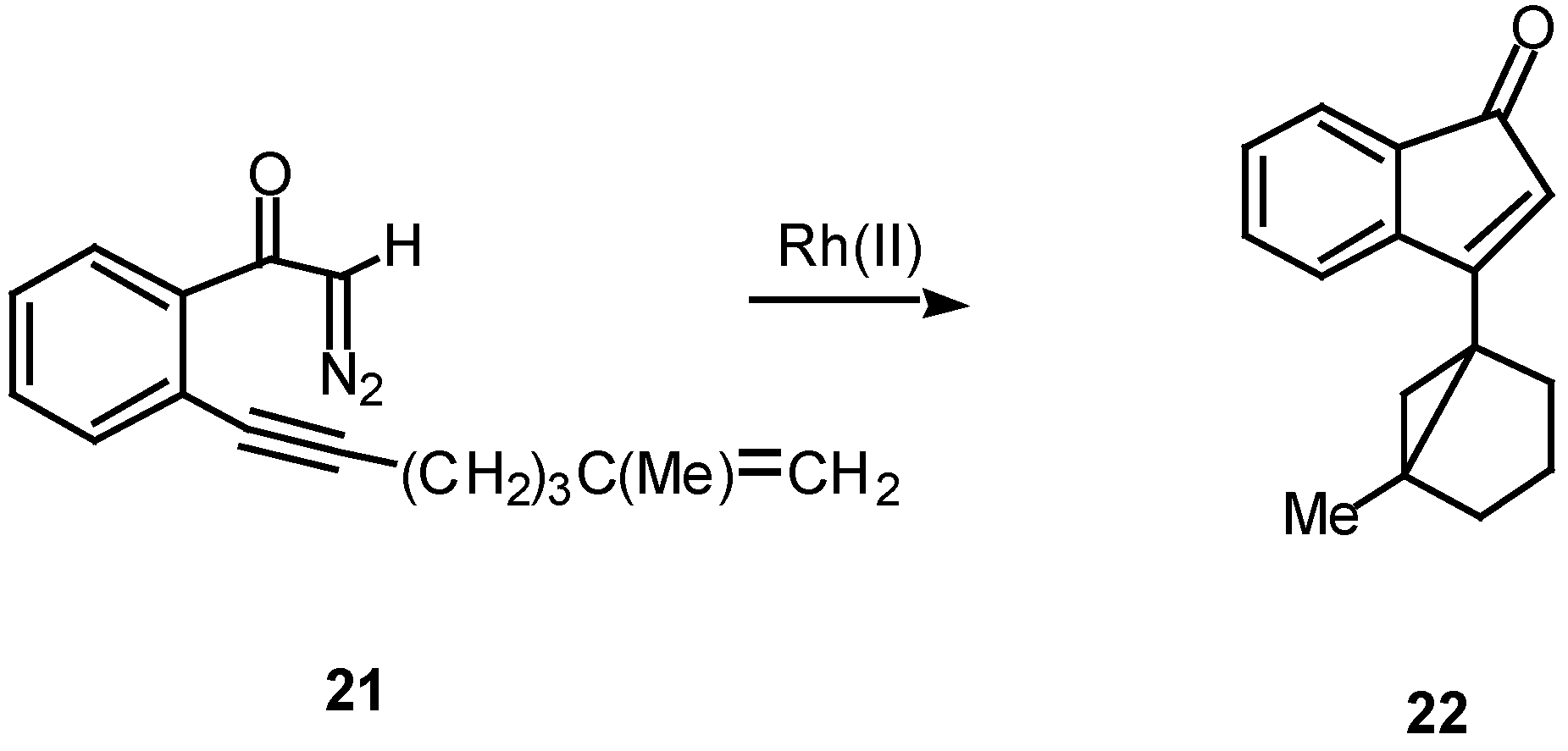Introduction
The chemistry of transition metal carbene complexes has been a subject of intense activity over the past two decades [
1]. Current interest in this field stems from the role of metal carbenes in alkene metathesis [
2], in alkene and alkyne polymerization [
3], in cyclopropanation chemistry [
4], and as intermediates in an impressive array of synthetic methodology [
5,
6]. The intramolecular reactions of metal carbene complexes derived from α-diazo carbonyl compounds have been extensively studied from both a mechanistic and synthetic viewpoint [
7]. Rhodium(II) carboxylates are particularly effective catalysts for the decomposition of diazo compounds and many chemical syntheses are based on this methodology [
8]. Among the more synthetically useful processes of the resulting carbenoid intermediates are intramolecular C-H insertion [
9], cyclopropanation [
10], and ylide generation [
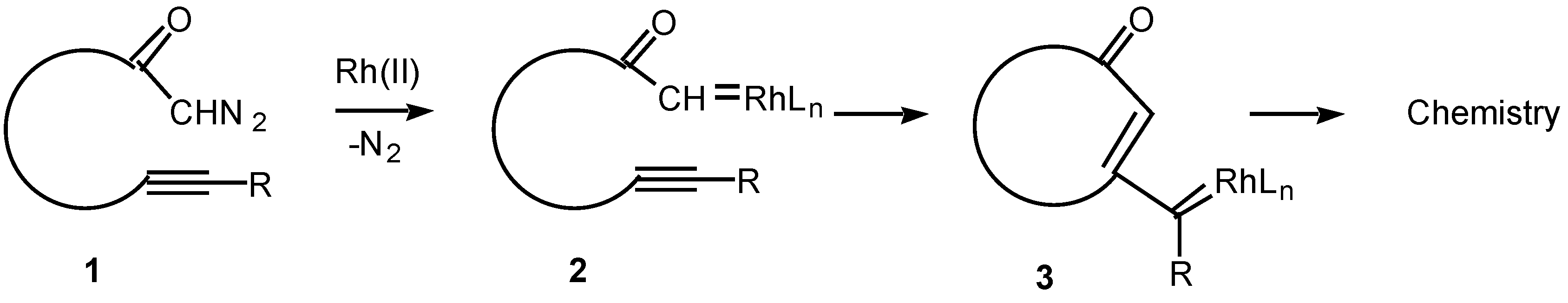
11]. In contrast to these processes, the corresponding reaction of alkynes with metal carbenes has been far less studied. Only in recent years has some attention been focused on the intramolecular cyclization reactions of α-diazo ketones containing tethered alkynes (
i.e.,
1) in the presence of transition metal catalysts. The overall reaction observed is believed to proceed
via an initial decomposition of the α-diazo ketone to generate a rhodium carbenoid intermediate
2. Attack on the carbenoid carbon by the tethered alkyne generates a new intermediate (
3) in which carbene-like character has been transferred to the beta-carbon of the alkyne. The intermediate vinyl carbenoid may then react further in either an intramolecular or intermolecular fashion to give novel products. This article describes some of our work in this area.
General Mechanistic Considerations
The mechanism of the diazo ketone-alkyne cyclization reaction has been the subject of some study over the past several years [
12,
13,
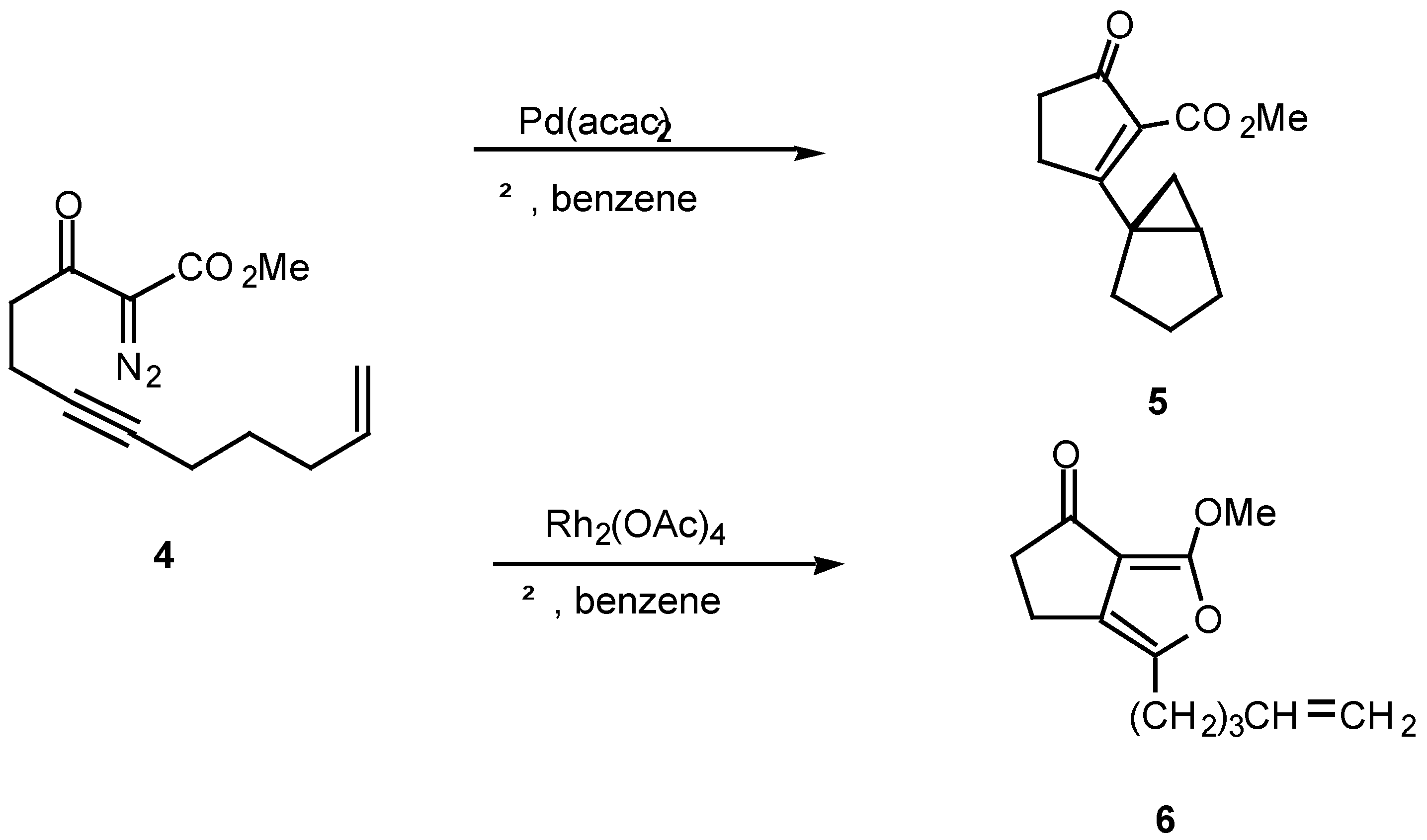
14]. For example, treatment of ketone, α-diazo ketoester
4 with catalytic palladium(II) acetoacetonate produced cyclopropane
5 in 78% yield, while the reaction with rhodium(II) acetate provided furan
6 in 56% yield. Furan
6 arises from a 1,5-electrocyclization of the initially produced vinyl carbenoid intermediate onto the adjacent carbonyl group (
vide infra).
The fact that the chemistry of
4 is catalyst dependent suggests that a metalated species is involved in the product-determining step [
12]. One possible mechanism to explain the products involves the initial decomposition of the α-diazo moiety to give the metal carbenoid
7. In the next step, the rhodium metal migrates from the original diazo carbon to the alkynyl carbon
via a metathesis reaction and ultimately produces metallocyclobutene
8. This intermediate could then ring open to furnish the vinyl carbenoid
10 which goes on to afford the observed products. Another possible variation would be formation of the highly strained cyclopropene
9. This intermediate could then be rapidly converted into the 5-
exo vinyl carbenoid
10 or the 6-
endo carbenoid
11, both of which can undergo further chemistry. This pathway has precedent from the known metal catalyzed ring opening of cyclopropenes to vinyl carbenes [
14].
Results in our laboratory showed that the reaction mechanism is markedly dependent on the solvent employed in these Rh(II)-catalyzed insertion processes. Thus, treatment of
12 with a catalytic amount of rhodium(II) acetate resulted in a 2:1-mixture of the
cis and
trans-alkenyl substituted indenones
13 (85% combined yield). No signs of cyclopropene
14 (<2%) could be detected in the crude reaction mixture by NMR spectroscopy. Interestingly, when pentane was used as the solvent, cyclopropene
14 (80%) was the exclusive product. The degree of chemoselectivity that was achieved in this reaction by simply changing the solvent from dichloromethane to pentane is most remarkable [
17]. A reasonable explanation that nicely accounts for the formation of indenone
13 involves stepwise cyclization of the initially formed keto carbenoid to give
15 (
Scheme 5). A 1,2-hydrogen shift results in the formation of allylic cation
16 and this is followed by collapse to
13 and regeneration of the rhodium catalyst. The intermediates involved in the formation of
13 are dipolar, which would explain why the formation of
13 is strongly inhibited in nonpolar solvents. When pentane is used as the solvent, metal migration occurs
via the metallocyclobutene intermediates
17 and
18 so as to avoid charge buildup [
17]. Thus, it would appear as though the reaction mechanism of these alkyne cyclizations is markedly dependent on the nature of the solvent used.
Cyclopropenation
Many of the earlier systems studied involved trapping the vinyl carbenoid intermediate as a cyclopropane
via reaction with external or tethered alkenes [
12,
18,
19]. What is most interesting about this reaction is the formation of three new rings in one step from an acyclic precursor. A typical example is outlined in
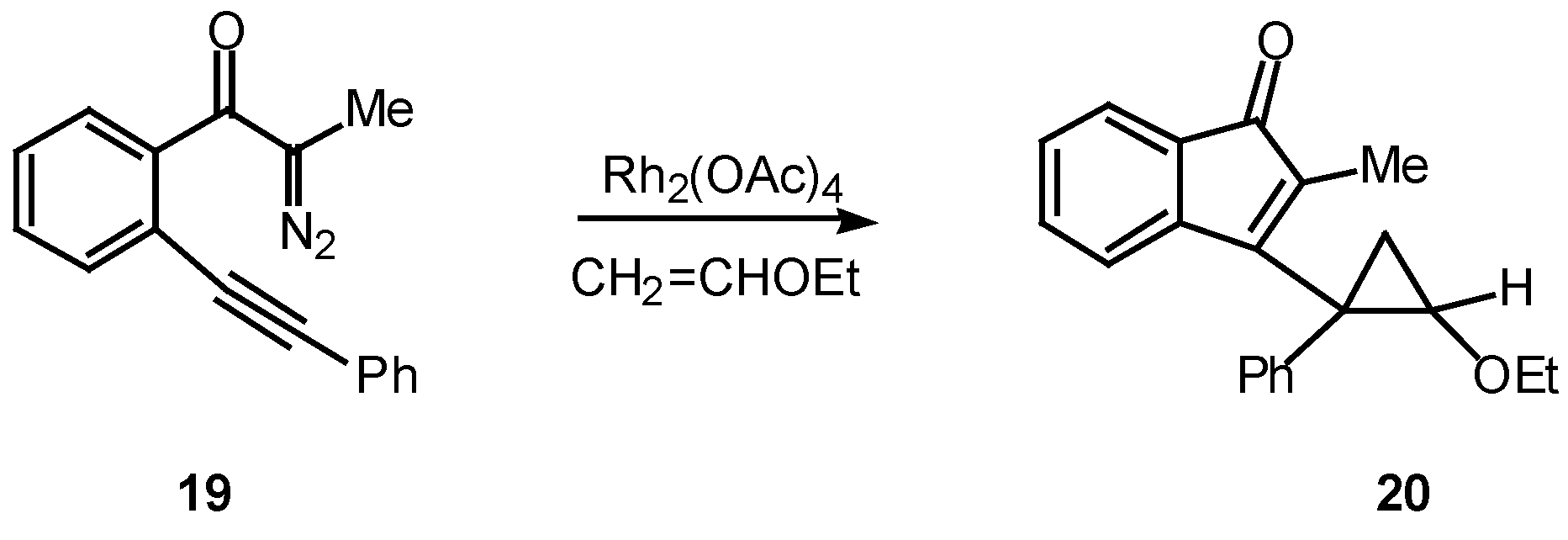
Scheme 6. Treatment of
19 with a catalytic quantity of rhodium(II) acetate in the presence of two equiv of vinyl ether afforded cyclopropane
20 in 91% yield [
18].
In the case of intramolecular trapping with tethered alkenes, two basic structural variations are possible. These will depend on the point of attachment of the alkenyl group and each variation will lead to very different cyclization products. In type I systems, the alkenyl group is tethered onto the alkynyl carbon atom and this is illustrated with α-diazo ketone 21. Treatment of 21 with a catalytic quantity of rhodium(II) acetate gave indenone 22 in 60% yield.16
A number of experiments designed to probe the scope and generality of the intramolecular alkyne cyclopropanation reaction were carried out in an effort to exploit this tandem sequence as a synthetic method. Initial efforts focused on the rhodium(II) catalyzed reaction of
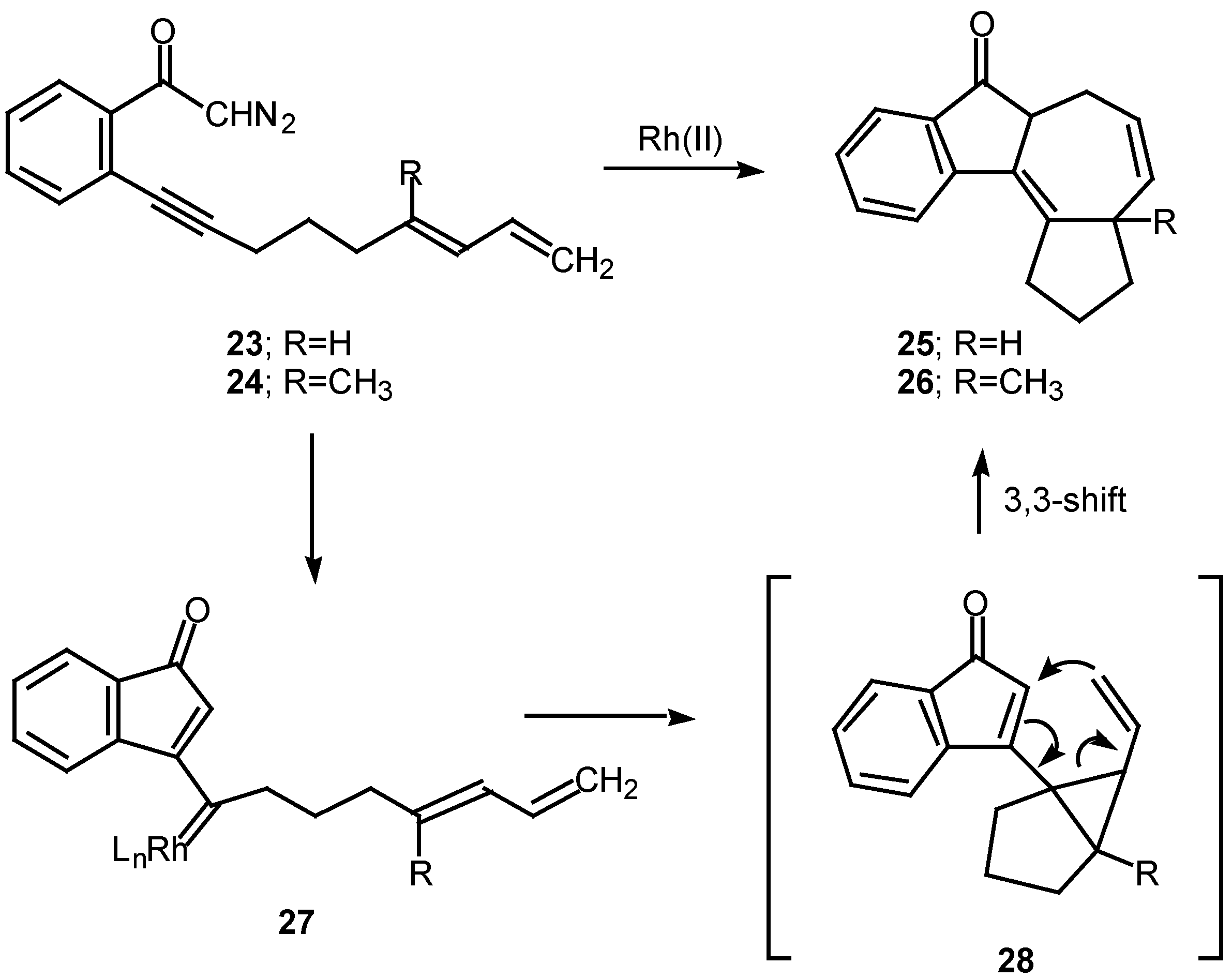
o-(6,8-nonadien-1-ynyl)-α-diazoacetophenone
23. Treatment of
23 with a catalytic quantity of rhodium(II) mandelate gave cycloheptadiene
25 in 58% yield. In a similar manner, treating the closely related diazo ketone
24 (R=CH
3) with rhodium(II) mandelate also gave cyclopent[
g]azulenone
26 [
13]. The formation of the fused cycloheptadienes
25 and
26 can be rationalized by assuming that the reaction proceeds through the divinylcyclopropane intermediate
28. When the internal double bond of the diene possesses the
E-geometry, intramolecular cyclopropanation gives rise to a
cis-divinyl cyclopropane, which rapidly undergoes a Cope rearrangement under the conditions used [
13]. It should be noted that intramolecular cyclopropanation of dienes by simple carbenoids followed by rearrangement of the resulting vinylcyclopropane has been effectively in several elegant syntheses. The overall process is also closely related to work by Davies who developed a synthesis of fused seven membered carbocycles based on a formal intramolecular [3+4]-cycloaddition of vinyl carbenoids with dienes [
20].
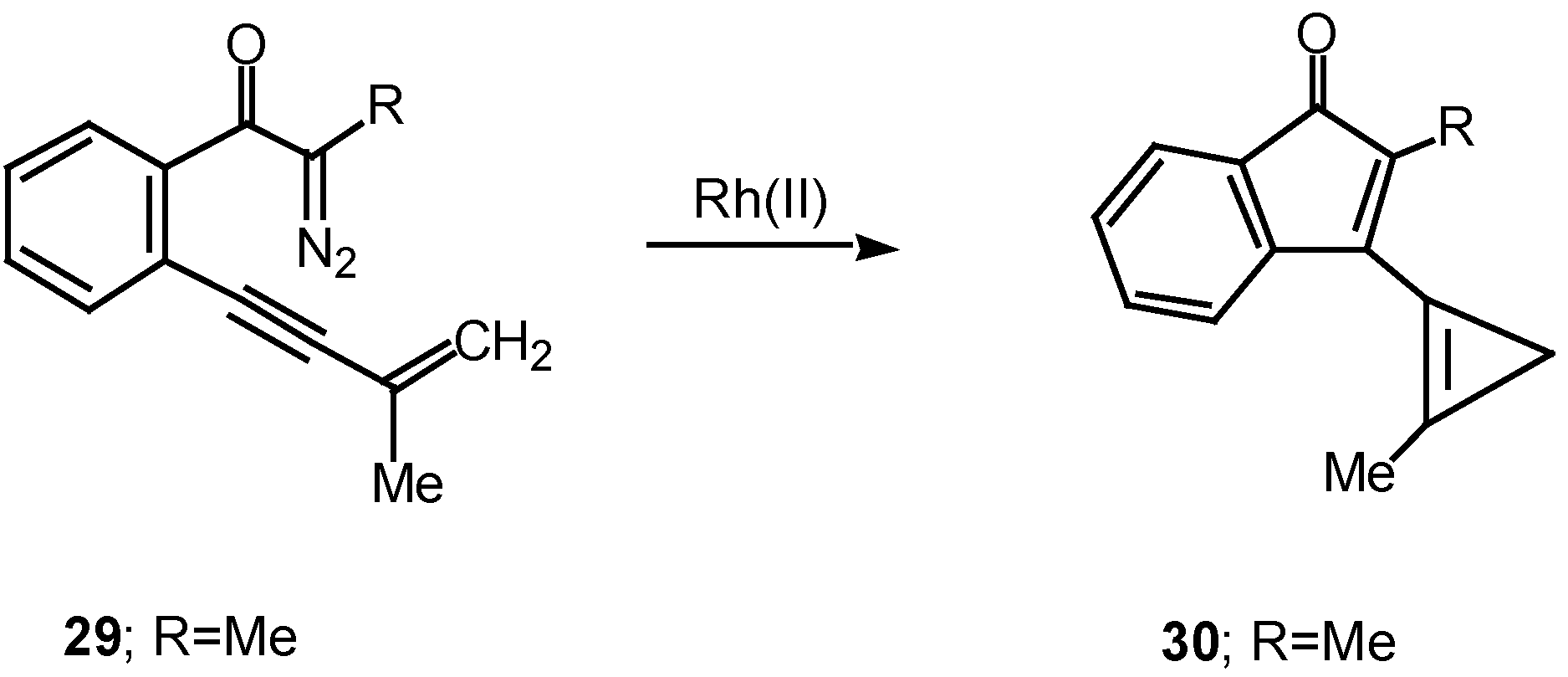
Cyclization of vinyl carbenoids to produce cyclopropenes is another common reaction that is often encountered with these systems [
21]. For example, treatment of α-diazo ketone
29 with a catalytic quantity of rhodium(II) acetate afforded cyclopropene
30 in 95% yield.
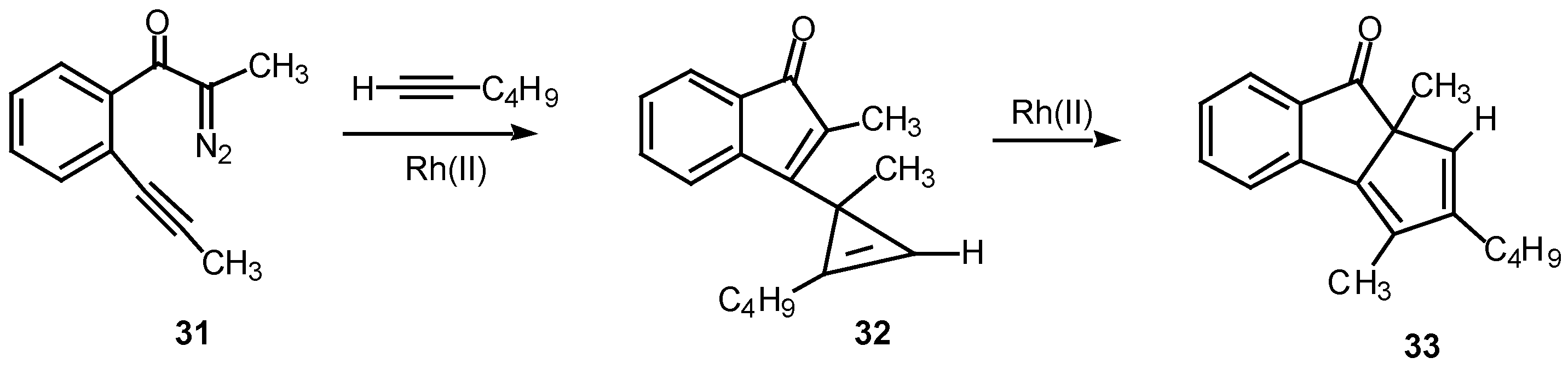
A
double internal/external alkyne cyclization of acetylenic α-diazo ketone
31 with 1-hexyne was also studied in our laboratory. Stirring this mixture in the presence of rhodium(II) acetate at 25°C for 1 h afforded the novel cyclopentadiene derivative
33 in 81% yield. Control experiments established that the initial product that was first formed was indenone
32. This product is the result of the vinyl carbenoid adding across the acetylenic π-bond of 1-hexyne. When the reaction was carried out for only 10 min at 25°C, indenone
32 could be isolated in 85% yield. Further stirring of
32 with rhodium(II) acetate induced a subsequent rearrangement and ultimately produced
33 in 92% yield [
22].
The ease with which these systems undergo the rhodium(II) catalyzed cyclization to give cyclopropenyl substituted indenones suggested that a similar transformation might occur with diacetylenic systems [
23]. Such a study was carried out using diazo ketone
34. A critical issue is whether the cyclization will occur to give products derived from the fully rearranged carbenoid
36 or from the initially formed carbenoid
35. In fact, treatment of
34 with a catalytic quantity of rhodium(II) acetate at 25°C in the presence of ethyl vinyl ether afforded cyclopropane
37 with notable efficiency (90% chemical yield) and selectivity (>95% isomeric purity). No signs of the isomeric cyclopropane
38 could be detected in the crude reaction mixture [
24]. The exclusive formation of cyclopropane
37 was attributed to a slower rate of trapping of vinyl carbenoid
35 by ethyl vinyl ether, perhaps as a consequence of a more congested transition state. Another possibility is that the equilibrium between the two carbenoids lies completely in favor of the more stable phenyl substituted isomer
36.
Diazoester Cyclizations
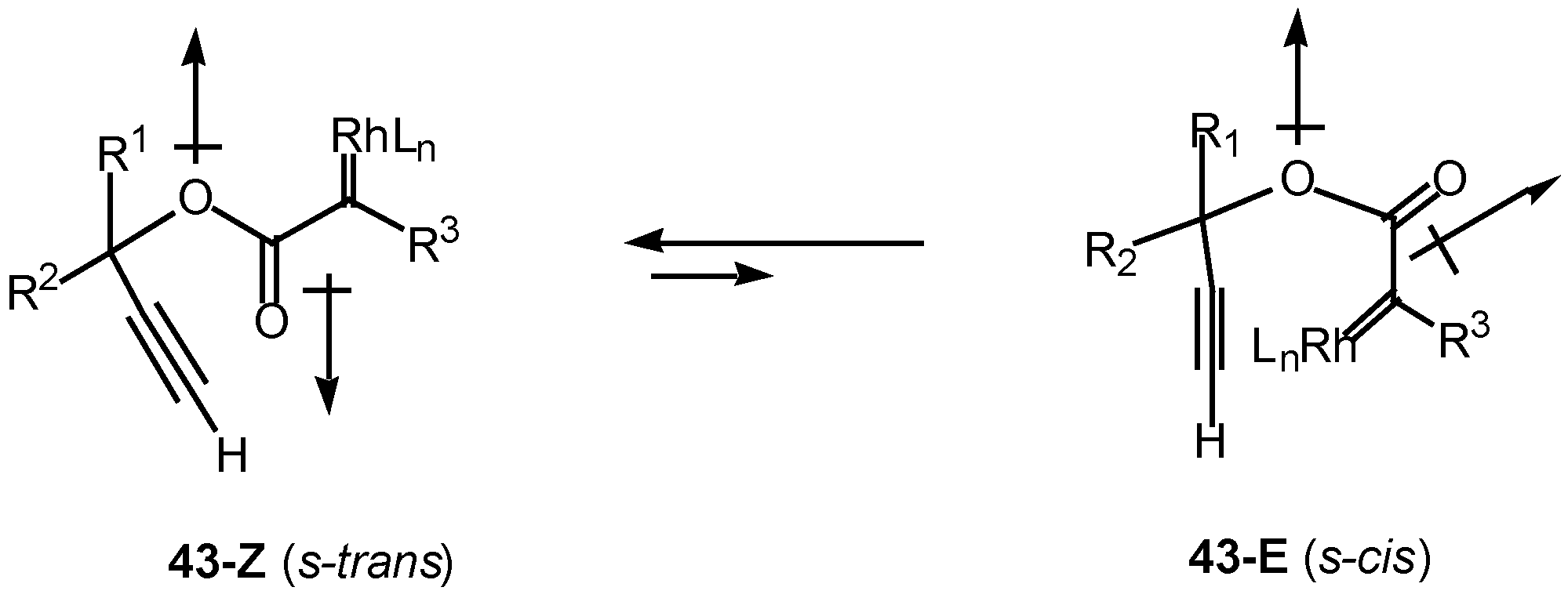
Introduction of a heteroatom α to the diazo carbonyl group may further complicate the cyclization chemistry. It is well known that esters exist primarily in the
Z or
s-trans (
i.e.,
43-Z) conformation about the carbonyl π-bond (
Scheme 13). Esters are more stable in this conformation for several reasons, one of which is to minimize the overall dipole effect. In this orientation, intramolecular cyclization of the rhodium carbenoid onto the alkyne π-bond cannot occur. In order for cyclization to take place, there must be rotation about the ester bond to generate the
E or
s-cis conformer
43-E, which can then achieve the necessary geometry to allow the cyclization to proceed [
26].
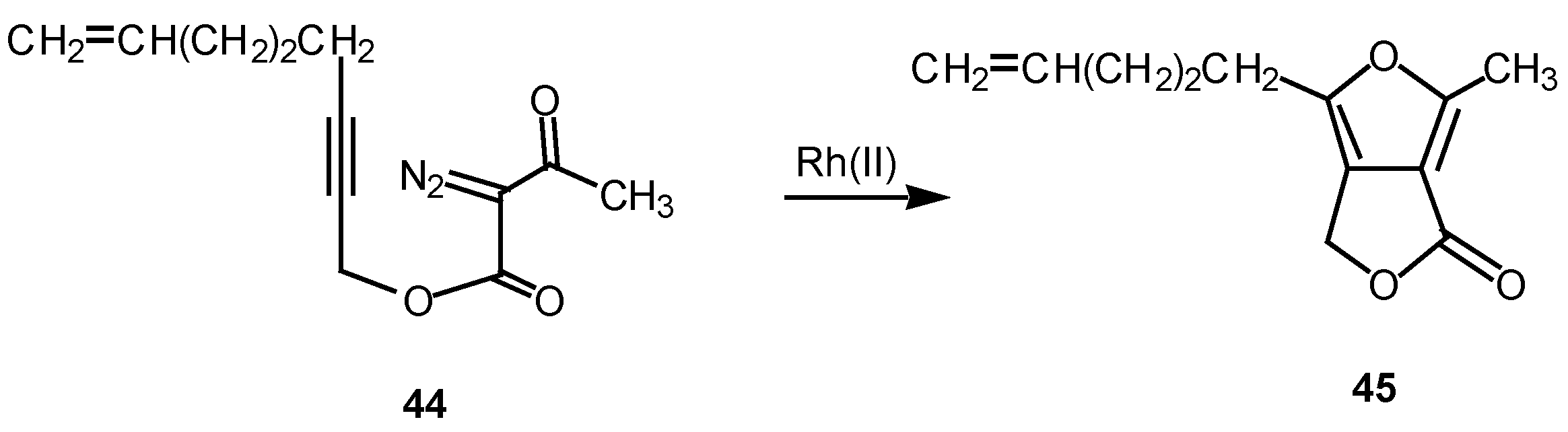
We have found that cyclization of the distabilized diazo ketoester
44 with rhodium(II) octanoate furnished furan
45 in 77% yield [
27]. This transformation proceeds by addition of the rhodium-stabilized carbenoid onto the acetylenic π-bond to produce an electrophilic vinyl carbenoid intermediate (
i.e.,
47) which is subsequently attacked by the adjacent carbonyl oxygen bond. The resulting dipole
48 undergoes subsequent collapses to give furan
49 [
28]. 6π-Electrocyclization reactions to produce five membered rings are well precedented transformations in heterocyclic chemistry [
29]. Related furan cyclizations have also been observed in
ortho constrained systems [
30].
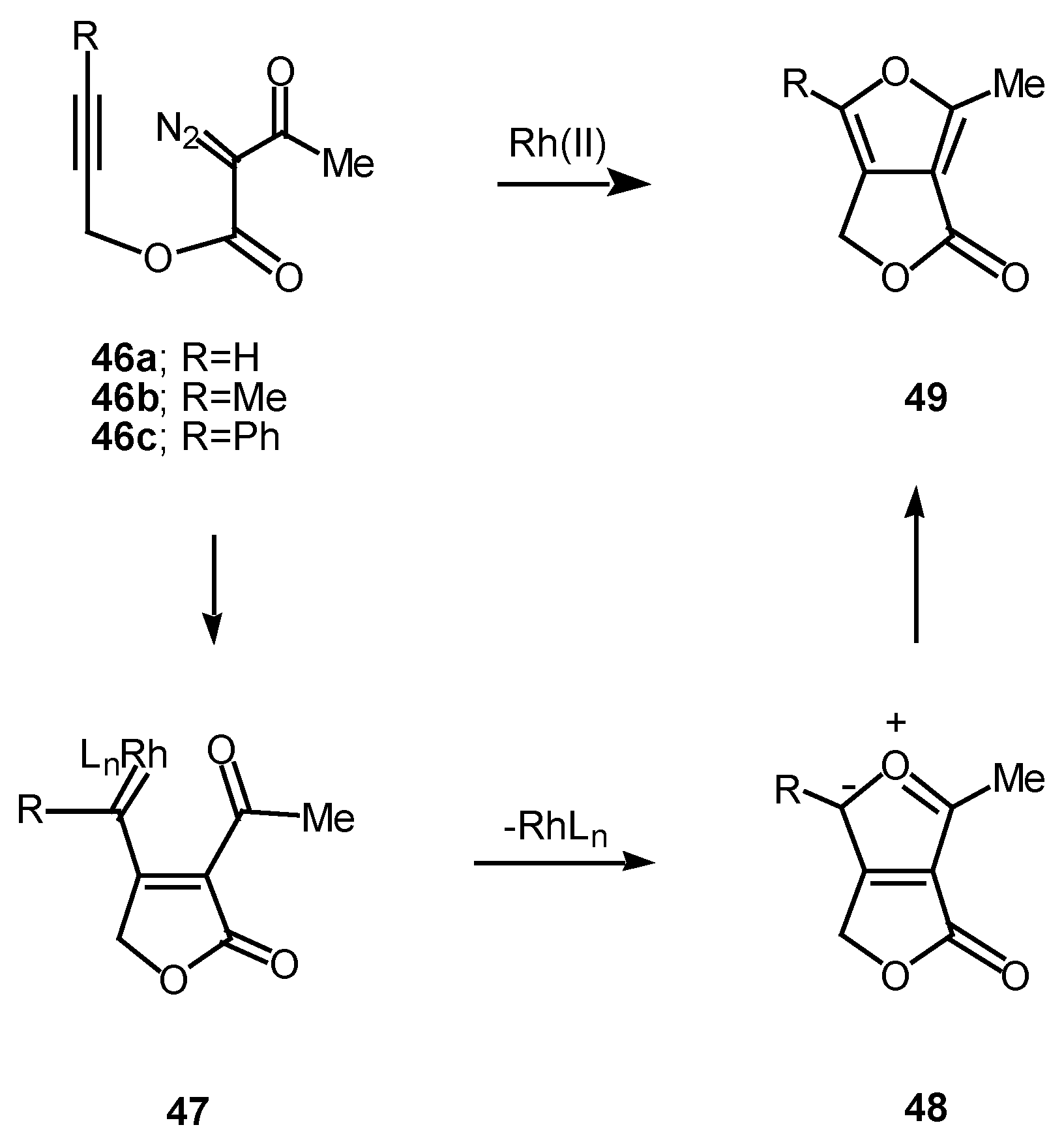
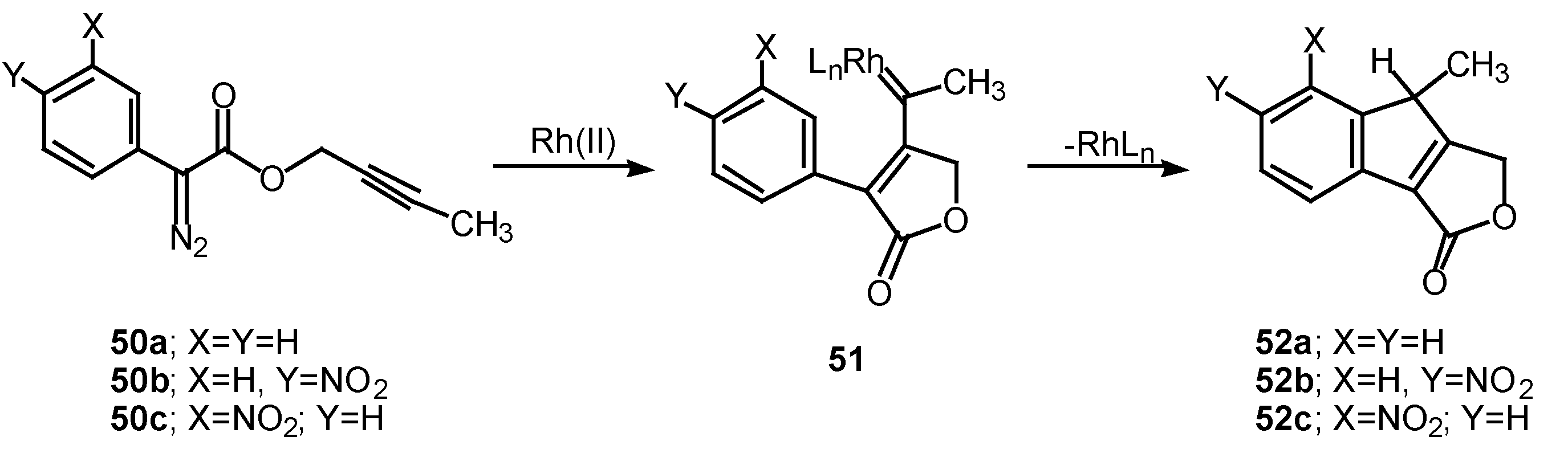
The 1,5-electrocyclization process involved in furan formation has also been utilized to produce indeno[1,2-c]furans such as
52a-
c in 45-60% yield. Treatment of the starting α-diazo esters
50a-c with rhodium(II) catalysts gave indenes
52a-c via an electrocyclization of the transient vinyl carbenoid
51 [
26]. There seemed to be little effect displayed by the nature of the substituent groups on the aromatic ring as indeno[1,2-c]furans
52b and
52c were isolated as the exclusive products. The fact that the insertion reactions occurs
ortho to the nitro group (
i.e.,
50c to
52c) rather than producing a mixture of
ortho and
para isomers, suggests that subtle factors play a role in this process as well.
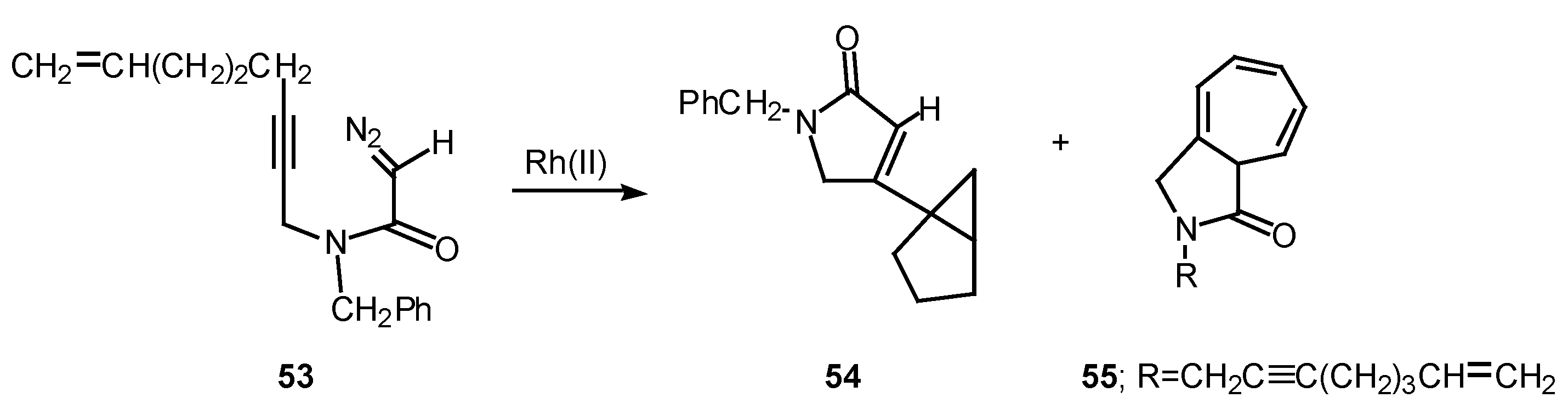
Rotamer population can play a significant role in determining the chemoselectivity of rhodium(II) catalyzed reactions of α-diazo amide systems containing tethered alkynes. The reaction of diazo amide 53 with rhodium(II) octanoate was found to undergo attack on the π-system of the acetylenic tether to give a transient vinyl carbenoid. The next step involved an internal cyclopropanation reaction to produce 54 in 41% yield. Cycloheptatriene 55, which is derived by insertion of the carbenoid into the N-benzyl substituent, was also isolated from this reaction in 33% yield. Rotamer populations nicely account for the behavior of this system.
Amide rotamers generally interconvert in solution with lifetimes of 10-1-10-2 sec. The geometry of a typical amide C-N bond will be fixed during the entire lifetime of the acyl rhodium carbenoid intermediate. Assuming that both amide rotamers are equally reactive toward π-addition, the relative amounts of compounds 54 and 55 that are formed are determined by the equilibrium concentration of the starting rotamers.
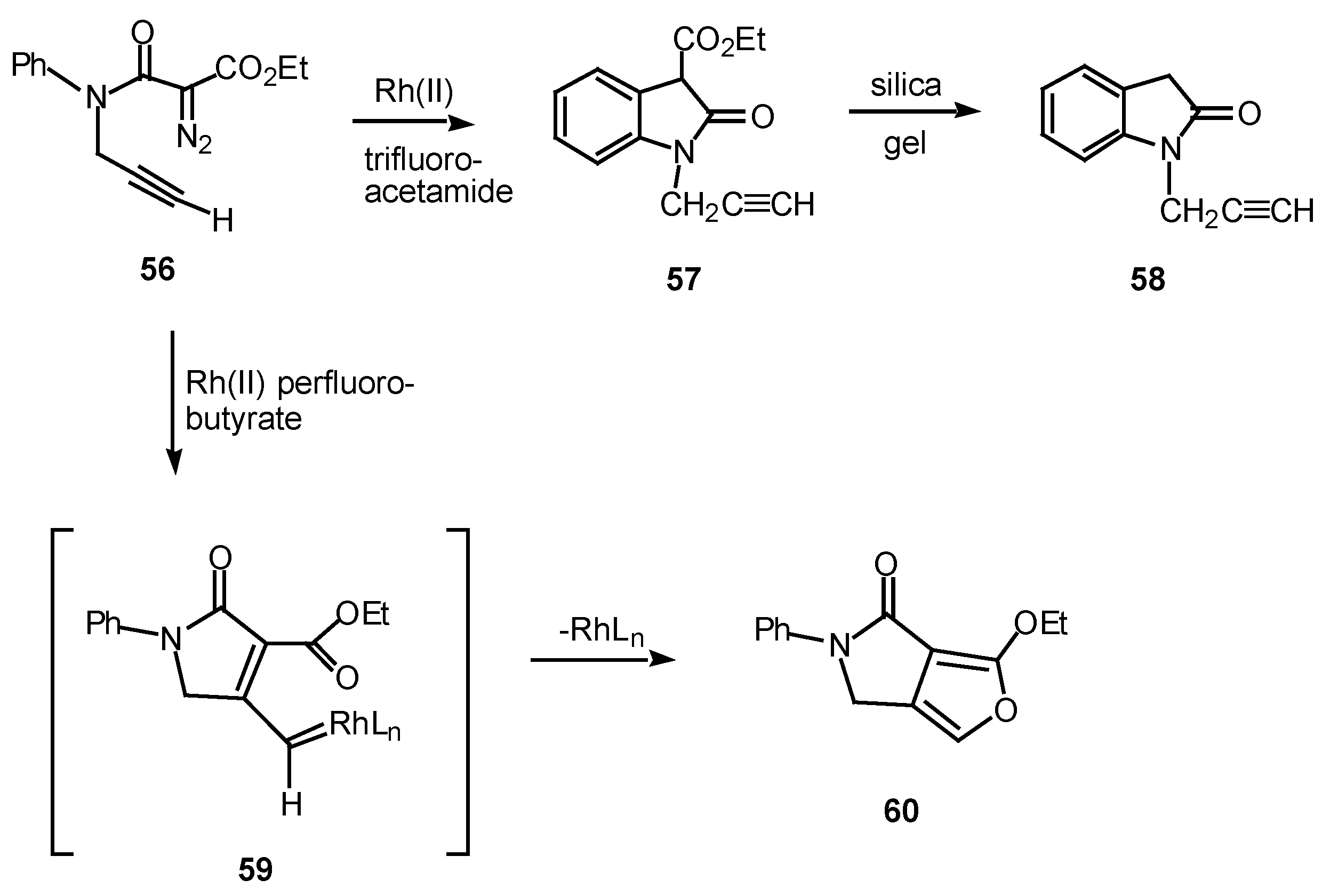
In one study, the mode of cyclization of a distabilized α-diazo amide was varied by changing the ligands on the rhodium catalyst. Reaction of
56 with rhodium(II) trifluoroacetamide in benzene at 25°C provided oxindole
57 in 87% yield. On the other hand, when rhodium(II) perfluorobutyrate was used as a catalyst, fluropyrrolone
60 was formed in 98% yield [
31], in line with previous observations [
32].