Synthesis of a Highly Functionalized Triquinane: Studies Towards a Total Synthesis of Subergorgic Acid and Its Analogues
Abstract
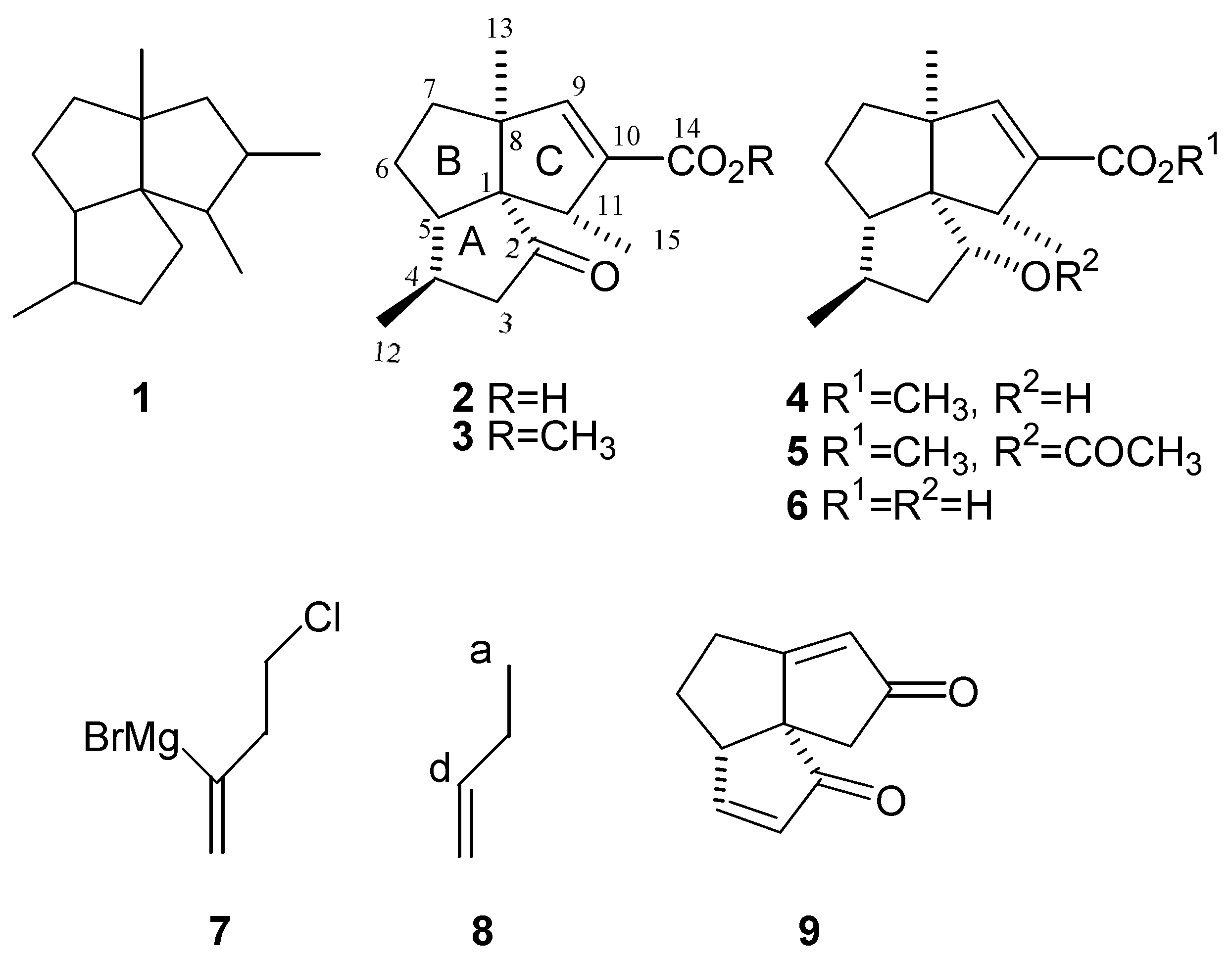
:Introduction
Results and Discussion
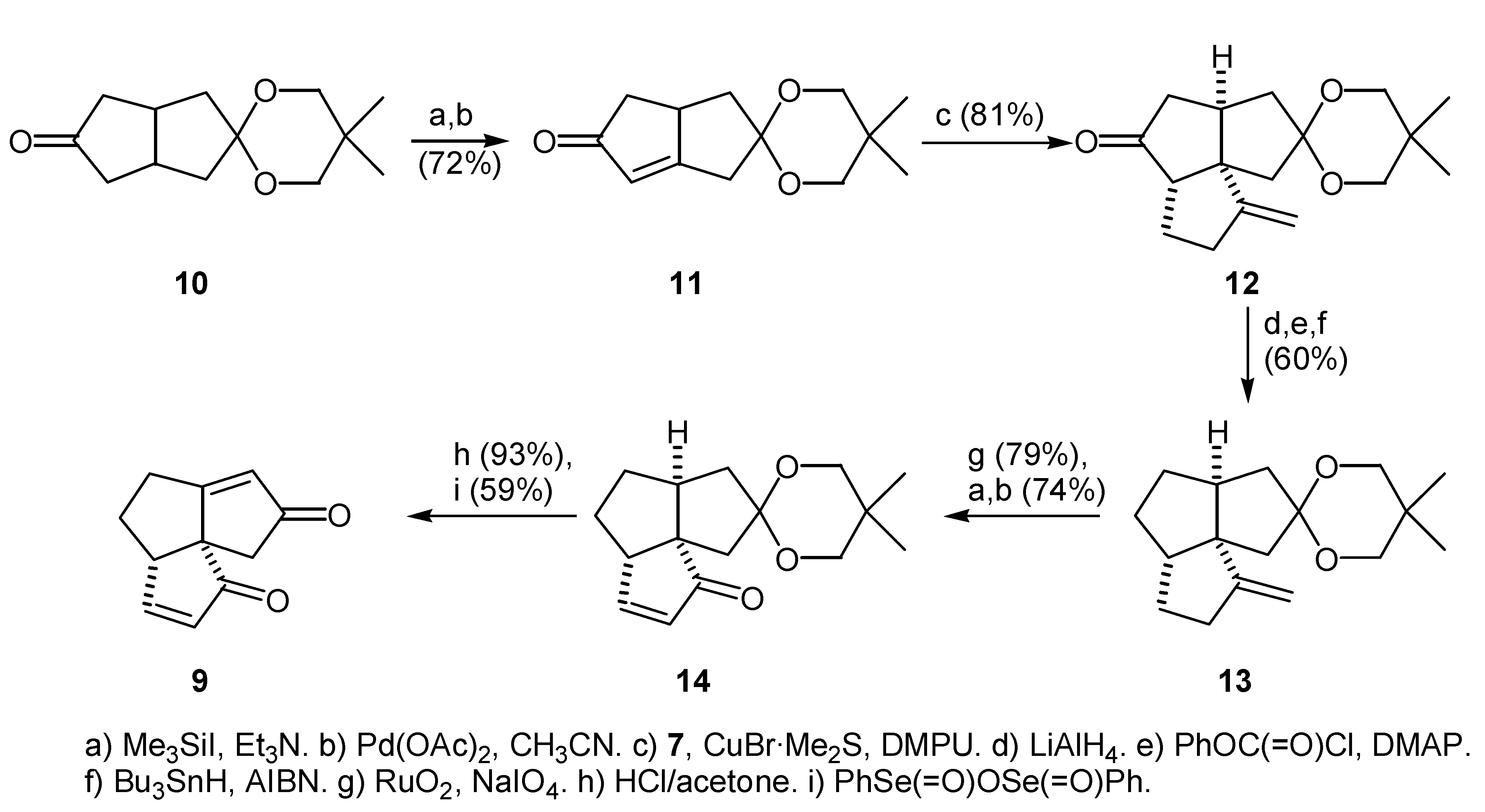
Preparation of the Enone Ketal (11)
Preparation of the Tricyclic Ketone (12)
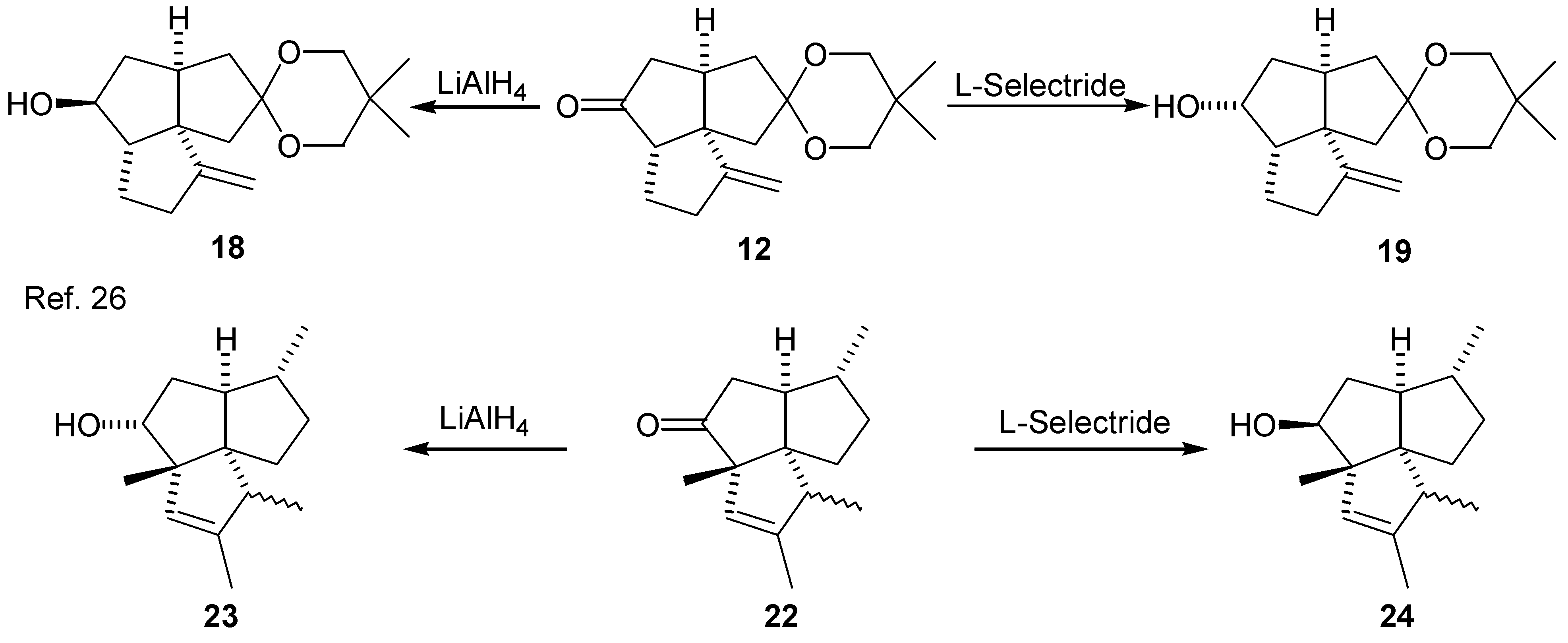
Deoxygenation of the tricyclic ketone (12)
Stereochemistry of the Alcohols (18) and (19), and the Phenyl Thionocarbonates (20) and (21)
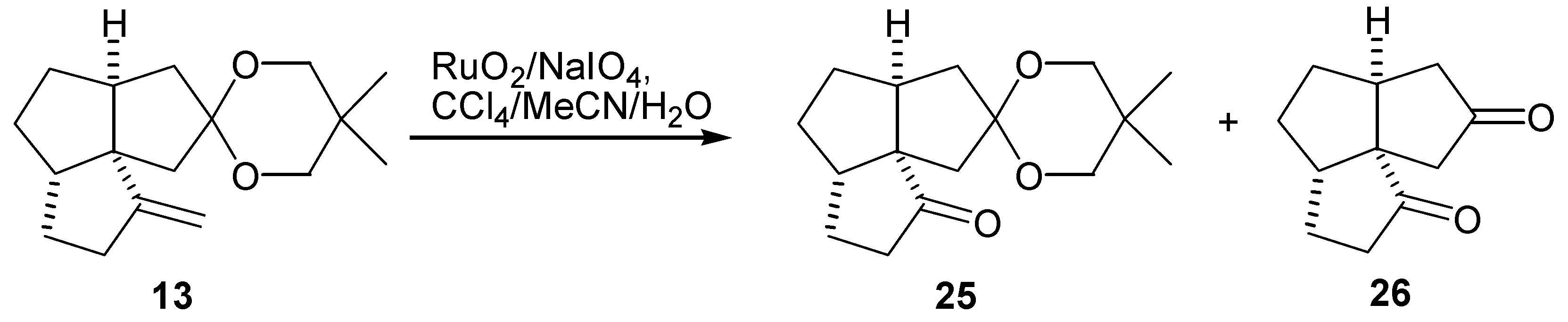
Preparation of the Tricyclic Keto Ketal (25)
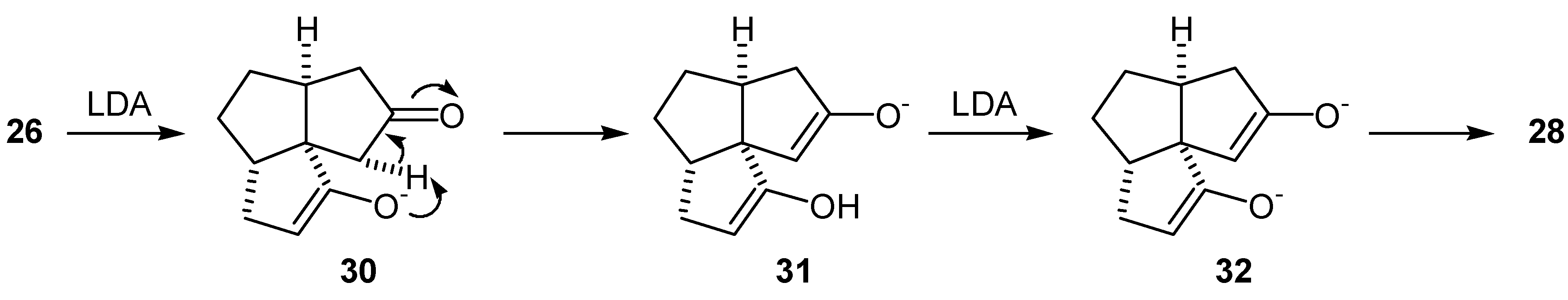
Preparation of the Diketone (26)
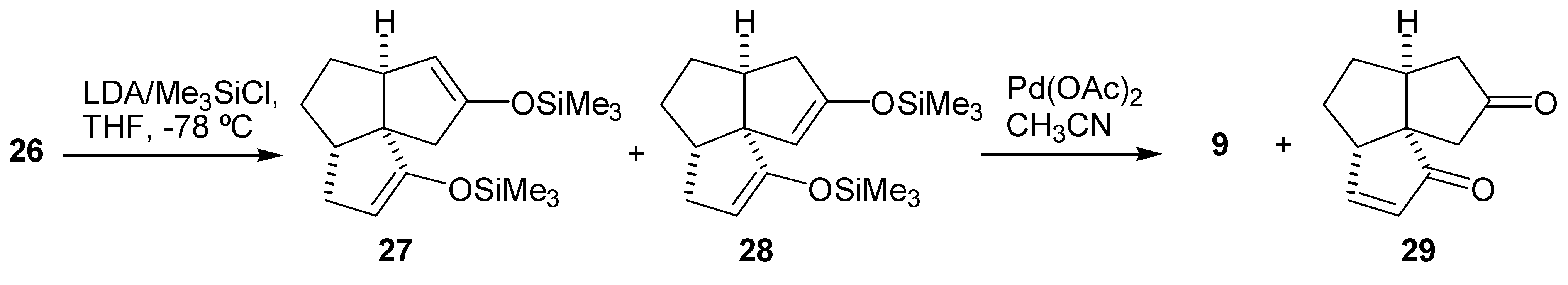
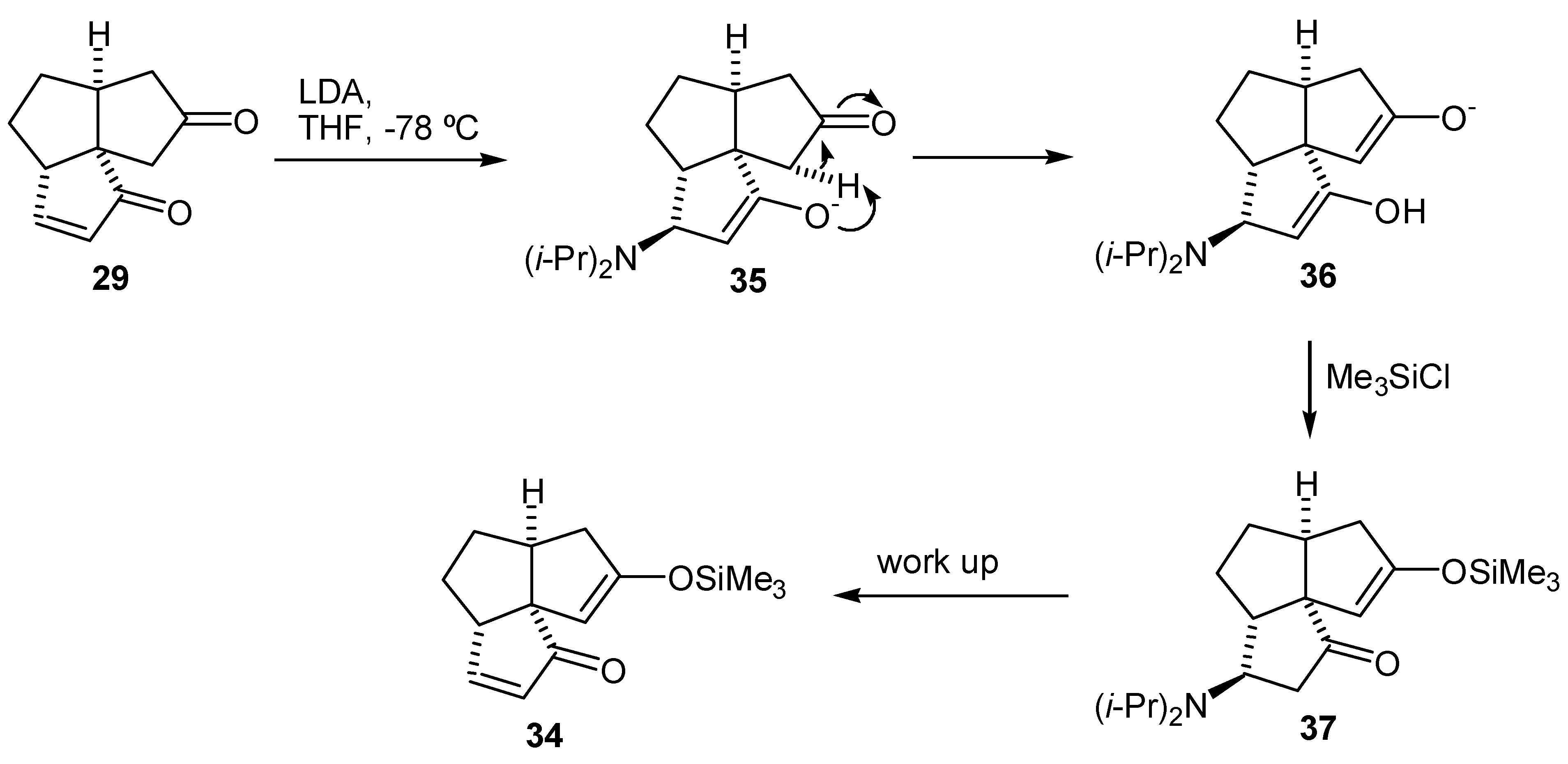
Preparation of the Tricyclic Enedione (29)
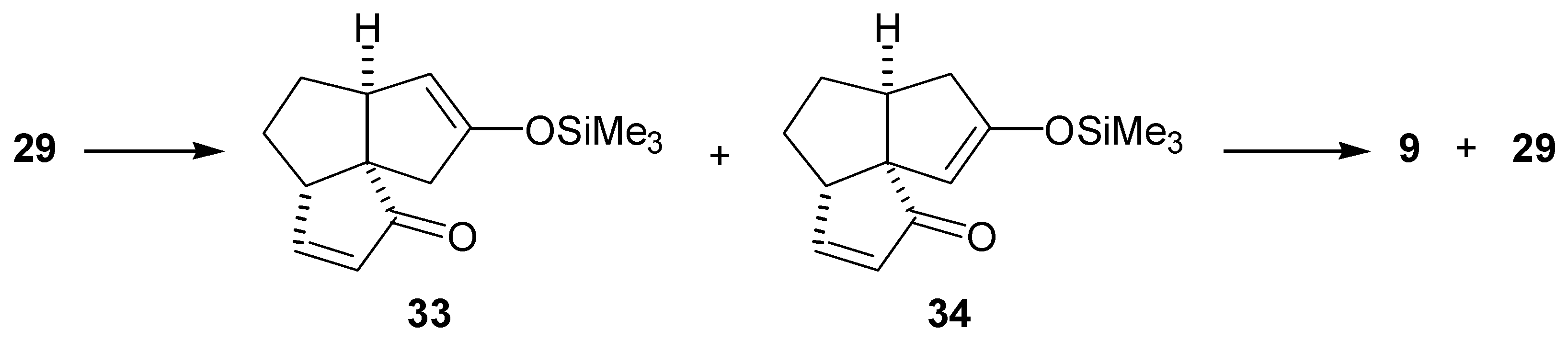
Preparation of the Tricyclic Dienedione (9)
Alkylation of the Dienedione (9)
Conclusion
Experimental
General
Enone Ketal (11)
Tricyclic Ketone (12)
LiAlH4 Reduction of the Ketone (12)
L-Selectride® Reduction of the Ketone (12)
Phenyl Thionocarbonate (21)
Phenyl Thionocarbonate (20)
Alkene (13) from Phenyl Thionocarbonate (20)
Alkene (13) from Phenyl Thionocarbonate (21)
Keto Ketal (25)
Diketone (26)
Enone Ketal (14)
Enedione (29)
Dienedione (9)
11-Methyl Dienedione (39)
Acknowledgement
References and Notes
- Paquette, L. A. The Development of Polyquinane Chemistry. Top. Curr. Chem. 1979, 79, 41–165. [Google Scholar]
- Paquette, L. A. Recent Synthetic Developments in Polyquinane Chemsitry. Top. Curr. Chem. 1984, 119, 1–160. [Google Scholar]
- Paquette, L. A.; Doherty, A. M. Polyquinane Chemistry. Reactivity and Structure Concepts in Or- ganic Chemistry 1987, 26, 1–225. [Google Scholar]
- Mehta, G.; Srikrishna, A. Synthesis of Polyquinane Natural Products: An Update. Chem. Rev. 1997, 97, 671–719. [Google Scholar] [CrossRef] [PubMed]
- Groweiss, A.; Fenical, W.; Cun-heng, H.; Clardy, J.; Zhongde, W.; Zhongnian, Y.; Kanghou, L. Subergorgic Acid, A Novel Tricyclopentanoid Cardiotoxin from the Pacific Gorgonian Coral Subergorgia Suberosa. Tetrahedron Lett. 1985, 26, 2379–2382. [Google Scholar] [CrossRef]
- Parameswaran, P. S.; Naik, C. G.; Kamat, S. Y.; Puar, M. S.; Das, P.; Hegde, V. R. Studies on the Secondary Metabolites from the Indian Gorgonian Subergorgia suberosa: Isolation and Characterization of Four Analogues of the Cardiotoxin Subergorgic Acid. J. Nat. Prod. 1998, 61, 832–834 and 1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Jiao, K.-F.; Ji, Q.-E.; Song, H.-Q. Studies on the Relationship between the MO Indices and Anticholinesterase Activity of Subergorgin. Journal of Molecular Structure (Teochem) 1989, 188, 167–174. [Google Scholar] [CrossRef]
- Tan, X.; Ye, H.; Zeng, L.; Cui, Z.; He, S. An Organophosphorus (Soman) Antidote from Gorgonian Coral Subergorgia Suberosa. Zhongguo Haiyang Yaowu 1990, 9, 11–12, (Chemical Abstracts 1991, 115: 35564g). [Google Scholar]
- Trost, B. M. Some Aspects of Organosulfur-Mediated Synthetic Methods. Acc. Chem. Res. 1978, 453–461. [Google Scholar] [CrossRef]
- Piers, E.; Karunaratne, V. Organitin-Based Bifunctional Reagents: 4-chloro-2-lithio-1-butene and Related Substances. Methylenecyclopentane Annulations. Total Synthesis of (±)-Δ9(12)-Capnellene. Tetrahedron 1989, 45, 1089–1104. [Google Scholar] [CrossRef]
- Piers, E.; Karunaratne, V. Bifunctional Reagents in organic Synthesis. Total Synthesis of the Ses- quiterpenoids (±)-Pentalene and (±)-9-epi-Pentalene. Can. J. Chem. 1989, 67, 160–164. [Google Scholar] [CrossRef]
- Piers, E.; Reanud, J. Annulations via Bifunctional Reagents. Total Synthesis of (±)-Methyl Canta- brenonate and (±)-Methyl Epoxycantabrenonate. Synthesis 1992, 74–82. [Google Scholar] [CrossRef]
- Iwata, C.; Takemoto, Y.; Doi, M.; Imanishi, T. Stereoselective Total Synthesis of (±)-Subergorgic Acid, A New Type of Angular Triquinane Sesquiterpene. J. Org. Chem. 1988, 53, 1623–1628. [Google Scholar] [CrossRef]
- Wender, P.; deLong, M. Synthetic Studies on Arene-Olefin Cycloadditions. XII. Total Synthesis of (±)-Subergorgic Acid. Tetrahedron Lett. 1990, 31, 5429–5432. [Google Scholar] [CrossRef]
- Crimmins, M.; Dudek, C.; Cheung, A. W.-H. A Fragmentation-Rearrangement Sequence of Cyclobutylcarbinyl Radicals. Tetrahedron Lett. 1992, 33, 181–184. [Google Scholar] [CrossRef]
- Paquette, L. A.; Meister, P. G.; Friedrich, D.; Sauer, D. R. Enantioselective Total Synthesis of (-)- Subergorgic Acid. J. Am. Chem. Soc. 1993, 115, 49–56. [Google Scholar] [CrossRef]
- Piers, E.; Moss, N. Thermal Rearrangement of Divinylcyclopropane Systems. A New Formal Total Synthesis of (±)-Quadrone. Tetrahedron Lett. 1985, 26, 2735–2738. [Google Scholar] [CrossRef]
- Miller, R. D.; McKaen, D. R. The Facile Silylation of Aldehydes and Ketones using Trimethylsilyl Iodide: An Exceptionally simple Procedure for the Generation of Thermodinamically Equilibrated Trimethylsilylenol Ethers. Synthesis 1979, 730–732. [Google Scholar] [CrossRef]
- Ito, Y.; Hirao, T.; Saegusa, T. Synthesis of α,β-Unsaturated Carbonyl Compounds by Palla-dium(II)-Catalyzed Dehydrosilylation of Silyl Enol Ethers. J. Org. Chem. 1978, 43, 1011–1013. [Google Scholar] [CrossRef]
- Piers, E.; Chong, J. M. Regioselective Addition of Trimethylstannylcopper-Dimethyl sulphide to 1-Alkynes: Synthesis of ω-Substituted 2-(Trimethylstannyl)-1-Alkenes. J. Chem. Soc. Chem. Commun. 1983, 934–935. [Google Scholar]
- Piers, E.; Karunaratne, V. 4-Chloro-2-lithio-1-butene, a Novel Donor-Acceptor Conjuctive Reagent. J. Org. Chem. 1983, 48, 1774–1776. [Google Scholar] [CrossRef]
- Taylor, R. J. K. Organocopper Conjugate Addition-Enolate Trapping Reactions. Synthesis 1985, 364–392. [Google Scholar] [CrossRef]
- Robins, M. J.; Wilson, J. S. Smooth and Efficient Deoxygentation of Secondary Alcohols. A General Procedure for the Conversion of Ribonucleosides to 2’-Deoxynucleosides. J. Am. Chem. Soc. 1981, 103, 932–933. [Google Scholar] [CrossRef]
- Robins, M. J.; Wilson, J. S.; Hansske, F. Nucleic Acids Related Compounds. 42. A General Procedure for the Efficient Deoxygenation of Secondary Alcohols. Regiospecific and Stereoselective Conversion of Ribonucleosides to 2’-Deoxynucleosides. J. Am. Chem. Soc. 1983, 105, 4059–4065. [Google Scholar]
- Story, B.-A. The Total Synthesis of (±)-β-Panasinsene. Ph. D. Thesis, University of British Columbia, 1991; pp. 87–92. [Google Scholar]
- Brendel, J.; Weyerstahl, P. Total Synthesis of (±)-Silphiperfol-5-en-3-ol. Tetrahedron Lett. 1989, 30, 2371–2374. [Google Scholar]
- Cram, D. J.; Abd Elhafez, F. A. Studies in Stereochemistry. X. The Rule of “Steric Control of Asymmetric Induction” in the Synthesis of Acyclic Systems. J. Am. Chem. Soc. 1952, 74, 5828–5835. [Google Scholar] [CrossRef]
- Chérest, M.; Felkin, H.; Prudent, N. Torsional Strain Involving Partial Bonds. The Stereochemistry of the Lithium Aluminium Hydride Reduction of Some Simple Open-Chain Ketones. Tetrahe- dron Lett. 1968, 2199–2204. [Google Scholar]
- Wipke, W. T.; Gund, P. Simulation and Evaluation of Chemical Synthesis. Congestion: a Conformation-Dependent Function of Steric Environment at a Reaction Center. Application with Torsional Terms to Stereoselectivity of Nucleophilic Additions to Ketones. J. Am. Chem. Soc. 1976, 98, 8107–8118. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Houk, K. N. Electronic and Conformational Effects of π-Facial Stereoselectivity in Nucleophilic Additions to Carbonyl Compounds. J. Am. Chem. Soc. 1987, 109, 908–910. [Google Scholar]
- Wu, Y.-D.; Houk, K. N.; Trost, B. M. Origin of Enhanced Axial Attack by Sterically Undemanding Nucleophiles on Cyclohexanones. J. Am. Chem. Soc. 1987, 109, 5560–5561. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Houk, K. N. Effect of Torsional Strain and Electrostatic Interactions on the Stereochemistry of Nucleophilic Additions to Cyclohexanone and Related Systems. Angew. Chem. Int. Ed. Engl. 1992, 31, 1019–1021. [Google Scholar] [CrossRef]
- Carlsen, P. H. J.; Katsuki, T.; Martin, V. S.; Sharpless, K. B. A Greatly Improved Procedure for Ruthenium Tetraoxide Catalyzed Oxidations of Organic Compounds. J. Org. Chem. 1981, 46, 3936–3938. [Google Scholar] [CrossRef]
- Corey, E. J.; Gross, A. W. Highly Selective, Kinetically Controlled Enolate Formation using Lithium Dialkylamides in the Presence of Trimethylchlorosilane. Tetrahedron Lett. 1984, 25, 495–498. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M. A.; Iqbal, J. An Efficient Regio and Stereoselective Synthesis of Silyl Enol Ethers. Synth. Commun. 1988, 18, 1679–1683. [Google Scholar] [CrossRef]
- Tsuji, J.; Minami, I.; Shimizu, I. A Novel Palladium-Catalyzed Preparative Method of α,β-Unsaturated Ketones and Aldehydes from Saturated Ketones and Aldehydes via Their Silyl Enol Ethers. Tetrahedron Lett. 1983, 24, 5635–5638. [Google Scholar] [CrossRef]
- Minami, I.; Takahashi, K.; Shimizu, I.; Tsuji, J. New Synthetic Methods for α,β-Unsaturated Ketones, Aldehydes, Esters and Lactones by Palladium-Catalyzed reactions of Silyl Enol Acetates with Allyl Carbonates. Tetrahedron 1986, 42, 2971–2977. [Google Scholar] [CrossRef]
- Mincone, E.; Ortaggi, G.; Sirna, A. The Regioselective α,β-Dehydrogenation of Ketosteroids by Palladium(II) Chloride. Synthesis 1977, 773–774. [Google Scholar] [CrossRef]
- Barton, D. H. R.; Lester, D. J.; Ley, S. V. Dehydrogenation of Steriodal Ketones using Benzeneseleninic Anhydride. J. Chem. Soc. Chem. Commun. 1978, 130–131. [Google Scholar] [CrossRef]
- Barton, D. H. R.; Lester, D. J.; Ley, S. V. Dehydrogenation of Steriodal and Triterpenoid Ketones using Benzeneseleninic Anhydride. J. Chem. Soc. Perkin Trans I 1980, 2209–2212. [Google Scholar] [CrossRef]
- Barton, D. H. R.; Morzycki, J. W.; Motherwell, W. B.; Ley, S. V. Oxygen Atom Transfer from Iodylbenzene to Diphenyl Diselenides – A Convenient Method for Dehydrogenation of Steroidal 3-Ketones. J. Chem. Soc. Chem. Commun. 1981, 1044–1045. [Google Scholar] [CrossRef]
- Dragojlovic, V. Preparation of Cyclopentenones by Benzeneseleninic Anhydride Oxidation of Cyclopentanones. J. Chem. Res. (S) 1999, 256–257. [Google Scholar] [CrossRef]
- Lee, R. A.; McAndrews, C.; Patel, K. M.; Reusch, W. Methylation of Kinetically Generated Dienolate Anions Derived from α,β-Unsaturated Ketones. Tetrahedron Lett. 1973, 965–968. [Google Scholar] [CrossRef]
- Olofson, R. A.; Dougherty, C. M. Lithium 2,2,6,6-Tetramethylpiperidide and Related, Strong, Proton-Specific Bases. Evaluation in Synthesis. J. Am. Chem. Soc. 1973, 95, 582–584. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J. Neue Sesquiterpen-Kohlenwasserstoffe mit Anomalen Kohlenstoffgerüst aus Silphium-Arten. Phytochemistry 1980, 19, 259–265. [Google Scholar] (the spectra have been erroneously reversed Wender, P.; Singh, S. K. Synthetic Studies on Arene-Olefin Cycloadditions-VIII. Total Synthesis of (±)-Silphiperfol-6-ene, (±)-7αH-Silphiperfol-5-ene and (±)-7βH-Silphiperfol-5- ene. Tetrahedron Lett. 1985, 26, 5987–5990. [Google Scholar] ).
- Jakupovic, J.; Zdero, C.; Paredes, L.; Bohlmann, F. Sesquiterpene Glycosides and Other Constituents from Osteospermum Species. Phytochemistry 1988, 27, 2881–2886. [Google Scholar] [CrossRef]
- Knudsen, M. J.; Shore, N. E. Synthesis of the Angularly Fused Triquinane Skeleton via Intramolecular Organometallic Cyclization. J. Org. Chem. 1984, 49, 5025–5026. [Google Scholar] [CrossRef]
- Huguet, J.; Karpf, M.; Dreiding, A. S. Synthesis of a Stereoisomer of Ptychanolide. Tetrahedron Lett. 1983, 24, 4177–4180. [Google Scholar] [CrossRef]
- Still, W. C.; Kahn, M.; Mitra, A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Kofron, W. G.; Baclawski, L. M. A Convenient method for Estimation of Alkyllithium Concentrations. J. Org. Chem. 1976, 41, 1879–1880. [Google Scholar] [CrossRef]
- Wuts, P. G. M. An Expedient Procedure for the Purification of the CuBr℘CH SCH Complex. Synth. Commun. 1981, 11, 139–140. [Google Scholar] [CrossRef]
- Sigma Chemical Co., S-6628 Silica-Gel for Thin Layer Chromatography, Type H, Size 10-40 μ, binder: none.
- Samples Availability: Not available.

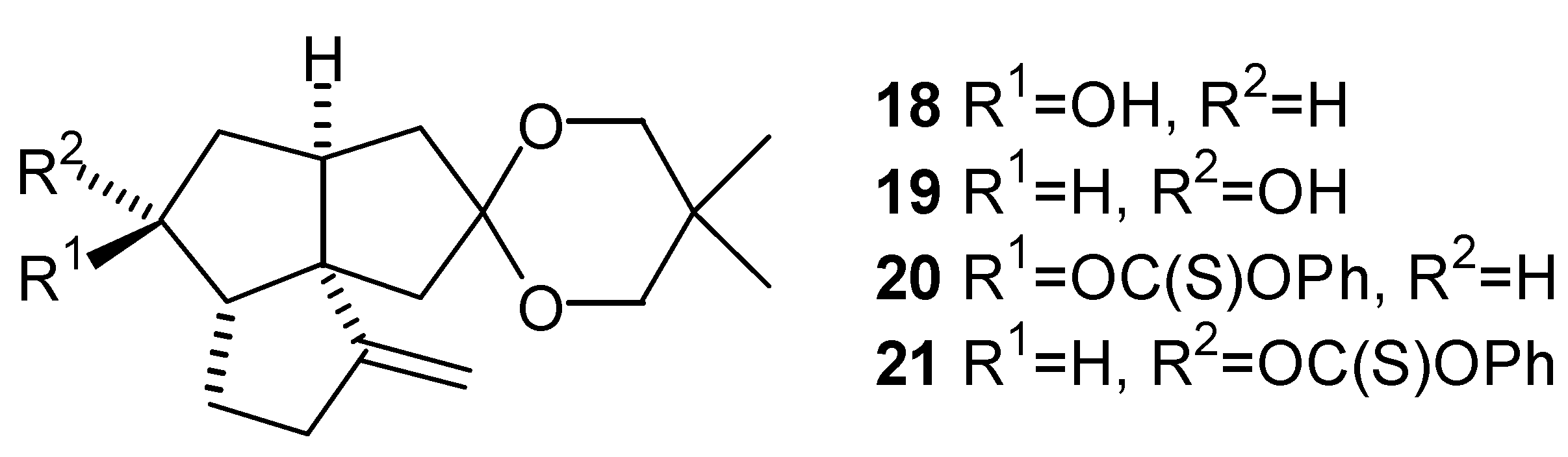

| Entry | Additive | (12)/(17)a | yield of (12)(%) |
| (1) | (none) | 50/50 | 74b |
| (2) | TMEDA (4 eq.) | 90/10 | 75 |
| (3) | HMPA (2 eq.) | 95/5 | 80 |
| (4) | DMPU (2 eq.) | 95/5 | 81 |
| Entry | Reducing Agent | (18)/(19)a | Yield (%) |
| (1) | LiAlH4 | >98/<2 | 97b |
| (2) | NaBH4 | 75/25 | 97c |
| (3) | DIBAL-H | 20/80 | 96c |
| (4) | L-Selectride® | 4/96 | 92b |
| Proton | δ | COSY correlationsb | NOEsb,c |
| phenylthionocarbonate (20) | |||
| H-4 α | 1.60 | H-4 β, H-5 | 1.87 (H-4 β) 5.32 (H-6) |
| H-4 β | 1.87 | H-4 α, H-5 | 1.60 (H-4 α) 2.69 (H-5) |
| H-5 | 2.69 | H-4 α & β, H-6 | 1.87 (H-4 β) 2.20 (H-11 β) 5.32 (H-6) |
| H-6 | 5.32 | H-5, H-7 α and β | 2.32-2.50 (H-3 α & H-7 α) 2.69 (H-5) |
| phenyl thionocarbonate (21)a | |||
| H-4 α | 1.61 | H-5 | |
| H-4 β | 1.71 | H-5 | 1.61 (H-4 α) 2.79 (H-5) |
| H-5 | 2.79 | H-4 α & β, H-6 | 1.71 (H-4 β) 2.19 (H-11 β) 5.81 (H-6) |
| H-6 | 5.81 | H-5, H-7 α & β | 1.90 (H-9 β) 2.07-2.15 (H-7 α & β) 2.79 (H-5) |
| H-9 α | 2.19 | H-9 β | |
| H-9 β | 1.90 | H-9 α | 2.19 (H-9 α) 5.81 (H-6) |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Dragojlovic, V. Synthesis of a Highly Functionalized Triquinane: Studies Towards a Total Synthesis of Subergorgic Acid and Its Analogues. Molecules 2000, 5, 674-698. https://doi.org/10.3390/50400674
Dragojlovic V. Synthesis of a Highly Functionalized Triquinane: Studies Towards a Total Synthesis of Subergorgic Acid and Its Analogues. Molecules. 2000; 5(4):674-698. https://doi.org/10.3390/50400674
Chicago/Turabian StyleDragojlovic, Veljko. 2000. "Synthesis of a Highly Functionalized Triquinane: Studies Towards a Total Synthesis of Subergorgic Acid and Its Analogues" Molecules 5, no. 4: 674-698. https://doi.org/10.3390/50400674




