An Improved Synthesis of 5-(2,6-Dichlorophenyl)-2-(phenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one (a VX-745 analog)
Abstract
:Introduction
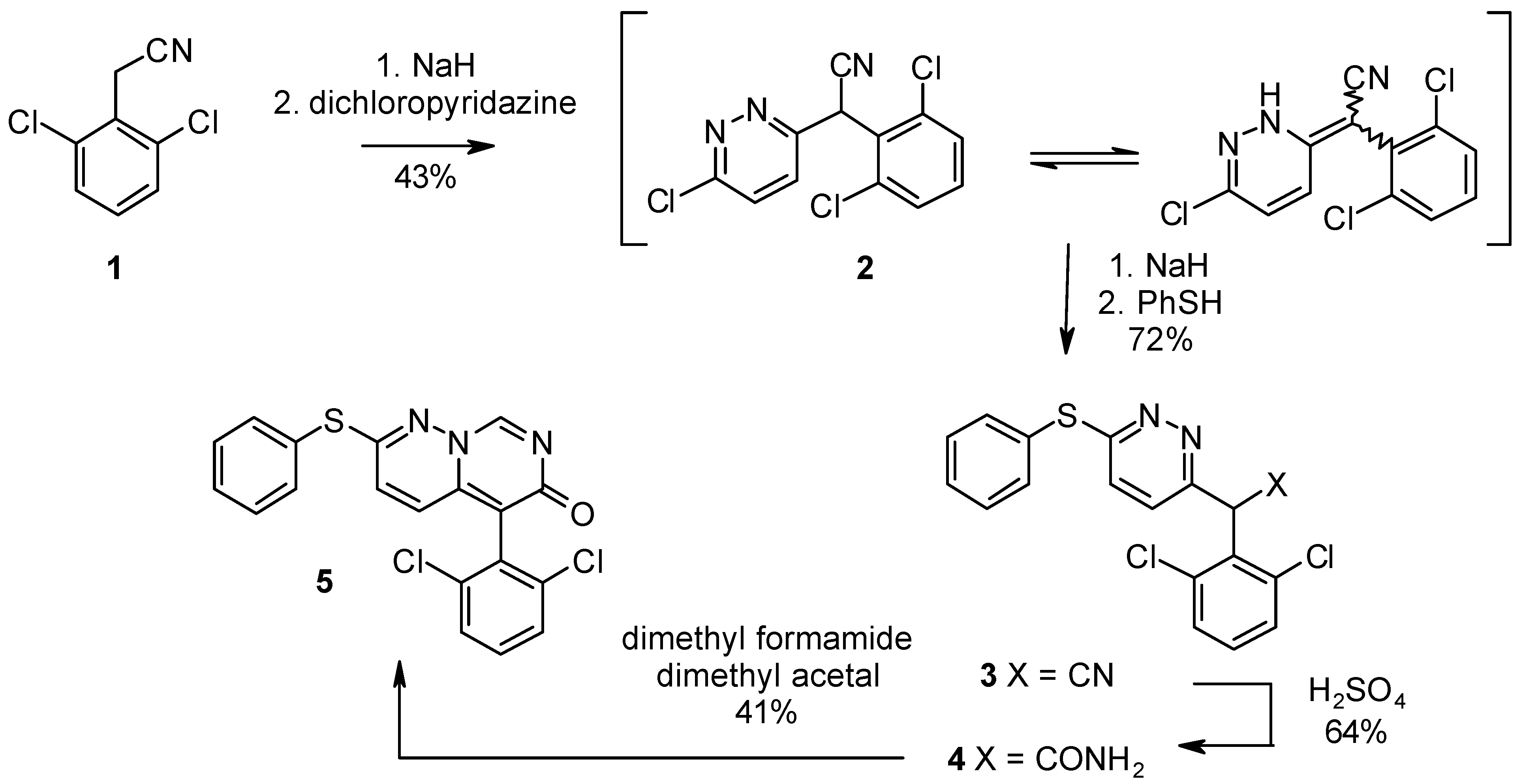
Results and Discussion
Conclusions
Experimental
General
6-Chloro-α-(2,6-dichlorophenyl)pyridazine-3-acetonitrile (2).
α-(2,6-Dichlorophenyl)-6-phenylthiopyridazine-3-acetonitrile (3).
α-(2,6-Dichlorophenyl)-6-phenylthiopyridazine-3-acetamide (4).
5-(2,6-Dichlorophenyl)-2-(phenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one (5).
| Time | Flow | %A | %B |
|---|---|---|---|
| 0.00 | 1.00 | 30.0 | 70.0 |
| 3.00 | 1.00 | 30.0 | 70.0 |
| 15.00 | 1.00 | 90.0 | 10.0 |
| 20.00 | 1.00 | 30.0 | 70.0 |
| 50.00 | 0.20 | 30.0 | 70.0 |
References
- Dillon, S. B.; Griego, S. D. Use of CSAIDs (cytokine suppressive antiinflammatory drugs) in rhinovirus infection. WO 01/19322, 22 March 2001. [Chem. Abstr. 2001, 134, 242657]. [Google Scholar] Ferraccioli, G. F. VX-745 Vertex Pharmaceuticals. Curr. Opin. Anti-Inflammatory Immunomodulatory Invest. Drugs 2000, 2, 74–77. [Google Scholar]
- Bemis, G. W.; Salituro, F. G.; Duffy, J. P.; Cochran, J. E.; Harrington, E. M.; Murcko, M. A.; Wilson, K. P.; Galullo, V. P. Preparation of annelated pyrimidinones and analogs as p38 kinase inhibitors. WO 98/27098, 25 Jun 1998. [Chem. Abstr. 1998, 129, 81749]. [Google Scholar]
- Bemis, G. W.; Salituro, F. G.; Duffy, J. P.; Harrington, E. M. Preparation of pyrido[1,2-c]-pyrimidin-3-ones or 1,2-dihydro-pyrido[1,2-c]pyrimidin-3-ones as inhibitors of p38. US 6147080, 14 Nov 2000. [Chem. Abstr. 2000, 133, 350242]. [Google Scholar]
- Sample Availability: Available from MDPI.
© 2001 by Molecular Diversity Preservation International (MDPI)
Share and Cite
Treu, M.; Jordis, U.; Lee, V.J. An Improved Synthesis of 5-(2,6-Dichlorophenyl)-2-(phenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one (a VX-745 analog). Molecules 2001, 6, 959-963. https://doi.org/10.3390/61200959
Treu M, Jordis U, Lee VJ. An Improved Synthesis of 5-(2,6-Dichlorophenyl)-2-(phenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one (a VX-745 analog). Molecules. 2001; 6(12):959-963. https://doi.org/10.3390/61200959
Chicago/Turabian StyleTreu, Matthias, Ulrich Jordis, and Ving J. Lee. 2001. "An Improved Synthesis of 5-(2,6-Dichlorophenyl)-2-(phenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one (a VX-745 analog)" Molecules 6, no. 12: 959-963. https://doi.org/10.3390/61200959




