Introduction
The phenomenon of induced defense and its implications has been well documented in several commercially important crops such as corn [
1,
2], cotton [
3,
4,
5], cabbage [
6] and potato [
7]. However, limited information exists on the response of terpene accumulating plants to attack by arthropods or microbial pathogens. To date studies on Grand fir oleoresin (
Abies grandis) have shown that stem wounding induces an increased activity of monoterpene cyclases [
8,
9] although a concomitant increase in monoterpene olefins in the stem could not be demonstrated [
10].
Ocimum minimum L. (Bush basil) (family Lamiaceae) is native to India and is known worldwide mainly due to the aromatic and medicinal properties of the essential oil obtained from its aerial parts [
11]. The acyclic monoterpene linalool and the allylphenol eugenol are known to be the major constituents of the oil from most chemotypes/varieties although methyl cinnamate-rich oils have also been described [
11,
12,
13].
This communication reports the changes, over a two day period, in the composition of the essential oil obtained from the leaves of eugenol-rich (>50%) O. minimum caused by mechanical damage. The significance and possible ecological implication of the findings is discussed.
Results and Discussion
The statistical analysis of the essential oil composition obtained from
O. minimum leaves highlighted a group of five compounds that changed their contribution to the essential oil as a result of mechanical wounding over the two-day period. As shown in
Table 1, the level of eugenol, linalool, camphor, myrcene and methyl eugenol in the oil changed significantly during the first 24 post-wounding hours. Eugenol and linalool were the compounds affected the most during that period. However, camphor was the only compound with significantly altered percent concentration during the second 24 post-wounding hours. These results indicate that in
O. minimum most of the essential oil transformations after injury occur during the first 24 post-wounding hours. After this period the wounded leaves start to return slowly to their pre-wounding metabolic state. Similar behaviour has been reported thus far for other non oil-bearing commercially important crops such as corn and cotton [
1,
14].
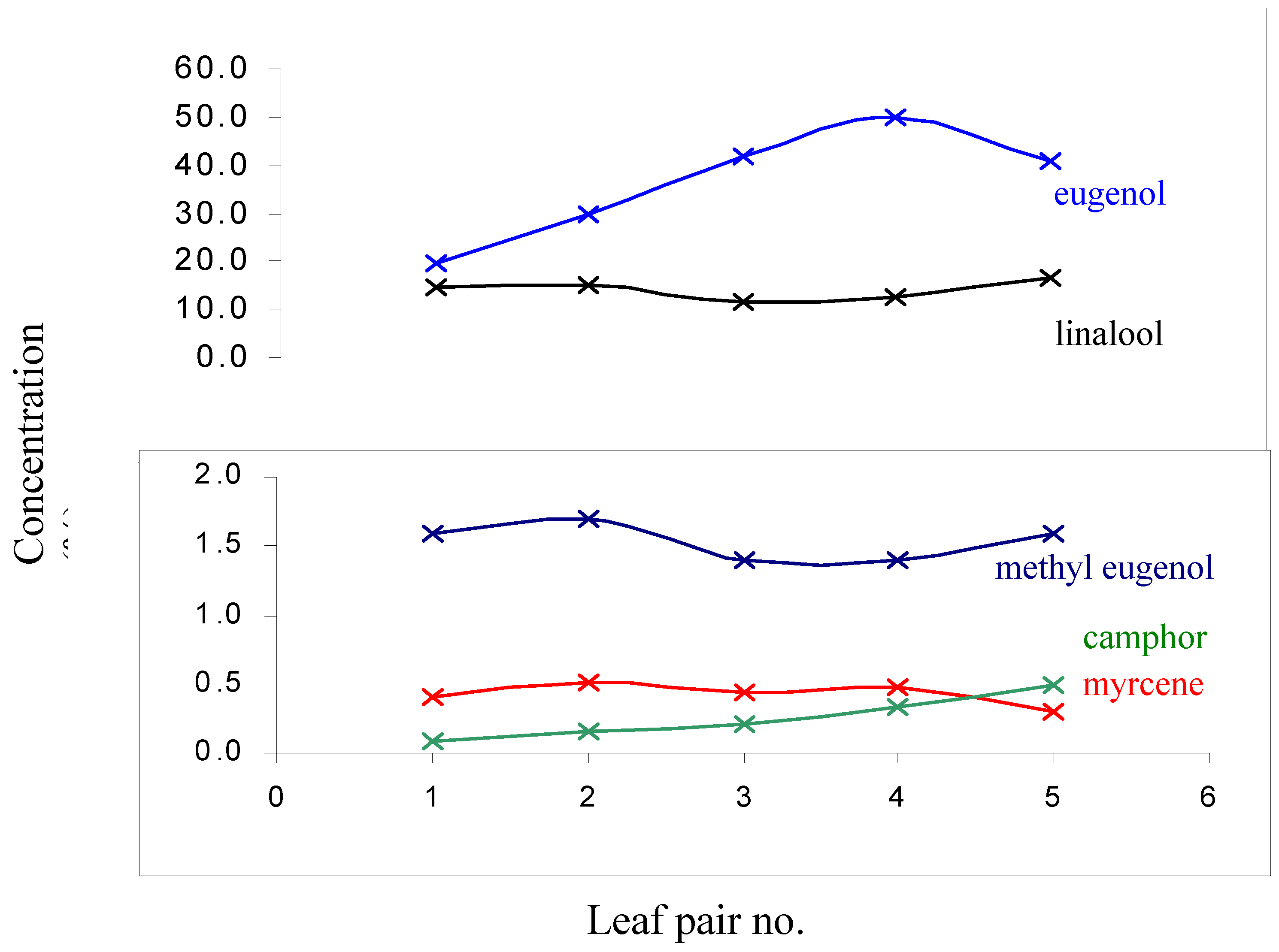
Developmental processes such as maturation are known to have an impact on the oil-profile of terpene accumulating plants especially in herbaceous species and is possible that the small but significant changes observed above are caused by such processes [
15]. To ensure the results were independent from such effects the oil composition of 5 pairs of leaves of
O. minimum was determined starting from the apex and moving down the branch [
16] (
Figure 1). The normal ontogenetical changes in
O. minimum could then be compared to the changes described in
Table 1.
The only considerable biogenetic transformation observed in
Figure 1 is the decrease in the concentration of eugenol as the leaf reaches full maturation. However, considering that the 3
rd and 4
th pair of leaves (from the apex) were used in this work it is evident (
Fig. 1) that this transformation does not account for the wounding response observed. All the other compounds of interest showed either negligible variation (considering that these transformations occur over a few months) or a trend that was opposite to that observed when the leaves were wounded. For example, camphor levels in
O. minimum decline after wounding (
Table 1) but the opposite is observed during the aging process of the plant (
Fig. 1) at least until the leaf reaches full maturation. Studies on common sage (
S. officinalis L.) showed that the key enzymes involved in the production of camphor in this species are at the highest levels during the period of leaf expansion [
15].
Supporting experiments (data not shown) were also carried out to test the validity of the assumption that To values represent pre-wounding levels. Analysis of the data showed that the cutting of a leaf has no detectable instantaneous effects on the composition of the oil in the leaf.
Table 1.
Effect of mechanical damage on oil composition of selected components in O. minimum leaves. Constituents with a concentration greater than 0.2% are shown. Asterisks indicate statistical significance of the difference between T0 and T24 values for that component within the same experiment (* at p<0.05; ** at p<0.02, Wilkoxon signed-ranks test, S.E.M = standard error of the mean).
Table 1.
Effect of mechanical damage on oil composition of selected components in O. minimum leaves. Constituents with a concentration greater than 0.2% are shown. Asterisks indicate statistical significance of the difference between T0 and T24 values for that component within the same experiment (* at p<0.05; ** at p<0.02, Wilkoxon signed-ranks test, S.E.M = standard error of the mean).
| Components | Oil composition (%)
(mean±S.E,M) (n=8) |
| After 24 hrs | After 48 hrs |
| T0 | T24 | T0 | T48 |
| α-Pinene | 0.55±0.04 | 0.59±0.02 | 0.71±0.08 | 0.60±0.10 |
| Sabinene | 0.35±0.03 | 0.42±0.01 | 0.32±0.07 | 0.41±0.06 |
| β-Pinene | 0.72±0.05 | 0.84±0.03 | 0.89±0.08 | 0.86±0.12 |
| Myrcene | 0.26±0.04 | 0.34±0.01* | 0.25±0.06 | 0.31±0.03 |
| Limonene/p-Cymene | 0.37±0.03 | 0.40±0.01 | 0.36±0.06 | 0.40±0.06 |
| 1,8-Cineole | 7.73±0.43 | 8.51±0.17 | 9.30±0.63 | 9.07±0.98 |
| trans-Sabinene hydrate | 0.54±0.03 | 0.58±0.02 | 0.53±0.09 | 0.64±0.06 |
| Linalool | 8.81±1.12 | 11.29±1.56** | 12.8±1.76 | 14.3±1.40 |
| Camphor | 0.87±0.12 | 0.67±0.10** | 0.93±0.10 | 0.69±0.11* |
| Terpinen-4-ol | 1.22±0.07 | 1.14±0.05 | 1.35±0.08 | 1.27±0.13 |
| α-Terpineol | 0.85±0.03 | 0.86±0.02 | 0.94±0.04 | 0.93±0.07 |
| Bornyl acetate | 0.76±0.06 | 0.80±0.04 | 0.90±0.10 | 0.88±0.12 |
| Eugenol | 58.62±1.76 | 53.79±1.97** | 50.4±2.43 | 48.5±2.95 |
| Methyl eugenol | 1.59±0.08 | 1.95±0.12** | 1.66±0.11 | 1.89±0.10 |
| Sesquiterpenes | 16.0±0.74 | 16.60±0.62 | 18.29±1.48 | 18.36±1.04 |
Figure 1.
Ontogenetical variation in the levels of selected constituents found in the oil from O. minimum leaves. The compounds shown are those implicated in the wounding response.
Figure 1.
Ontogenetical variation in the levels of selected constituents found in the oil from O. minimum leaves. The compounds shown are those implicated in the wounding response.
The different types of the compounds involved in the response suggests that they possibly serve distinct purposes. The acyclic terpenes linalool and myrcene are known to be induced on Lima beans, cotton and corn by the feeding of herbivores [
1,
14,
17]. Therefore it is likely that their role during the wounding response in
O. minimum is involved with the protection of the wounded leaves from further damage. Similar function could be attributed to methyl eugenol considering its known insect antifeedant and deterrent properties [
18]. Increased levels of methyl eugenol in the leaves after wounding are likely to be associated with the observed loss of eugenol. However, the amount of eugenol lost from the leaves upon wounding is much greater than the amount of methyl eugenol produced. Given that no other (known) volatile metabolites of eugenol were detected in
O. minimum and that glycosidic-bound eugenol is known to be abundant in the Lamiaceae [
19], it is possible that the unaccounted for eugenol is converted to a non-volatile derivative. A similar behaviour is likely to be exhibited by camphor and therefore accounts for the amount of the ketone lost during the post-wounding period. Work on common sage has already shown that camphor undergoes catabolism by conversion to 1,2-campholide (a lactone) and then to β-D-glucoside-6-o-glucose ester of the corresponding hydroxy acid [
15]. The ester is then transported to the roots where it is re-utilised by the plant [
15]. In this case eugenol and camphor may be utilised during repairing processes operating in
O. minimum wounded leaves.
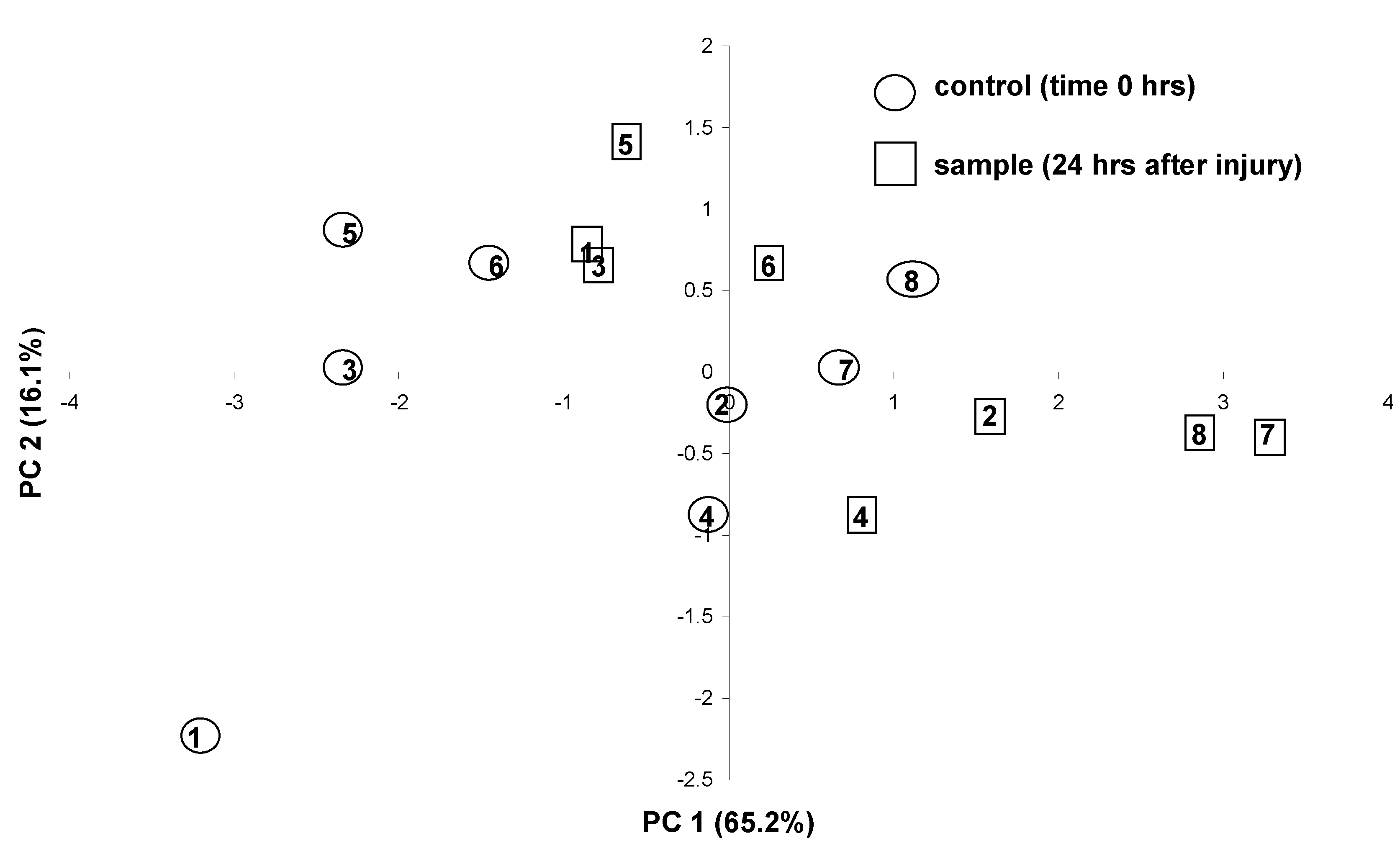
The detected compositional change caused by the wounding response was further elucidated by principal component analysis (PCA). This feature-reduction technique allowed the visualization of the response in a two-dimensional score plot (
Fig. 2). A plot defined by the first two principal components was sufficient for that purpose as it could explain most of the variation in the data (> 81.3 %).
Figure 2 shows that the wounding response in each leaf (observed as a shift of the points mostly along the PC1 axis) is independent of the pre-wounding levels of the particular compound expressing the response. Also, the magnitude of the response (distance traveled) appears to be similar between replicates, despite the large inter-plant variation which is evident from the scattering of the positions of the control points on the plot.
Figure 2.
PCA scatterplot of oil-profiles from O. minimum leaves on the first 2 principal components extracted using the group of five compounds as variables (unwounded (T0) leaves in circles, wounded (T24) leaves in squares). Identical numbers indicate same leaf (before – after wounding). Individuals 5-8 were sampled one month after individuals 1-4 to test the reproducibility of the response.
Figure 2.
PCA scatterplot of oil-profiles from O. minimum leaves on the first 2 principal components extracted using the group of five compounds as variables (unwounded (T0) leaves in circles, wounded (T24) leaves in squares). Identical numbers indicate same leaf (before – after wounding). Individuals 5-8 were sampled one month after individuals 1-4 to test the reproducibility of the response.
The oil-profile differences demonstrated above between unwounded and wounded leaves are small in magnitude especially when compared with results obtained from non-terpene accumulating plants [
1,
5,
14]. However, it is possible that inducible defenses in oil-bearing plants are limited due to the large amount of constitutive defense present in these plants in the form of essential oils. Also, an elicitor may be required for a greater response to be induced. Studies on cabbage and corn seedlings have shown that the wound-response in these plants is much greater when an elicitor (for example enzymes found in the predator’s saliva) is present rather than the response obtained by simple mechanical damage [
6,
20]. Work in progress is currently testing the validity of this hypothesis in relation to terpene accumulating plants.
Experimental
Plants. O. minimum plants were obtained from a local nursery and were kept under field conditions. Five-month old plants were used for the experiments.
Experimental design. Three leaves (3rd-4th pair from the apex) were cut in half laterally at 0 hour, combined, weighed and immediately subjected to solvent extraction. After 24 hours the other half of the leaves (that had remained on the branch) was taken and treated similarly. The above process was carried out on eight different plants divided into two groups. The two groups were sampled one month apart in order to test the reproducibility of the results. The 48 hours treatment was carried out in the same way but this time the second half of the leaves was sampled 48 hours after hour zero. Different plants were used for each treatment.
Field conditions. The experiments were carried out during spring (temperature 21-26 °C, humidity 65-80 %). Sampling time was midday. The plants were irrigated daily and each plant was wounded only once.
Chemical analysis. After sampling the leaf material (15-25 mg) was immersed in CH
2Cl
2 spiked with 400 ppm octane for 24 hours at room temperature in the dark [
21]. Extracts were then subjected to GC and GC-MS analysis. GC analysis was carried out using a HP 6890 gas chromatograph equipped with a flame ionisation detector and a HP 7673 GC/SFE auto-injector. The column used was 50QC2/BPX-5 (50 m length x 0.22 μm ID x 0.25 μm film thickness) (SGE Scientific, Melbourne, Australia). The injection volume was 2 μL, inlet temperature and pressure were 280 °C and 20 psi respectively, carrier gas was H2 (40 mL/min.), split ratio was 1:10, detector temperature at 280 °C, and the oven program was: initial temperature 60 °C for 5 min., increased to 180 °C at 4 °C/min., final temperature maintained for 5 min.
GC-MS analysis conditions were as above with He as the carrier gas. The instrument used was a Varian 3800 gas chromatograph connected to a Varian 2000 ion trap detector (0.9 scans/sec, 20 μA). Compounds were identified by comparison of their mass spectra and retention indices (based on
n-alkanes) with those of authentic standards (Fluka Chemicals, NSW, Australia). The relevant literature was also consulted [
11,
22].
Statistical analysis. The contribution of each compound (and of the sesquiterpenes measured as a group) in the oil was expressed as a percent value based on the peak areas of the integrated chromatograms. This was necessary in order to compare the oil-profiles before and after wounding. If absolute values (e.g., area counts/g) were to be used for the comparison, then factors such as moisture and total oil content would have been superimposed on the data and the results obtained would be misleading.
The difference between the percentage contribution of each compound in the oil before and after wounding was tested for statistical significance using the Wilkoxon signed ranks test (paired samples) [
23]. A correlation matrix was then built (based on the actual percentages) using as variables the compounds found to be statistically significant. PCA was then performed on the correlations and the eigenvalues obtained were used to visualize the relationship between objects (leaves) in two-dimensional score plots. The software used for all data analyses was JMP-IN
® (version 3 for Windows) (SAS Institute Inc., USA).