Spiro Cyclohexadienones from the Reaction of Phenolic Enaminone Derivatives with Hypervalent Iodine Reagents
Abstract
:Introduction
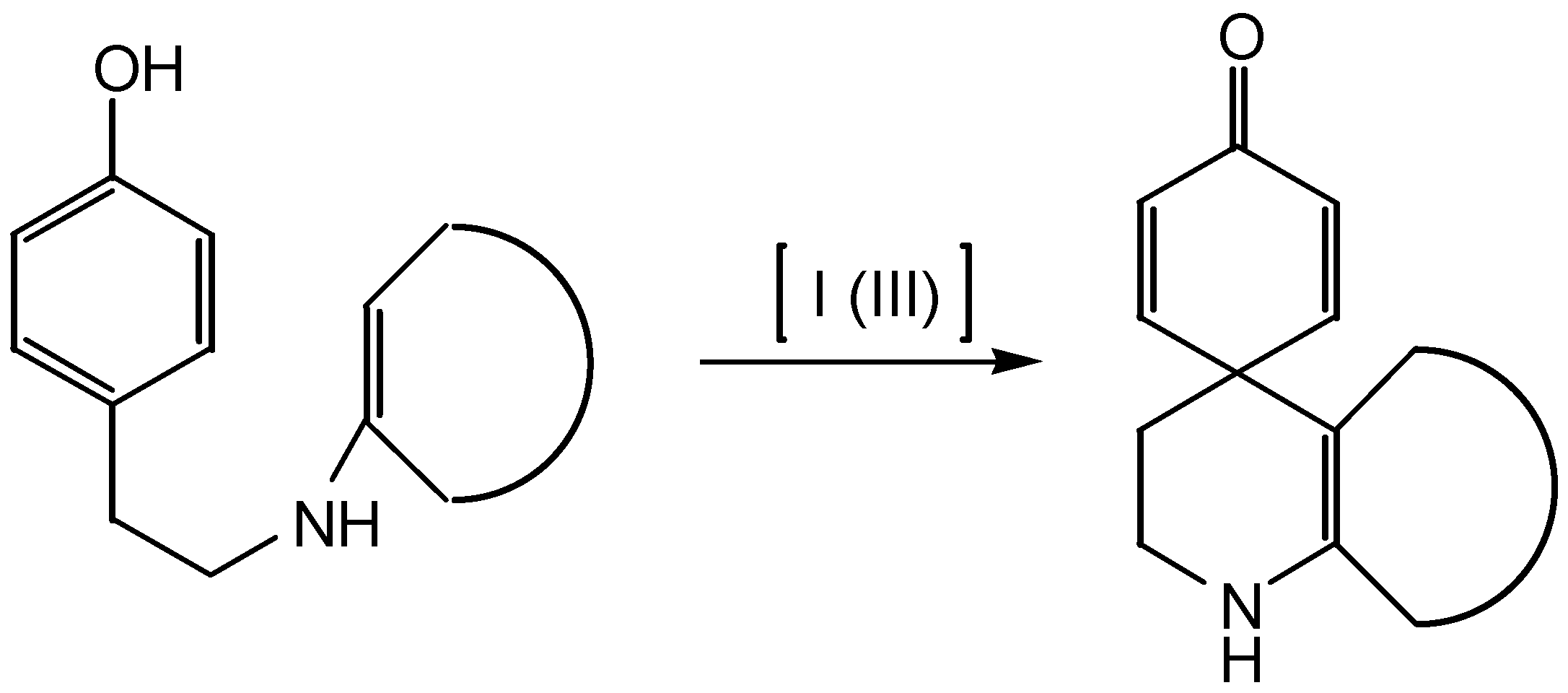
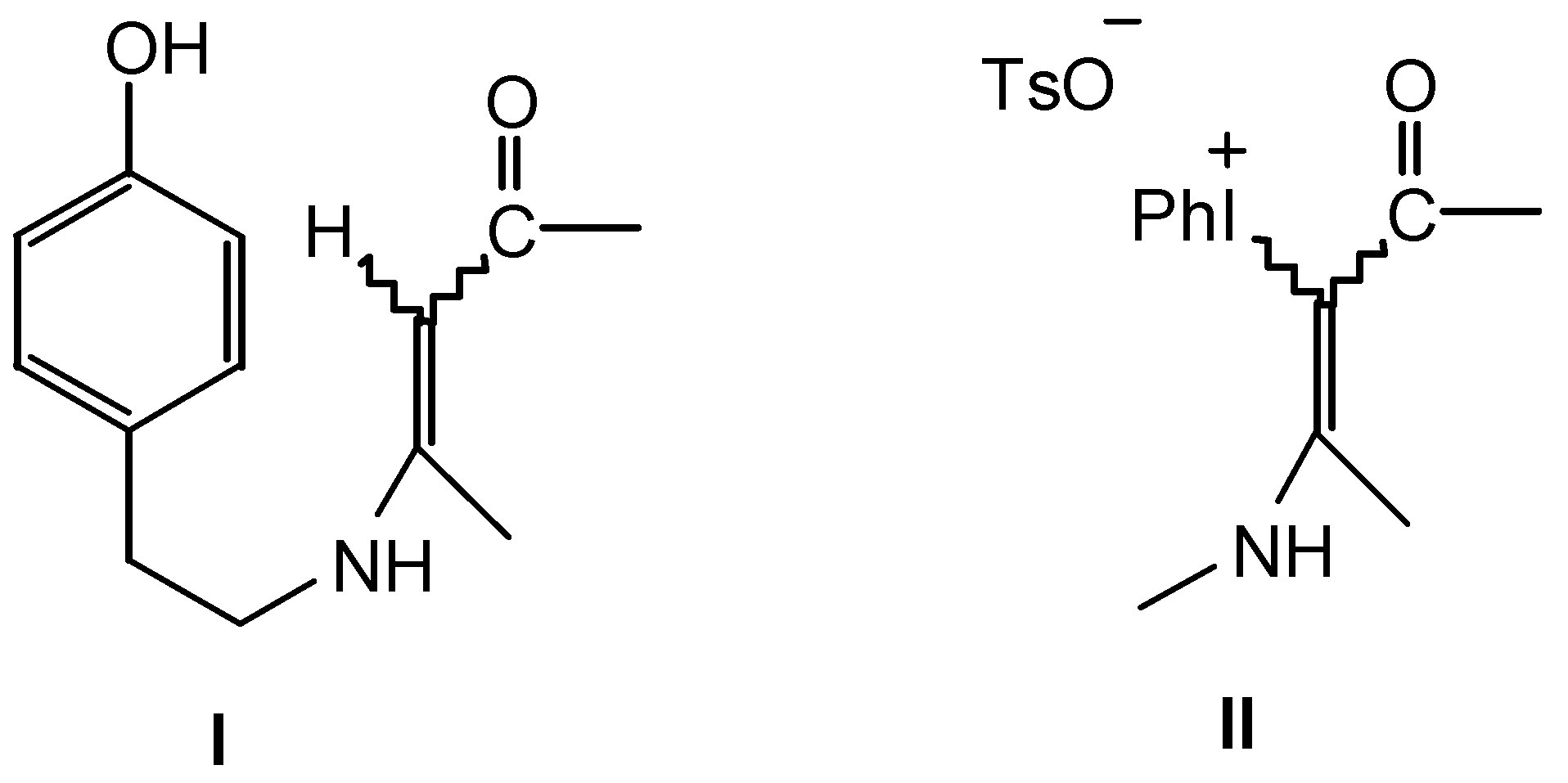
Results and Discussion
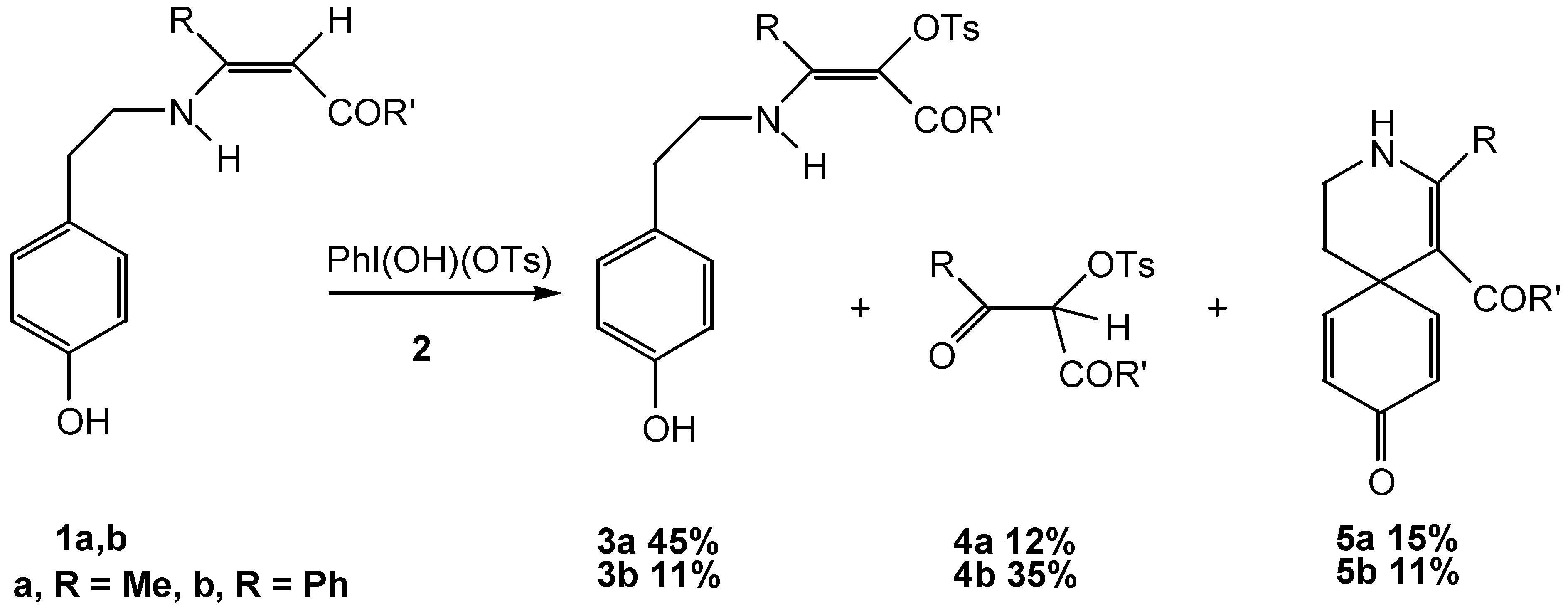
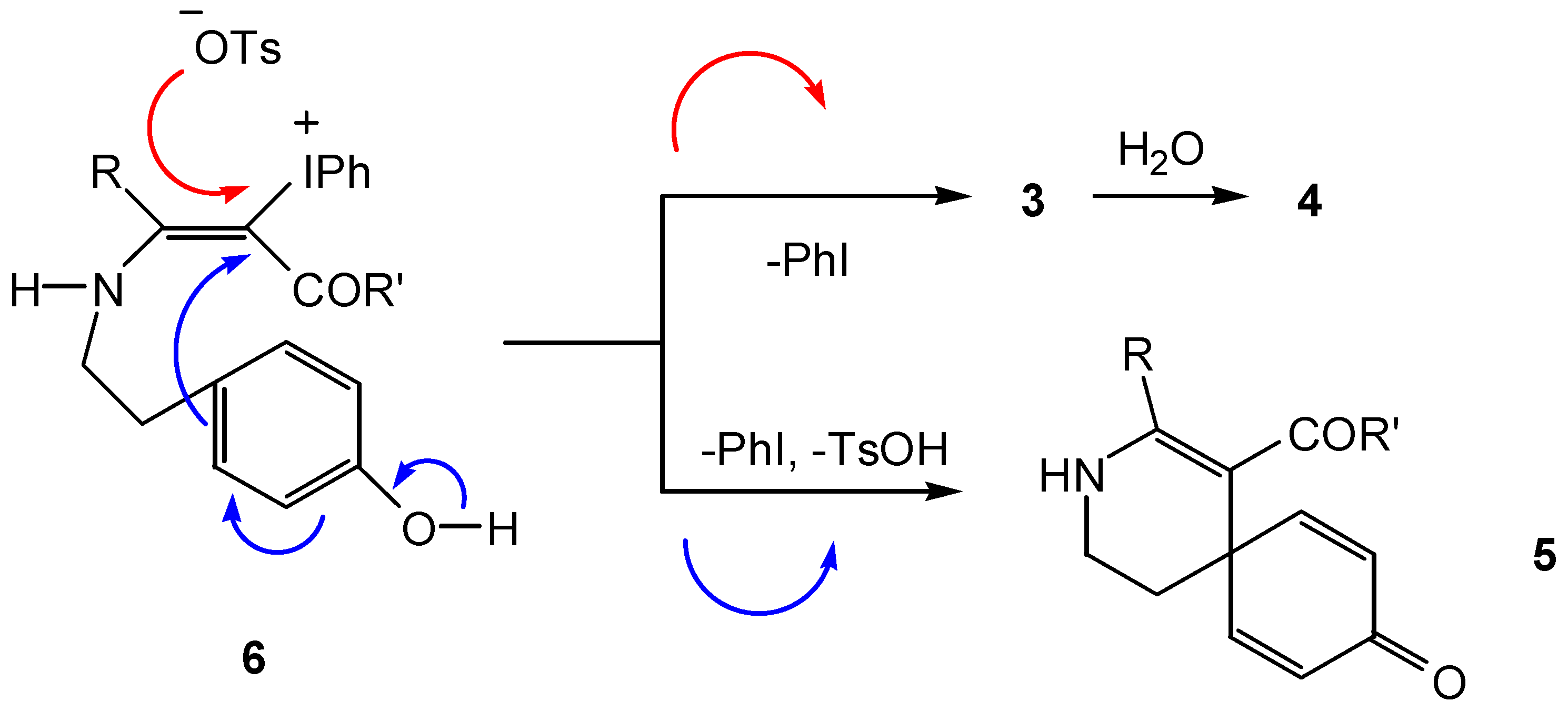
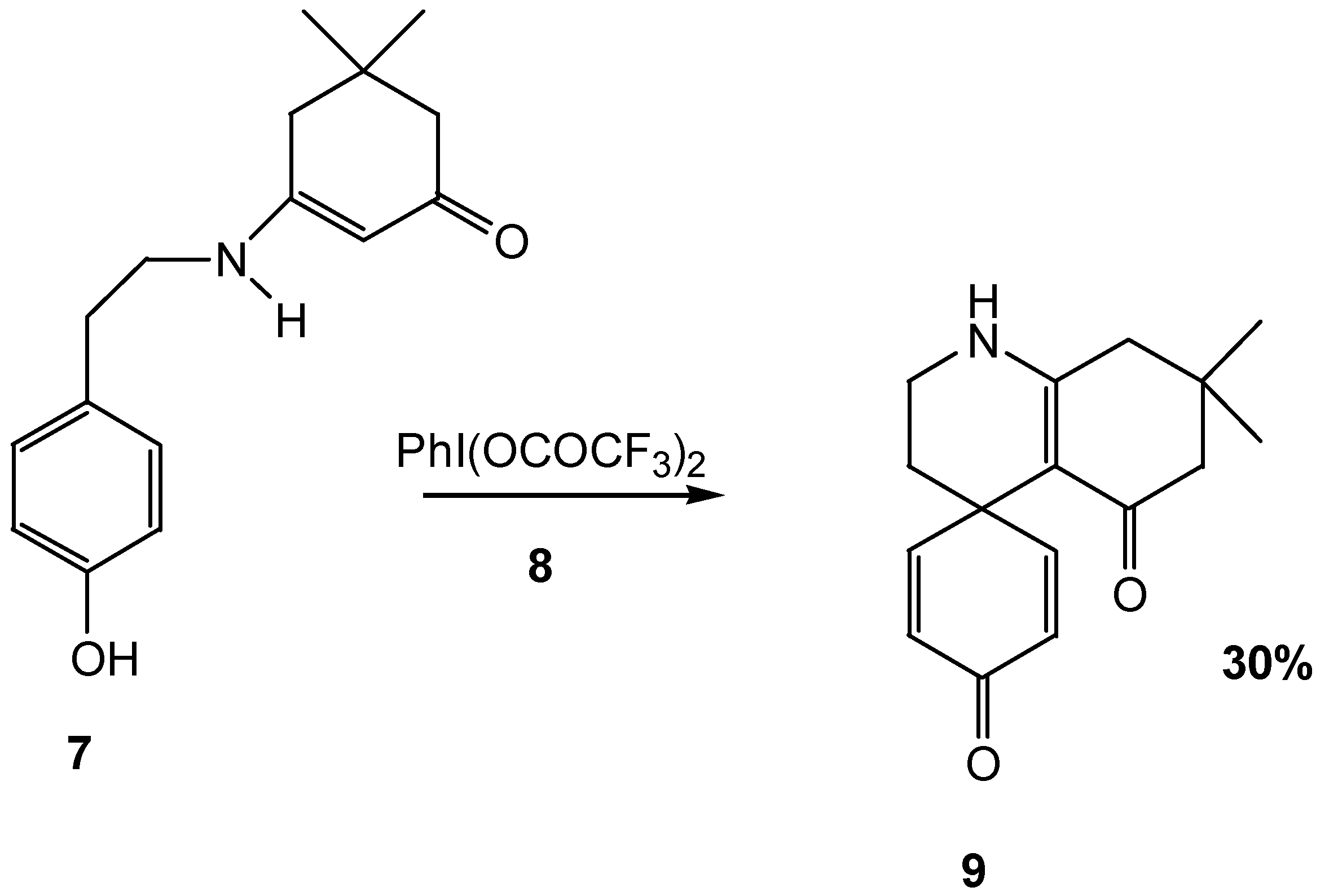
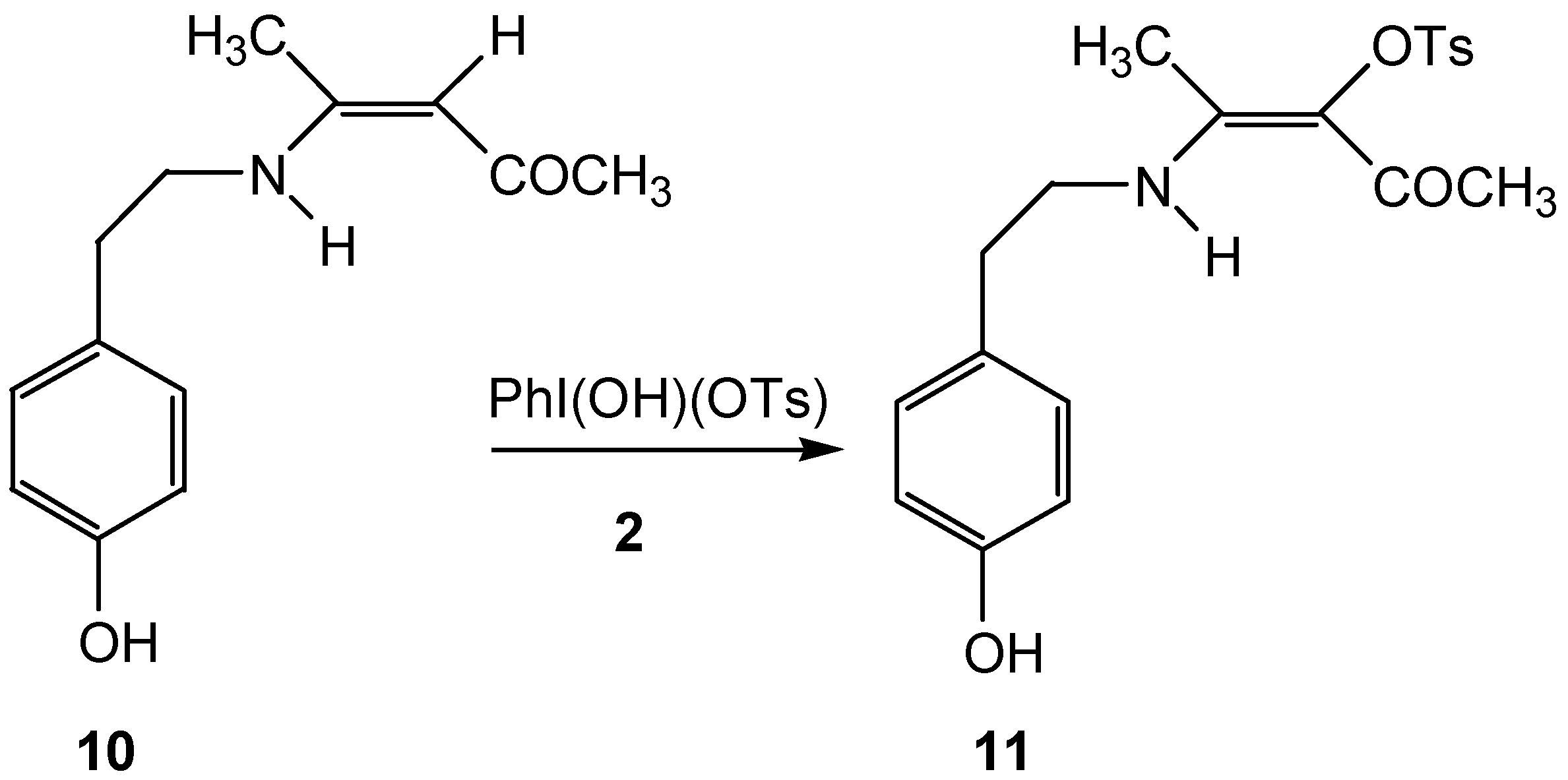
Conclusion
Experimental
Typical procedure for the preparation of 5 and 9
2-Methyl-3-carboxyethyl-1,4,5,6-tetrahydropyridino-4-spiro-4'-cyclohexa-2',5'-dien-1'-one 5a
2-Phenyl-3-carboxyethyl-1,4,5,6-tetrahydropyridino-4-spiro-4'-cyclohexa-2',5'-dien-1'-one 5b
7,7-Dimethyl-1,2,3,4,5,6,7,8-octahydro-quinoline-5-one-4-spiro-4'-cyclohexa-2',5'-dien-1'-one 9
References and Notes
- Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; VCH: New York, 1992; pp. 80–82. [Google Scholar]
- Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: London, 1997; pp. 56–60. [Google Scholar]
- Moriarty, R.M.; Prakash, O. Synthesis of heterocyclic compounds using organohypervalent iodine reagents. Advances in Heterocyclic Chemistry; Academic Press: London, 1998; Volume 69, pp. 1–86. [Google Scholar]
- Kita, Y.; Takada, T.; Gyoten, M.; Tohma, H.; Zenk, M.; Eichhorn, J. An oxidative intra-molecular phenolic coupling reaction for the synthesis of amaryllidaceae alkaloids using a hypervalent iodine(III) reagent. J. Org. Chem. 1996, 61, 5857–5864, and references cited therein. [Google Scholar] [CrossRef]
- Varvoglis, A.; Spyroudis, S. Hypervalent iodine chemistry: 25 years of development at the University of Thessaloniki. SynLett. 1998, 221–232. [Google Scholar] [CrossRef]
- Papoutsis, I.; Spyroudis, S.; Varvoglis, A.; Raptopoulou, C.P. Aryliodonium derivatives of 2-amino-1,4-naphthoquinones: preparation and reactivity. Tetrahedron 1997, 53, 6097–6112. [Google Scholar] [CrossRef]
- Papoutsis, I.; Spyroudis, S.; Varvoglis, A. Reactivity of a new alkenyl phenyliodonium tosylate derived from methyl 3-amino crotonate. Tetrahedron 1998, 54, 1005–1012. [Google Scholar] [CrossRef]
- Papoutsis, I.; Spyroudis, S.; Varvoglis, A.; Callies, J.A.; Zhdankin, V.V. Novel trifluoroethyliodonium salts from cyclic enaminones and their thermal decomposition. Tetrahedron Lett. 1997, 38, 8401–8404. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Papoutsis, I.; Spyroudis, S.; Varvoglis, A. Cyclisation of tryptamine enaminones to functionalised tetrahydro-β-carbolines induced by [bis(trifluoroacetoxy)iodo] benzene. Tetrahedron Lett. 1998, 39, 2865–2866. [Google Scholar] [CrossRef]
- Sukari, M.A.; Vernon, J.M. Oxidation of enamine esters with lead tetraacetate – part 3. Tetrahedron 1983, 39, 793–796. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Asmanidou, A.; Papoutsis, I.; Spyroudis, S.; Varvoglis, A. Spiro Cyclohexadienones from the Reaction of Phenolic Enaminone Derivatives with Hypervalent Iodine Reagents. Molecules 2000, 5, 874-879. https://doi.org/10.3390/50600874
Asmanidou A, Papoutsis I, Spyroudis S, Varvoglis A. Spiro Cyclohexadienones from the Reaction of Phenolic Enaminone Derivatives with Hypervalent Iodine Reagents. Molecules. 2000; 5(6):874-879. https://doi.org/10.3390/50600874
Chicago/Turabian StyleAsmanidou, Anna, Ioannis Papoutsis, Spyros Spyroudis, and Anastasios Varvoglis. 2000. "Spiro Cyclohexadienones from the Reaction of Phenolic Enaminone Derivatives with Hypervalent Iodine Reagents" Molecules 5, no. 6: 874-879. https://doi.org/10.3390/50600874



