Intramolecular Acylation of Aryl- and Aroyl-Aliphatic Acids by the Action of Pyrophosphoryl Chloride and Phosphorus Oxychloride
Abstract
:Introduction:
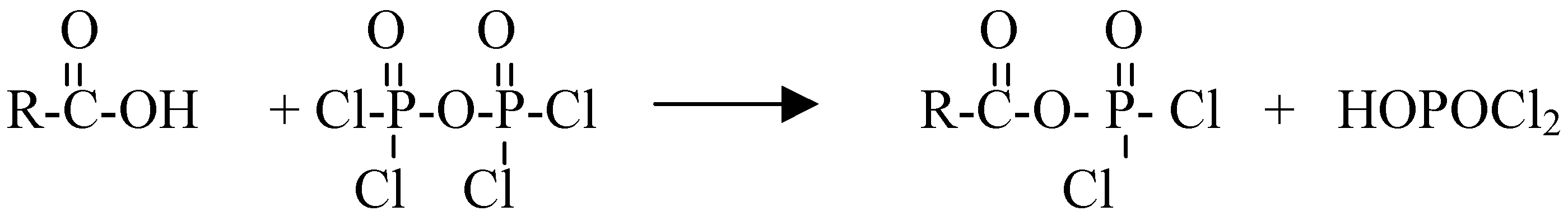
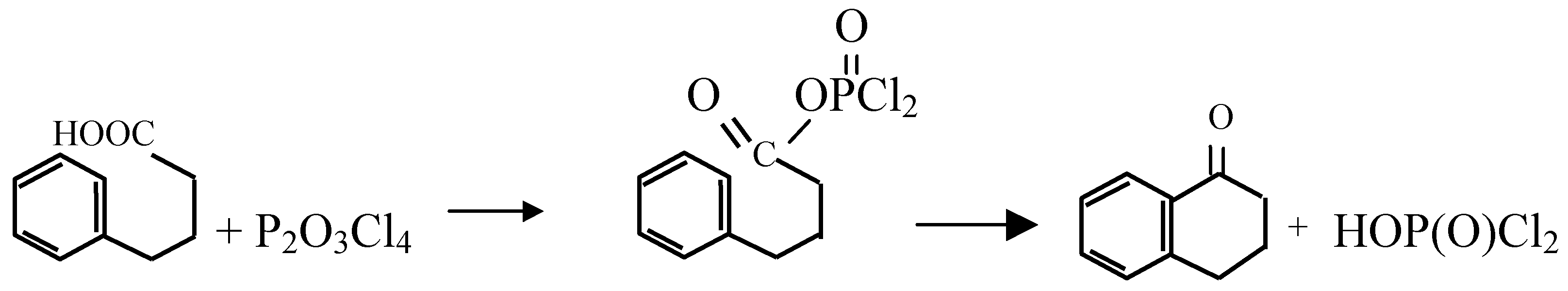
Results and Discussion:
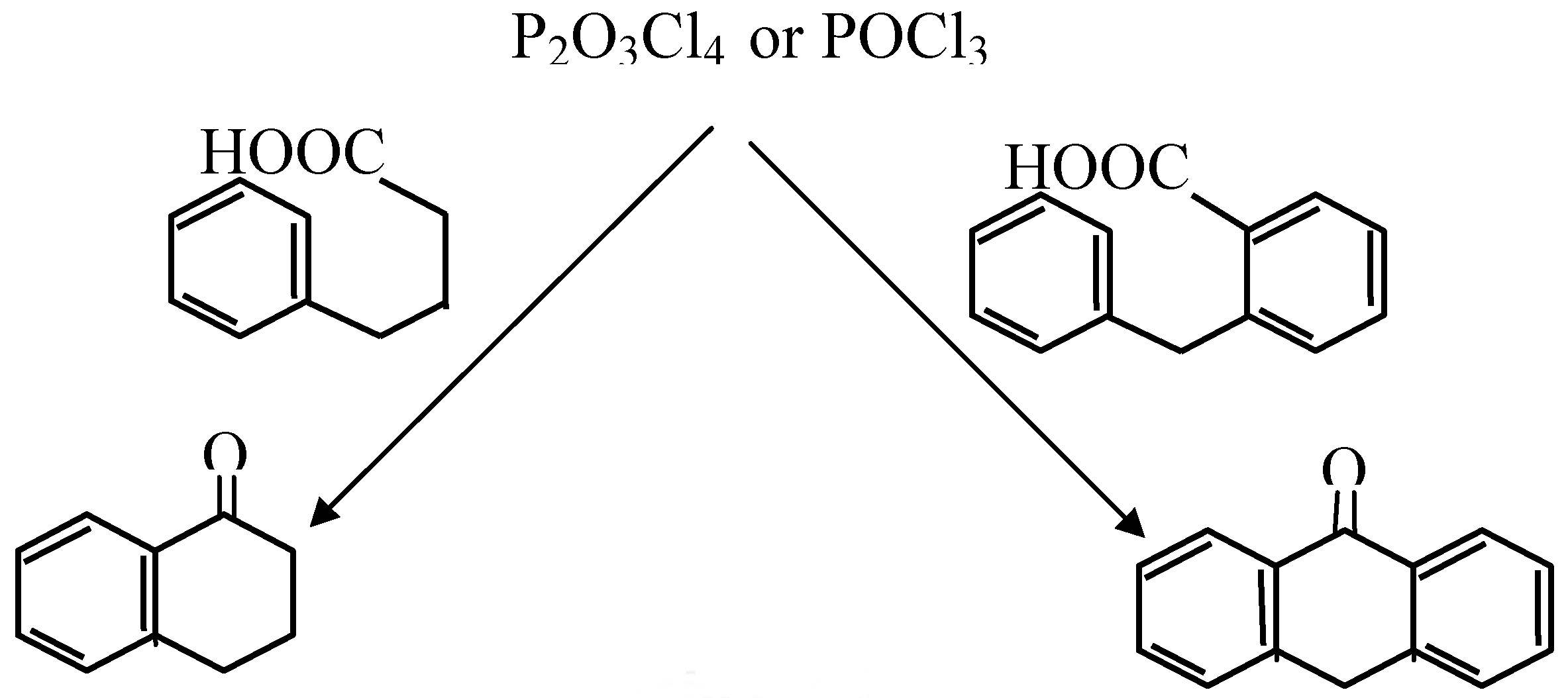

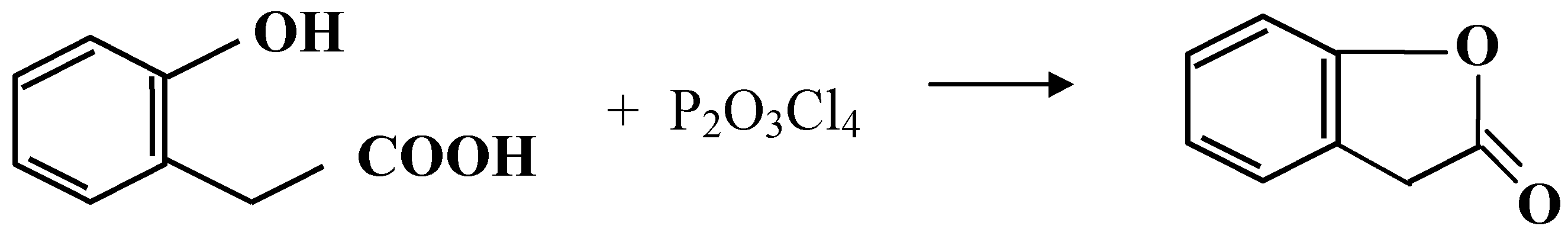

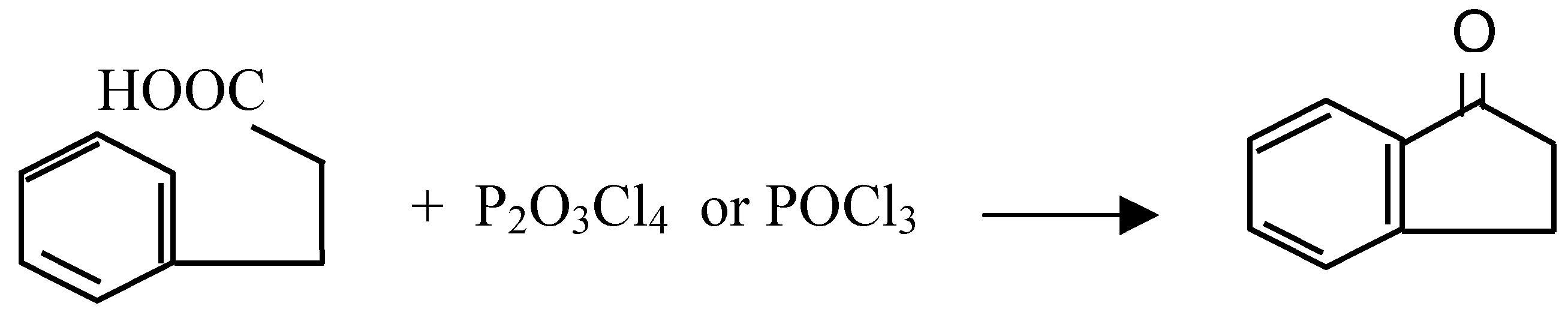
| Cyclized Product | Dehydrating Agent: POCl3 % Yield / Conditions | Dehydrating Agent: P2O3Cl4 % Yield / Conditions | ||
| Anthraquinone | 53 | reflux 3 hrs | 65 | Reflux in CH3NO2, 2.5 hrs |
| Anthrone | 90 | reflux 30 min | Quant * | Room temperature, 30 min. |
| 2-Methoxyanthraquinone | 43 | reflux 1.5 hrs | 75 | Warm at 40°, 2 hrs |
| 2-Chloroanthraquinone | 20 | reflux 3 hrs | 20 | Heat at 150°, 1 hr |
| 1-Tetralone | 70 | room temp, 2 days | 90 | Room temperature, 30 min. |
| 1-Indanone | 18 | reflux 5 min | 20 | Warm at 35°C, 30 min., |
| 2-Coumaranone | 90 | reflux 5 min. | Quant* | Warm at 35°C, 20 min., |
Conclusions
Experimental
General
Synthetic procedures
Spectral Data
Acknowledgements
References
- Fieser, L.F.; Hershberg, E.B. Use of Hydrogen Fluoride in Acylations and Cyclizations. J. Am. Chem .Soc. 1940, 62, 49–53. [Google Scholar] [CrossRef]
- Haworth, R.D. Syntheses of Alkyl Phenanthrenes I. 1-,2-,3-,4-Methylphenanthrenes. J. Chem. Soc. 1932, 1125–1133. [Google Scholar] [CrossRef]
- Cook, J.W. Polycyclic Aromatic Hydrocarbons X.1,2,7,8-Dibenzanthracene. J. Chem. Soc. 1932, 1472–1484. [Google Scholar] [CrossRef]
- Koo, J. New Simple Cyclization of 2-Anilinopropionic acids to 4-Oxo-1,2,3,4-tetrahydro-quinolines with Polyphosphoric Acid. J. Org. Chem. 1963, 28, 1134–1135. [Google Scholar] [CrossRef]
- Baker, W.; Coates, G.E.; Glockling, F. Fluorosulfonic Acid as a Cyclodehydrating Agent. J. Chem. Soc. 1951, 1376–1377. [Google Scholar]
- Shah, V.R.; Bose, J.W.; Shah, R.C. New Synthesis of 4-Hydroxycoumarins. J. Org. Chem. 1960, 25, 677–679. [Google Scholar] [CrossRef]
- Schroeter, G.; Muller, H.; Huang, J.Y.S. Hydrogenation of Phenanthrene II. Ber. 1929, 62, 645–658. [Google Scholar]
- Crofts, P.C.; Downie, I.M.; Heslop, R.P. Preparation of Pyrophosphoryl Chloride from Phosphoric Oxide and Phosphorus Pentachloride, and a Radiochemical Investigation of the Reaction. J. Chem. Soc. 1960, 3673–3675. [Google Scholar] [CrossRef]
- Effenberger, F.; Konig, G. Synthesis of Carboxylic Dihalophosphoric Anhydrides. Chem Ber. 1981, 114, 916–925. [Google Scholar] [CrossRef]
- Effenberger, F.; Konig, G.; Klenk, H. Mixed Carboxylic-Dichlorophosphoric Anhydrides- Reactive Intermediates in Acyl Chloride Synthesis with POCl3. Angew. Chem. Inter. Ed. 1978, 17, 695–696. [Google Scholar] [CrossRef]
- Effenberger, F.; Konig, G.; Klenk, H. Acylation of Benzenes with Carboxylic Dihalophosphoric Anhydrides. Chem Ber.Ger 1981, 114, 926–936. [Google Scholar] [CrossRef]
- Heaney, H.; Shuhaibar, K.F. Acyliminium Ions Derived from the Rearrangement of Bischler- Napieralski Cyclisation Products. Tet.Lett. 1994, 35, 2751–2752. [Google Scholar] [CrossRef]
- Meyer, K.H. Anthrone. Org.Syn. Coll.Vol.1. 1941, 60–61. [Google Scholar]
- Downing, R.G.; Pearson, D.E. Rates of Cyclization of some o-Benzoylbenzoic acids and of o- Phenoxybenzoic acid in Polyphosphoric Acid. J. Am. Chem. Soc. 1962, 84, 4956–4962. [Google Scholar] [CrossRef]
- Feiser, L.H. Organic Experiments. Heath: New York, 1968; pp. 199–204. [Google Scholar]
- Bruce, D.B.; Sorrie, A.J.S.; Thomson, R.H. Reactions in Fused Aluminum Chloride-Sodium Chloride. J. Chem. Soc. 1953, 2403–2406. [Google Scholar] [CrossRef]
- Johnson, W.S.; Bauer, V.J.; Margrave, J.L.; Frisch, M.A.; Dreger, L.H.; Hubbard, W.N. The Energy Difference between the Chair and Boat Forms of Cyclohexane. J. Am. Chem. Soc. 1961, 83, 606–614. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
El-Sayrafi, S.; Rayyan, S. Intramolecular Acylation of Aryl- and Aroyl-Aliphatic Acids by the Action of Pyrophosphoryl Chloride and Phosphorus Oxychloride. Molecules 2001, 6, 279-286. https://doi.org/10.3390/60300279
El-Sayrafi S, Rayyan S. Intramolecular Acylation of Aryl- and Aroyl-Aliphatic Acids by the Action of Pyrophosphoryl Chloride and Phosphorus Oxychloride. Molecules. 2001; 6(3):279-286. https://doi.org/10.3390/60300279
Chicago/Turabian StyleEl-Sayrafi, Sami, and Saleh Rayyan. 2001. "Intramolecular Acylation of Aryl- and Aroyl-Aliphatic Acids by the Action of Pyrophosphoryl Chloride and Phosphorus Oxychloride" Molecules 6, no. 3: 279-286. https://doi.org/10.3390/60300279




