Synthesis and Fungicidal Activity of 2-Imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and Their 5-Arylidene Derivatives
Abstract
:Introduction
Results and Discussion
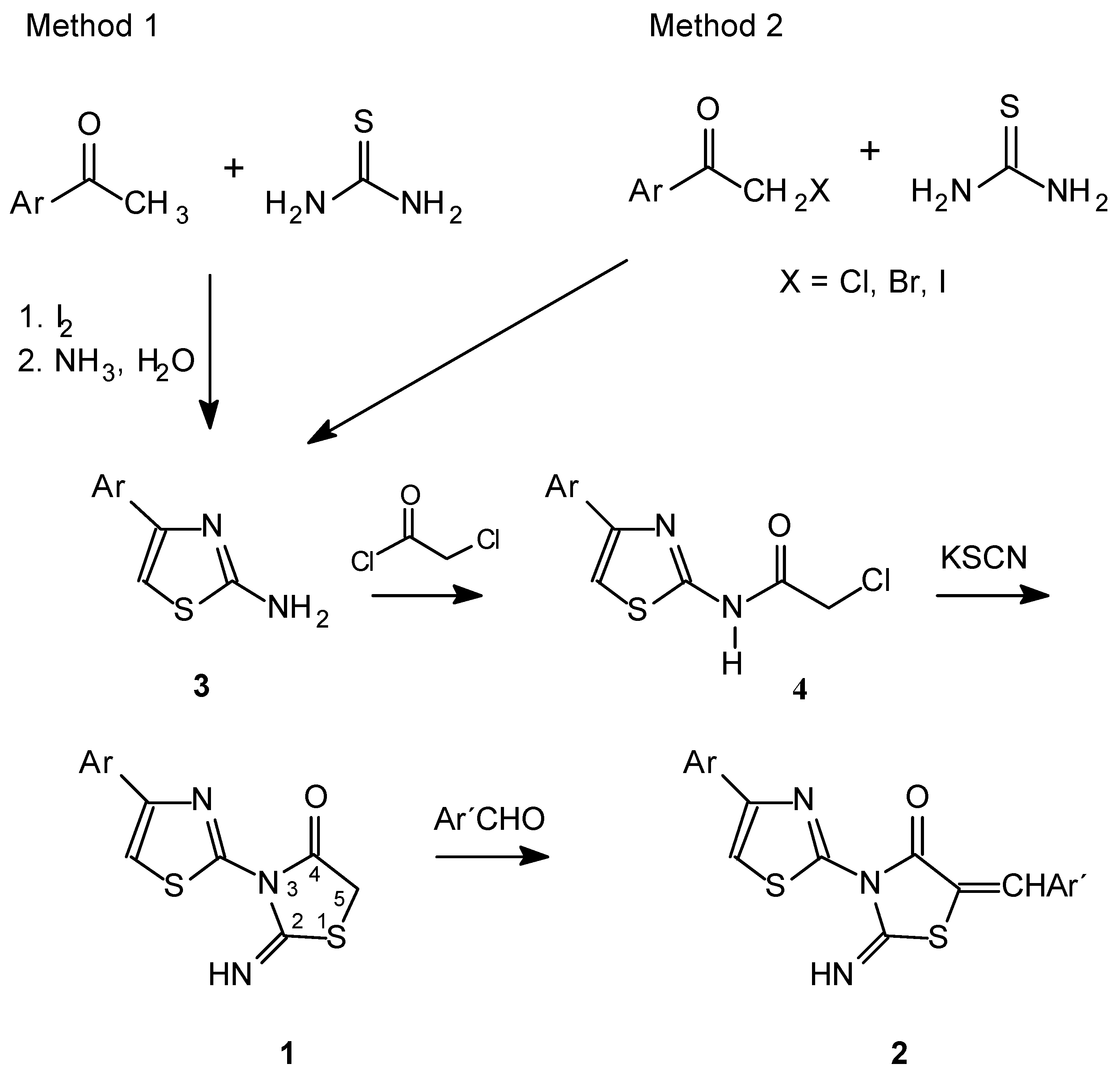
Syntheses
Fungicidal Activity
Experimental
General
Test for Fungicidal Activity
2-Amino-4-phenylthiazole (3a). Method 1
2-Amino-4-(2,4-dichlorophenyl)thiazole (3e). Method 2
2-Chloroacetamido-4-(4-chlorophenyl)thiazole (4b)
2-Imino-3-[4-(2,4-dichloro-5-fluorophenyl)thiazol-2-yl]-thiazolidin-4-one (1d)
5-(3-Nitrobenzylidene)-2-imino-3-(4-phenylthiazol-2-yl)-thiazolidin-4-one (2b)
Acknowledgments
References and Notes
- Doran, W. J.; Shonle, H. A. Dialkyl thiazolidinones. J. Org. Chem. 1938, 3, 193–197. [Google Scholar] [CrossRef]
- Troutman, H. D.; Long, L. M. The synthesis of 2,3-disubstituted-4-thiazolidones. J. Am. Chem. Soc. 1948, 70, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Rout, M. K.; Mahapatra, G. N. 2-β-Naphtyliminino-4-thiazolidone and its derivatives. J. Am. Chem. Soc. 1955, 77, 2427–2428. [Google Scholar] [CrossRef]
- Gaikwad, N. J.; Tirpude, R. N. Substituted 4-thiazolidones as anticonvulsants. Indian Drugs 1994, 31, 593–594. [Google Scholar]
- El-Gendy, Z.; Abdel-Rahman, R. M.; Fawzy, M. M.; Mahmoud, M. B. Biologically active thiazolidinone. Part II. Synthesis and fungitoxicities of isolated and fused thiazolidinones derived from thiosemicarbazones. J. Ind. Chem. Soc. 1990, 67, 927–929. [Google Scholar]
- Diurno, M. V.; Mazzoni, O.; Piscopo, E.; Calignano, A.; Giordano, F.; Bolognese, A. Synthesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 1992, 35, 2910–2912. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Pant, C. K.; Joshi, P. C. Synthesis and antifungal activity of some bis (2-arylimino-3-yl-thiazolidinones) and bis (1-aryl-3-yl-thiohydantoins). Asian J. Chem. 1993, 5, 83–88. [Google Scholar]
- Brown, F. C. thiazolidin-4-ones. Chem. Revs. 1961, 61, 463–521. [Google Scholar] [CrossRef]
- Newcome, G. R.; Nayak, A. thiazolidin-4-ones. Adv. Heterocycl. Chem. 1979, 25, 83–112. [Google Scholar]
- Lakhan, R.; Singh, O. P. Potential fungicides. Part III. Synthesis and evaluation of 5-methyl-thiazolidin-4-ones and 5-methyl-2,thiazolidin-4-ones. J. Ind. Chem. Soc. 1984, 61, 784–787. [Google Scholar]
- Bhargava, P. N.; Prakash, S.; Lakhan, R. Synthesis of 2-(4´,5´-disubstituted thiazol-2´-ylimino)-3-(m-methylphenyl)-5-methyl (or H)-thiazolidin-4-ones and their fungicidal activity. Ind. J. Chem. 1981, 20B, 927–929. [Google Scholar]
- Lakhan, R. Potential fungicides: Studies of 2-arylimino-3-aryl-thiazolidin-4-ones, their 1,1-dioxides and 5-phenylazo derivatives. Agric. Biol. Chem. 1982, 46, 557–560. [Google Scholar]
- Alaimo, R. J. N-(6-Ethyl-4-thiocyanato-2-benzothiazolyl)-5-nitrofuramide. U.S. Patent 4012409, 1977. [Google Scholar]
- Bhagarva, P. N.; Lakhan, R.; Tripathi, R. Local anestics. Part II. Synthesis of 2-(N,N-disubstituted aminoacetamido)-4-p-fluorophenyl and -m-methoxyphenyl thiazoles. J. Ind. Chem. Soc. 1982, 59, 773–775. [Google Scholar]
- Moulard, T.; Lagorce, J. F.; Thomas, J. C.; Raby, C. Biological evaluation of compounds with -NCS-group or derived from thiazole and imidazole-Activity on prostaglandin synthetase complex. J. Pharm. Pharmacol. 1993, 45, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, A.; Kaku, Y. Y.; Kakinuma, H.; Tsukada, I.; Yanagishawa, M.; Naito, T. Synthesis and antifungal activity of novel thiazole-containing triazole antifugals. Chem. Pharm. Bull. 1997, 45, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R. M.; Carroll King, L. The reaction of ketones with halogens and thiourea. J. Am. Chem. Soc. 1945, 67, 2242–2243. [Google Scholar] [CrossRef] [PubMed]
- Carroll King, L.; Hlavek, R. J. The reaction of ketones with iodine and thiourea. J. Am. Chem. Soc. 1950, 72, 3722–3725. [Google Scholar] [CrossRef]
- Naoto, I.; Masumi, N.; Hiroshi, A. Production of 2-amino-4-substituted thiazole. J.P. 60056970A, 1985. [Google Scholar]
- Samples Availability: Not available.
| Substituent Ar | Substituent Ar´ | Mp,oC | Yield,% | |
| 1a | C6H5 | 240-242 | 61 | |
| 1b | p-ClC6H4 | 290-291 | 81 | |
| 1c | p-O2NC6H4 | 187-190 | 75 | |
| 1d | 2,4-(Cl)2-5-FC6H2 | 173-175 | 70 | |
| 1e | 2,4-(Cl)2C6H3 | 232-234 | 60 | |
| 2a | C6H5 | o-O2NC6H4 | 256-260 | 52 |
| 2b | C6H5 | m-O2NC6H4 | 288-290 | 74 |
| 2c | C6H5 | o-ClC 6H4 | 278-280 | 21 |
| 2d | p-ClC6H4 | o-O2NC6H4 | 238-240 | 58 |
| 2e | p-ClC6H4 | m-O2NC6H4 | 286-287 | 75 |
| 2f | p-ClC 6H4 | o-ClC 6H4 | 282-286 | 25 |
| 2g | p-O2NC6H4 | o-O2NC6H4 | >300 | 61 |
| 2h | p-O2NC6H4 | m-O2NC6H4 | >300 | 75 |
| 2i | p-O2NC6H4 | o-ClC 6H4 | >300 | 17 |
| 2j | 2,4-(Cl)2-5-FC6H2 | C6H5 | 258-260 | 27 |
| 2k | 2,4-(Cl)2-5-FC6H2 | o-O2NC6H4 | 289-292 | 53 |
| 2l | 2,4-(Cl)2-5-FC6H2 | m-O2NC6H4 | 270-274 | 70 |
| % Inhibition of 1a-1e and 2a-2l at 50 ppm against F1-F7 | |||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| 1a | 82.6 | 54.3 | 8.8 | 60.0 | 21.1 | 58.8 | 3.5 |
| 1b | 72.4 | 52.3 | 3.8 | 51.3 | 29.7 | 58.8 | 0 |
| 1c | 12.4 | 56.2 | 8.8 | 26.7 | 2.2 | 38.1 | 0 |
| 1d | 95.0 | 58.8 | 12.5 | 91.3 | 38.8 | 76.3 | 38.2 |
| 1e | 97.2 | 62.8 | 0 | 86.7 | 48.6 | 79.4 | 0 |
| 2a | 84.1 | 54.3 | 3.8 | 0 | 61.9 | 20.2 | 64.7 |
| 2b | 5.9 | 49.7 | 25.0 | 0 | 20.6 | 3.2 | 0 |
| 2c | 0 | 23.6 | 17.2 | 0 | 37.4 | 0 | 27.5 |
| 2d | 84.8 | 60.8 | 12.5 | 0 | 58.8 | 14.8 | 66.7 |
| 2e | 84.1 | 58.8 | 16.3 | 0 | 61.9 | 26.5 | 71.3 |
| 2f | 0 | 40.8 | 46.2 | 0 | 31.8 | 0 | 0 |
| 2g | 7.2 | 62.8 | 16.3 | 0 | 24.7 | 3.2 | 33.3 |
| 2h | 29.8 | 52.3 | 0 | 0 | 51.6 | 0 | 4.7 |
| 2i | 0 | 42.7 | 28.0 | 37.1 | 28.0 | 2.1 | 60.8 |
| 2j | 11.5 | 47.7 | 0 | 0 | 38.1 | 4.1 | 28.7 |
| 2k | 15.2 | 49.7 | 0 | 0 | 48.5 | 50.4 | 2.0 |
| 2l | 32.6 | 69.3 | 33.8 | 0 | 45.4 | 11.7 | 24.7 |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Liu, H.-L.; Lieberzeit, Z.; Anthonsen, T. Synthesis and Fungicidal Activity of 2-Imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and Their 5-Arylidene Derivatives. Molecules 2000, 5, 1055-1061. https://doi.org/10.3390/50901055
Liu H-L, Lieberzeit Z, Anthonsen T. Synthesis and Fungicidal Activity of 2-Imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and Their 5-Arylidene Derivatives. Molecules. 2000; 5(9):1055-1061. https://doi.org/10.3390/50901055
Chicago/Turabian StyleLiu, Hui-Ling, Zongcheng Lieberzeit, and Thorleif Anthonsen. 2000. "Synthesis and Fungicidal Activity of 2-Imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and Their 5-Arylidene Derivatives" Molecules 5, no. 9: 1055-1061. https://doi.org/10.3390/50901055




