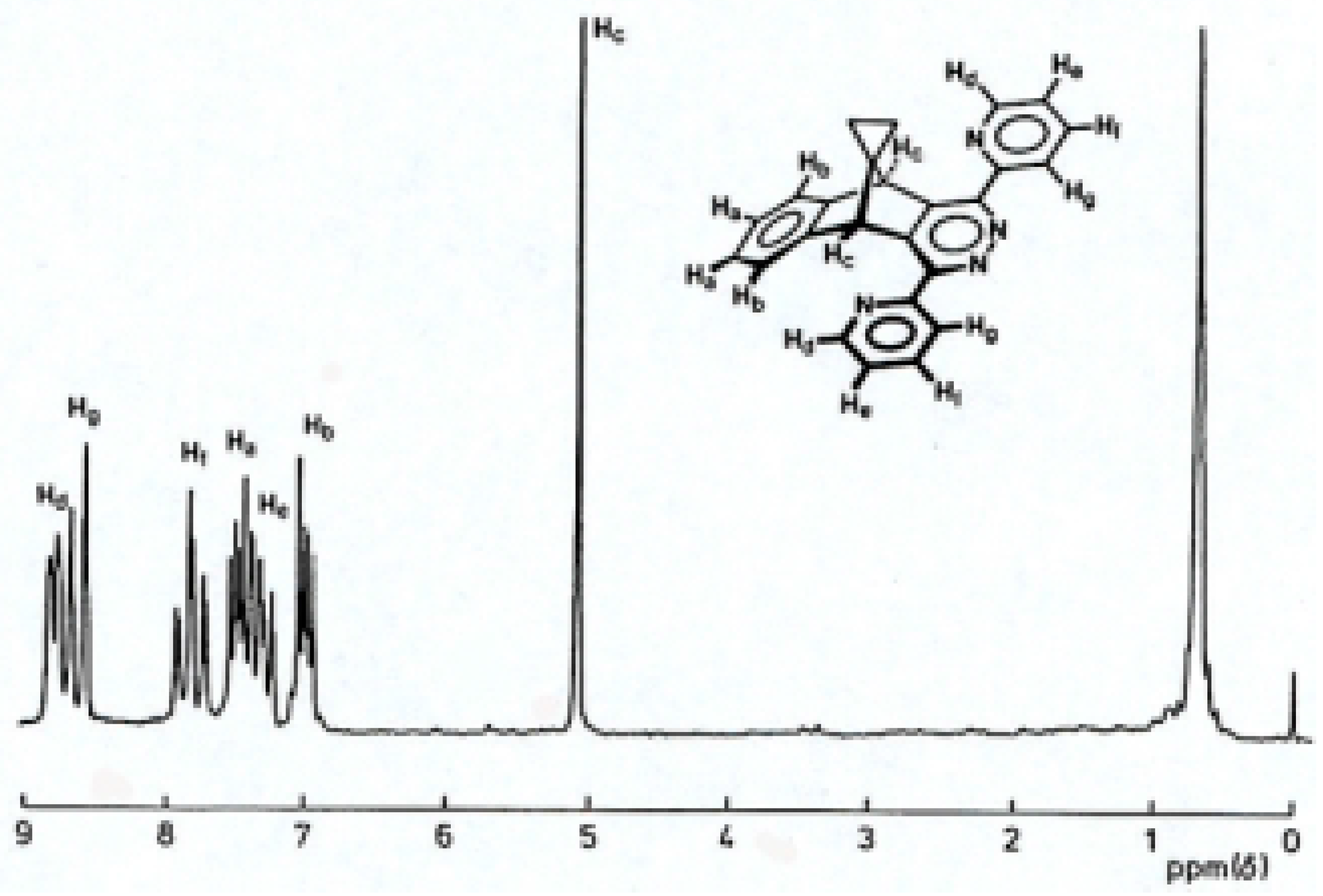
33, mass spectrum (M+
m/z 374), was produced from enriched mixtures favouring each product. The
1H-NMR of
33 was consistent with that expected for a structure of high symmetry (
Figure 4).
Figure 4.
1H-NMR spectrum (80 MHz; CDCl3) of pyridazine 33
Figure 4.
1H-NMR spectrum (80 MHz; CDCl3) of pyridazine 33
The structure of 1,4-dihydropyridazine
34 was assigned very much by analogy with
29 and in an attempt to form a derivative suitable for characterisation, it was treated with acetyl chloride in pyridine at reflux overnight. The isolated product had the expected mass spectral peak at
m/z = 418 for an acetyl derivative and an M-43 peak, corresponding to a loss of -COCH
3, while the
1H-NMR spectrum (
Figure 5) indicated that rearrangement had occurred. The fact that there was vicinal coupling between protons Ha and Hb (δ 4.0, δ 4.1
J = 3.8 Hz) excluded the structure
35 (
endo-Hb) and supported the structure
38 (exo-Hb)
. This assignment was also supported by the increased dispersion of the aromatic resonances
, a manifestation of the proximity of the phenyl and one of the pyridyl rings [
13]. The expected acetate methyl group occurred as a characteristic, sharp singlet (δ 2.32).
Figure 5.
1H-NMR spectrum (80 MHz; CDCl3) of N-acetyl 1,4-dihydropyridazine 38
Figure 5.
1H-NMR spectrum (80 MHz; CDCl3) of N-acetyl 1,4-dihydropyridazine 38
A clue to the mechanism for inversion of stereochemistry which occurred during the acetylation, is apparent in the deuterium exchange spectrum (
Figure 3, trace c and above) of the starting material
35 where Hb was exchanged under the basic conditions as well as the NH proton. This implicated the anion
36, demonstrating the acidity of the methine proton at the bridgehead of the heterocyclic ring. Accordingly, formation of the acetate
35 may well occur but isomerisation to the observed product would ensue since formation of anion
36 would be favoured under the basic conditions of the reaction which is conducted in pyridine at reflux, and protonation from the favoured
exo-face would yield the observed product
38.
The reaction between benzonorbornadiene 21 and s-tetrazine 2, which was essentially complete after 2 hours at room temperature, occurred at least an order of magnitude faster than that observed for spirocyclopropyl derivative 23. What, then, if the norbornene π-bond was even more screened by the 7-substituents?
Figure 6.
1H-NMR spectra (80 MHz; CDCl3) of the reaction between s-tetrazine 2 and 7,7- dimethyl benzonorbornadiene 25 after (a) 1 hour at 30 °C; (b) 12 hours at 60 °C; (c) 36 hours at 60 °C
Figure 6.
1H-NMR spectra (80 MHz; CDCl3) of the reaction between s-tetrazine 2 and 7,7- dimethyl benzonorbornadiene 25 after (a) 1 hour at 30 °C; (b) 12 hours at 60 °C; (c) 36 hours at 60 °C
The time-dependent
1H-NMR study of the reaction of
s-tetrazine
2 with the 7,7- dimethylbenzonorbornadiene
25 (
Figure 6) confirmed that reaction was even slower, with essentially no change having occurred at room temperature after 1 hour (
Figure 6, trace a). Increasing the temperature to 56 °C confirmed that reaction would proceed, but there were differences. First, the reaction yielded the dehydrogenated pyridazine
41 as the major reaction product after 36 hours (
Figure 6, trace c). Secondly, the spectral data indicated that initial attack had occurred from the
endo-face of
25.
There was also evidence to support an apparent equilibrium between symmetric dihydropyridazine
39 and the unsymmetrical isomer
40 being maintained prior to dehydrogenation to the pyridazine
41. The resonance ascribed to
exo-protons Hb (annotated in
Figure 5) in either dihydropyridazine
39 or
40 showed no evidence for deuterium exchange which reflected their crowded environment as expected if the
exo-stereochemical assignment was correctly assigned.
Figure 7.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s-tetrazine 2 and 7-spirocyclopentyl-benzonorbornadiene 27 after (a) 1 hour at 30 °C; (b) 12 hours at 60 °C; (c) 36 hours at 60 °C
Figure 7.
1H-NMR spectra (80 MHz; CDCl3) of products resulting from the reaction between s-tetrazine 2 and 7-spirocyclopentyl-benzonorbornadiene 27 after (a) 1 hour at 30 °C; (b) 12 hours at 60 °C; (c) 36 hours at 60 °C
This change from
exo-attack on
23 to
endo-attack on
25 by
s-tetrazine
2 was entirely in keeping with the known ability of
syn-substituents on the methano-bridge to screen the π-bond, and has been observed in other systems. Thus, it was not surprising to find that the predicted
endo-attack for the reaction of
2 with the spirocyclopentylbenzonorbornadiene
27 was also observed in practice. The slow rate of attack at the π-bond of
27 by
2 was very similar to that for the 7,7-dimethyl derivative
25, but conversion of the intermediate dihydropyridazines
42 and
43 to the pyridazine
44 was even faster. The time-dependent
1H-NMR study (
Figure 7) provided evidence for
endo-attack of
2 at the π-bond of
27, and was again based on the coupling between Ha and Hb (see annotations for relevant proton assignments). Rearrangement to the 1,4-isomer was accompanied by substantial oxidation to the pyridazine
44 upon raising the temperature to 60 °C, however, oxidation was still incomplete after 36 hours.
Experimental General
Melting points were determined on a Reichert hot-stage microscope, and are uncorrected. Microanalyses were performed by the Australian National University Microanalytical Service. Ultraviolet spectra were recorded on a Unicam SP800 spectrophotometer, using matched 5 or 10 mm silica cells. IR spectra were recorded on either a Unicam SP200G spectrophotometer or a Perkin-Elmer 283 spectrophotometer. Unless otherwise specified, IR spectra were obtained using a Nujol mull between NaCl discs. Where spectra were recorded on solutions, they were obtained using 1 mm NaCl cavity cells. The intensities of IR absorptions are reported as s (strong, > 50% intensity of maximum absorption), m (medium, 25-50% max.) or w (weak, 12-25% max.). 1H-NMR spectra were recorded on a Varian CFT20 (80 MHz, Fourier mode), a Jeol JNM-MH-100 (100 MHz, continuous wave), or a Bruker HFX-270 (270 MHz, Fourier mode). l3C-NMR spectra were recorded on a Jeol JNM-FX-60 (15.04 MHz), a Varian CFT-20 (20.00 MHz) or a Bruker HFX-270 (67.89 MHz) NMR spectrometer. 1H- and 13C-NMR spectra were obtained using solutions in 5 and 10 mm tubes respectively, with tetramethylsilane (TMS) as internal standard. All chemical shifts are expressed in parts per million (ppm) downfield from TMS (δ scale). Coupling constants (J) are given in Hertz, with multiplicity patterns designated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and br (broad). Simulated spectra were calculated using the NTCSIM or ITRCAL procedures, available as part of the Nicolet 1180 Fourier Package. Low resolution mass spectra were recorded on either a Varian MAT CH7 or an A.E.I. MS902 mass spectrometer. The latter instrument was used for high resolution mass measurements. Unless otherwise stated, all peaks greater than 5% of the intensity of the base peak are reported. Combined GC-MS was carried out using a Varian MAT 111 (0.125 inch column, 2% OV-17) system. Gas chromatographic analyses, and preparative separations were performed on either a Hewlet- Packard Model 5754B (12 ft x 0.25 inch metal column, 10% SE 30 on 60-100 mesh Embacel) or a Packard 7400 Series (2 m x 1 cm glass column, 10% SE 30 on 44-60 mesh Embacel) instrument. A Waters Associates Series 6000 system was used for analytical HPLC. Preparative thin layer chromatography was carried out on 20 x 100 cm glass plates using silica gel (Merck HP254 as absorbent, or on 20 x 20 cm Merck precoated (2 mm, 60 F254) PLC plates. Either Spence Type H activated alumina or May and Baker chromatography silica gel was employed in column chromatography.
l',4'-Dihydrospiro[cyclopentane-l,9'-[l,4]methononaphthalene] (27): Spiro[4,4]nona-l,3-diene
26 [
10] (40 g, 0.33 mol) was added to a solution of isoamylnitrite (58 g, 66 ml, 0.5 mol) in methylene chloride (400 mL). The resulting solution was heated to reflux and treated slowly, over 2 h, with a solution of anthranilic acid (68.6 g, 0.5 mol) in the minimum volume of acetone (
ca. 300 mL). The mixture was maintained under reflux for a further 2 h, and the bulk of the solvent removed by distillation. Water (500 mL) was added, and the resulting mixture extracted with light petroleum (2 x 400 mL). The combined organic extracts were washed with 5% aq. NaOH (3 x 500 mL) and brine (2 x 500 mL), and dried. The solvent was removed under reduced pressure and the residual brown coloured liquid purified by distillation. The product
27 was collected at 80-87 °C/ 0.5 mbar as a colourless liquid. The distillation residue was chromatographed on a column of alumina (6 cm x 20 cm), using light petroleum as eluent, to yield a further portion of the product
27 (total yield, 32.3 g, 50%). An analytical sample of the title compound
27 was obtained by short path distillation (b.p. 50 °C / 0.1 mbar); Found: C, 91.9; H, 8.2. C
15H
16 requires C, 91.8; H, 8.2%. U.V. λ
max (ε) 264 (560), 270 (600), 272 infl. (540), 278 (480) nm. IR ν
max(liquid film) 3070s, 3050m, 3020m, 2970s, 2870s, 1455s, 1325w, 1300m, 1245w, 1195w, 1140w, 1010w, 925w, 900w, 855w, 790s, 740s, 695s, 665m cm.
-1 1H-NMR spectrum, (80 MHz, CDCl
3): δ 1.1-1.9 (8H, m, cyclopentane protons), 3.46 (2H, dd
Ja,b = 2.0 Hz,
Ja,b' = 2.0 Hz, Ha), 6.70 (2H, dd
Ja,b = 2.0 Hz,
Ja',b = 2.0 Hz, Hb), 6.8-7.2 (4H, m, aromatic protons).
13C-{
lH}-NMR spectrum, (20 MHz, CDCl
3): δ 25.3 (C3, C4), 33.3 / 33.8 (C2,C5), 59.1 (C1', C4'), 91.1 (C1), 121.8 / 124.0 (C5', C6', C7', C8'), 142.5 (C2', C3'), 151.9 (C4'a, C8'a). Mass spectrum:
m/z (%) 197 (18), 196 (M+, 100), 195 (10), 181 (50), 167 (72), 166 (17), 165 (28), 155 (34), 154 (50), 153 (37), 152 (25), 142 (27), 141 (42), 129 (14), 128 (59), 127 (11), 115 (20), 39 (12).
1,4-dihydro-9,9-dimethyl-1,4-methanonaphthalene (25): Compound
25 was prepared in the same way as
27, in this case from 5,5-dimethylcyclopentadiene
24 [
9] by reaction with benzyne
20. The title compound
25 was separated by preparative tlc (silica / n-hexane) and purified by short-path distillation (50 °C / l0-2 mbar), as a colourless liquid (40%). Found, C, 91.8; H, 8.3. Cl
3H
l4 requires C, 91.7; H, 8.3%. U.V. λ
max (ε) 263 (580), 271 (610), 273 infl. (540), 279 (480) nm. IR ν
max (liquid film) 3070s, 3020m, 2970s, 2930s, 2910m, 2870s, 1470m, 1455s, 1445m, 1385m, 1365m, 1300m, 1265m, 1220w, 1135m, lOlOw, 925w, 915w, 855w, 790s, 740s, 700s, 670m cm
-1;
1H-NMR spectrum (80 MHz, CDCl
3): δ 0.79 (3H, s,
anti-CH
3), 1.25 (3H, s,
syn CH
3), 3.34 (2H, dd
Ja,b = 2.1 Hz,
Ja,b' = 2.1 Hz, Ha), 6.65 (2H, dd
Ja,b = 2.1 Hz,
Ja',b = 2.1 Hz, Hb), 6.8-7.2 (4H, AA'BB' pattern, aromatic protons).
13C-{
lH }NMR spectrum (20 MHz, CDCl
3): δ 22.8 / 23.4 (C10, C11), 60.2 (C1, C4), 78.6 (C9), 122.1 (C5, C8), 124.0 (C6, C7), 141.8 (C2, C3), 151.6 (C4a, C8a). Mass spectrum:
m/z 170 (M+, 30%), 156 (14), 155 (100), 153 (14), 128 (18).
Reaction of 1',4'-dihydro-spiro[cyclopropane-1,9'-[1,4]methanonaphthalene] (23) with 3,6-di(2'- pyridyl)-s-tetrazine (2): A solution of the olefin 23 (2.5 g, 14.9 mmol) and s-tetrazine (2) (3.6 g, 14.9 mmol) in CHCl3 (10 mL) was heated under reflux overnight. The resulting solution was filtered through a bed of alumina and freed of solvent to yield the crude, crystalline product mixture. Recrystallization from benzene / n-hexane afforded a mixture of (cis-4'a-cisoid4'a, 5'-cis-5')-1', 2": 4', 2"-dipyridyl4'a, 5', 10', 10'a-tetra-hydrospiro[cyclopropane-1,11'-[5, 10]methano benzo[g]phthalazine (31) and (cisoid- 4'a,5'-cis-5')-1',2":4',2"'dipyridyl-4'a, 5', 10'-tetrahydro spiro[cyclopropane-1,11'[5,10]methanobenzo [g]phthalazine] (34) (ratio ca 1:4) (4.6 g, 83%) as buff coloured prisms. Found: M+, 376.1687. C25H20N4 requires 376.1688; IR: vmax 3350m (>NH) cm.-1; 1H-NMR spectrum (80 MHz, CDCl3): δ -0.2-0.6 (m, cyclopropane protons), 2.76 (s, Hb), 3.21 (s, Ha), 3.64 (s, Ha'), 3.67 (s, Hb), 4.14 (s, Hd'), 7.0-8.7 (m, aromatic protons), 9.14 (br s, exchanged with DO- / D2O, Hc'). Mass spectrum: m/z 376 (M+, 70%) , 299 (58), 272 (72), 142 (31), 78 (100).
(Cis-4'a-transoid-4'a,5'-cis-5')-1',2":4',2"'-dipyridyl-4'a,5',10',10'a-tetrahydro-spiro[cyclo-pentane-1,11'-[5,10]methanobenzo[g]phthalazine] (42) and (transoid-4'a,5'-cis-5')-1',2":4',2"- dipyridyl-2',4'a,5',10'-tetrahydrospiro[cyclopropane-1,11'-[5,10]methanobenzo[g]-phthalazine] (43): Similar treatment of 1', 4'-dihydrospiro[cyclopentane-l, 9'-[1,4]methano naphthalene] (27) with s- tetrazine (2) afforded a mixture of (42) and (43) The mixture was not purified, and characterised only by lH-NMR spectroscopy. 1H-NMR spectrum, (80 MHz, CDCl3): δ 1.0-2.0 (m, cyclopentane protons), 3.61 (dd Jab = 2.1 Hz, Ja',b = 2.1 Hz, Hb), 3.80 (d Ja'b' = 3-5 Hz, Hb'), 4.03 (s, Hd'), 4.25 (dd Ja'b' = 3.5 Hz, Ja'd' = 1 4 Hz, Ha'), 4.33 (dd Jab = 2.1 Hz, Jab' = 2.1 Hz, Ha), 6.0-9.0 (m, aromatic protons), 8.59 (s, exchanged with DO-/ D2O, Hc').
(Cis-4a-transoid-4a,5-cis-5)-11,11-dimethyl-1,2':4,2"-dipyridyl-4a,5,10,10a-tetrahydro-5,10- methanobenzo[g]phthalazine (39) and (transoid-4a, 5-cis-5)-11,11-dimethyl-l,2':4, 2"-dipyridyl- 2,4a,5,10-tetrahydro-5,10-methanobenzo[g]phthalazine (40): Similarly, treatment of 1,4-dihydro9,9- dimethyl-1,4-methanonaphthalene (25) with (2) gave a mixture of (39) and (40) The mixture was not purified, and characterised only by lH-NMR.spectroscopy. 1H- NMR spectrum (80 MHz, CDC13): δ 0.65, 0.81, 1.38,1.42 (4x s, methyl protons), 3.52 (dd Jab = 2.0 Hz, Ja'b = 2.0 Hz, Hb), 3.88 (d Ja'b' = 3.6 Hz, Hb'), 3.92 (s, Hd'), 4.16 (dd Ja'b' = 36 Hz, J a'd' = 1.4 Hz, Ha'), 4.43 (dd Ja,b = 2.0 Hz, Ja,b' = 2-0 Hz, H a), 6.0-9.0 (m, aromatic protons), 8.62 (s, exchanged with DO-D2O, Hc').
(transoid-4'a, 5'-cis-5')-2-Acetyl-1',2":4',2"'-dipyridyl2',4'a,5',10'-tetrahydrospiro [cyclopropane- l, 11'-[5,10]methanobenzo[g]phthalazine] (38): A solution of the mixture of (31) and (34) (150 mg, 0.4 mmol) and pyridine (4 drops) in benzene (5 mL) was heated to reflux and treated, slowly, with a solution of acetyl chloride (200 μl, 2.4 mmol) in benzene (2 mL). The resulting mixture was stirred and heated under reflux overnight. The mixture was allowed to cool, ether (50 mL) added, and washed with brine (2 x 50 mL). The resulting solution was dried and freed of solvent to yield the crude product. Recrystallization from chloroform / n-hexane gave the product as colourless prisms (137 mg, 82%), m.p. 221-222 °C. U.V. λmax (ε) 243 (16000), 273 (13700), 280 infl. (13300), 322 (2970) nm. I.R. νmax 1685s, 1590s, 1570s, 1470s, 1465s, 1440s, 1380s, 1360s, 1405s, 1400s, 1370m, 1350m, 1310w, 1180s, 1155s, 1130w, 1115m, lO90w, 1080m, 1045m, 1020m, 995s, 990s, 950w, 940m, 890m, 880w, 805w, 785s, 760s, 750s, 735s, 705s, 680m, 665s, 650s, 640s, 625m cm.-1 1H-NMR spectrum (80 MHz, CDCl3): δ 0.4-1.0 (4H, m, cyclopropane protons), 2.32 (3H, s, CH3), 3.64 (lH, d Ja,c = 1 4 Hz, Hc), 4.00 (1H, dd Ja,b = 3.8 Hz, Ja,c = 1.4 Hz, Ha), 4.12 (lH, d, Ja,b = 3 8 Hz, Hb), 6.05 (lH, dd, J = 1.7, 6.1 Hz, Hd), 6.3-8.8 (11H, m, aromatic protons). Mass spectrum: m/z 419 (12%), 418 (M+, 32). Found: M+, 418.1794 (C27H22N40 requires 418.1794), 417 (12), 376 (33), 375 (56), 360 (18), 359 (47), 358 (12), 347 (15), 315 (26), 314 (100), 298 (33), 297 (13), 281 (11), 272 (27), 271 (34), 256 (11), 255 (18), 254 (11), 248 (27), 247 (52), 105 (14), 79 (10), 78 (23), 43 (30).
(cis-5')-5',10'-Dihydro-1', 2": 4', 2"'-dipyridyl-spiro[cyclopropane1,11'-[5,10]methanobenzo [g]-phthalazine] (33): A solution of the mixture of (31) and (35) (376 mg, 1.0 mmol), DDQ (250 mg, 1.1 mmol) and acetic acid (5 drops) in dioxane (10 mL) was stirred for 1 h. The resulting mixture was filtered and freed of solvent under vacuum to yield the crude product. Purification by preparative tlc (silica / 1:2 ethyl acetate / n-hexane) afforded compound 33 as buff coloured prisms (265 mg, 71%), m.p. 201-202 °C Found: C, 80.2; H, 4.7; N, 14.8. C25Hl8N4 requires C, 80.2; H, 4.9; N, 15.0%. U.V. λmax (ε) 288 (31700) nm. I.R. νmax 3100w, 3065m, 3045m, 3025m, 1940w, 1590s, 1575s, 1550m,1475s, 1460s, 1440m, 1425s, 1380s, 1365s, 1285w, 1245s, 1240m, ll90m, 1165w, 1150m, 1135m, lllOs, 1085m, 1045w, 1020w, 1015m, 1005w, 995m, 990m, 985w, 975m, 950w, 920w, 910m, 885w, 855w, 800s, 780s, 765m, 755w, 745s, 735s, 710s, 670s, 665m, 655s, 625m, 610s cm.-1 1H-NMR spectrum (80 MHz, CDCl3): δ 0.7 (4H, m, cyclopropane protons), 5.08 (2H, s, Hc), 6.99 (2H, dd Ja,b = 5.3 Hz, Ja',b = 3.2 Hz, Hb), 7.31 (2H, ddd Jd,e = 4.7 Hz, Je,f =7.6 Hz, Je,g = 1.3 Hz, He), 7.49 (2H, dd Ja,b =5.3 Hz, J a,b' =3.2 Hz, Ha), 7.83 (2H, ddd Jd,f = 1 9 Hz, Je,f = 7.6 Hz, Jf,g =7.8 Hz, Hf), 8.62 (2H, ddd Jd,g = 1.2 Hz, Je,g 1.3 Hz, Jf,g = 7 8 Hz, Hg ), 8.79 (2H, ddd Jd,e = 4.7 Hz, Jd,f = 1 9 Hz, Jd,g = 1.2 Hd). mass spectrum: m/z 375 (29%), 374 (M+, 100), 373 (44), 346 (21), 345 (18),296 (18), 269 (7), 142 (15), 141 (21).
(cis-5')-5', 10'-dihydro-1', 2'':4', 2"'dipyridylspiro[cyclopentane-1,11'[5,10]methanobenzo- [g]phthalazine] (44): Oxidation of a mixture of (42) and (43), as above, afforded the title compound (44) as buff-coloured prisms from chloroform/n-hexane (63%), m.p. 149-150 °C. Found: C, 80.4; H, 5.6; N, 13.8. C27H22N4 requires C, 80.6; H, 5.5; N, 13.9%. U.V. λmax (ε) 287 (29400), 338 infl. (640) nm. I.R. νmax 3060s, 3030s, 3010s, 1990w, 1950w, l910w, 1890w, 1870w, 1590s, 1580s, 1555s, 1480s, 1440s, 1430s, 1370s, 1340w, 1310w, 1290w, 1250s, 1200s, 1170w, 1150w, 1135s, lllOs, 1080s, 1045m, lOlOm, 995s, 970w, 955w, 935w, 895w, 880w, 800s, 775s, 740s, 720s, 665s, 645s, 620s, 615s cm.-1 1H-NMR spectrum (80 MHz, CDCl3): δ 1.53 (8H, m, cyclopentane protons), 5.29 (2H, s, Hc), 6.95 (2H, dd Jab = 5.2 Hz, Ja',b = 3 1 Hz,Hb), 7 28 (2H, ddd Jd,e = 4.8 Hz, Je,f = 7-6 Hz, Je,g = 1.4 Hz, He) 7.44 (2H, dd Jab = 5.2 Hz, Jab' = 3 1 Hz, Ha), 7.80 (2H, ddd Jd,f = 1 9 Hz, Je,f = 7.6 Hz, Jf,g = 7.8, Hf), 8.60 (2H, ddd Jd,g = 1.2 Hz, Je,g = 1.4 Hz, Jf,g = 7.8 Hz, Hg) 8.81 (2H, ddd Jd,e = 4.8 Hz, Jd,f = 1 9 Hz, Jd,g = 1.9 Hz, Hd). Mass spectrum: m/z 403 (31), 402 (M+, 100), 401 (23), 385 (26), 384 (10), 375 (17), 374 (60), 373 (17), 335 (16), 333 (17), 332 (7), 325 (11), 324 (44), 321 (12), 320 (42), 307 (16), 296 (20), 295 (58), 256 (11), 186 (11), 141 (11), 79 (10), 78 (39).
(cis-5)-5,10-dihydro-11,11-dimethyl-1,2':4,2"-dipyridyl-5,lO-methanobenzo[g]-phthalazine (41): Oxidation of a mixture of (39) and (40) afforded the title compound (41) as buff-coloured prisms from chloroform / n-hexane (68%), m.p. 199-201 °C. Found: C, 79.4; H, 5.4; N, 15.0. C25H20N4 requires C, 79.8; H, 5.4; N, 14.9%. U.V.λmax (ε) 287 (22000) nm. I.R. νmax 3060m, 3040m, 1590s, 1580s, 1550s, 1475s, 1455s, 1430s, 1365s, 1260m, 1250s, 1225m, 1200w, 1180w, 1165w, 1145w, 1130m, llOOs, 1080m, 1040m, lOlOm, 990m, 970w, 930w, 890w, 885w, 880w, 800s, 780w, 770m, 760m, 740s, 715s, 680m, 655s, 630s, 625m cm-l. 1H-NMR spectrum (80 MHz, CDCl3): δ 1.03 (3H, s, CH3) , 1.05 (3H, s, CH3), 5.17 (2H, s, Hc), 6.96 (2H, dd Ja,b = 5.2 Hz, Ja',b = 3 1 Hz, Hb), 7.37 (2H, ddd Jd,e = 4.7 Hz, Je,f = 7.6 Hz, Je,g = 1.2 Hz, He), 7.42 (2H, dd Ja,b = 5.2 Hz, Ja,b' = 3.1 Hz, Ha), 7.86 (2H, ddd Jd,f = 1.8 Hz, Je,f = 7.6 Hz, Jf,g = 7.8 Hz, Hf), 8.59 (2H, ddd Jd,g = 1 1 Hz, Je,g = 1.2 Hz, Jf,g = 7.8 Hz, Hg), 8.82 (2H, ddd Jd,e = 4.7 Hz, Jd,f = 18 Hz, Jd,q = 1.1 Hz, Hd). Mass spectrum: m/z 377 (20), 376 (M-, 69), 363 (29), 362 (100), 335 (23), 333 (10), 321 (15), 298 (17), 78 (21).