Synthesis of Asymmetrical Porphyrins Substituted in the meso-Position from Dipyrrolomethanes
Abstract
:Introduction
Experimental
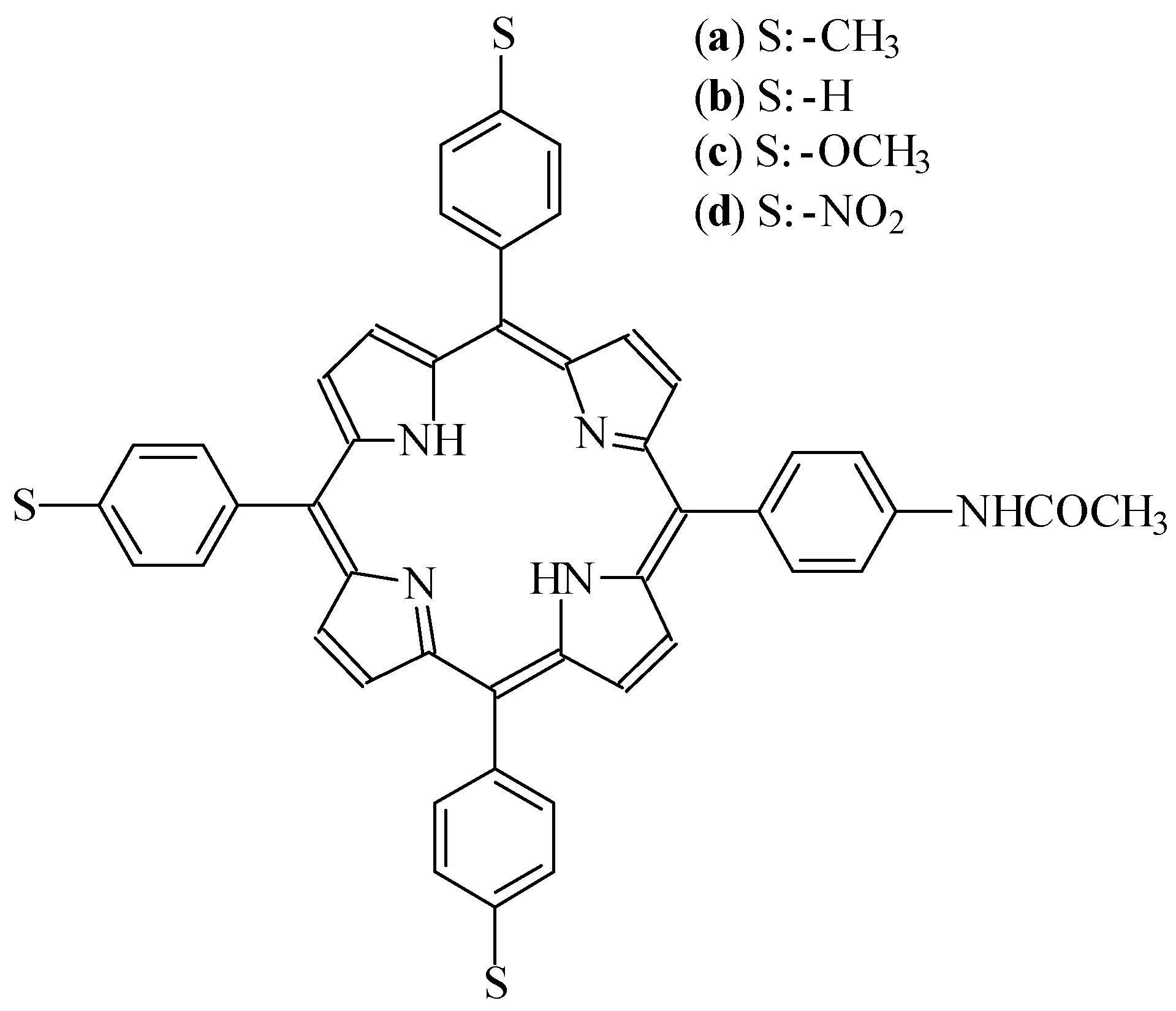
Results and Discussion
Synthesis of dipyrrolomethanes
Synthesis of asymmetric tetraphenylporphyrins
Acknowledgements
References and Notes
- Steinberg-Yfrach, G.; Rigaud, J.-L.; Durantini, E.N.; Moore, A.L.; Gust, D.; Moore, T.A. Nature (London) 1998, 392, 479.
- Han, W.; Durantini, E.N.; Moore, T.A.; Moore, A.L.; Gust, D.; Rez, P.; Leatherman, G.; Seely, G.; Tao, N.; Lindsay, S.M. J. Phys. Chem. B 1997, 101, 10719. [CrossRef]
- Durantini, E.N.; Silber, J.J. Synth. Commun. 1999, 29, 19.
Share and Cite
Milanesio, E.; Chiacchiera, S.M.; Silber, J.J.; Durantini, E.N. Synthesis of Asymmetrical Porphyrins Substituted in the meso-Position from Dipyrrolomethanes. Molecules 2000, 5, 531-532. https://doi.org/10.3390/50300531
Milanesio E, Chiacchiera SM, Silber JJ, Durantini EN. Synthesis of Asymmetrical Porphyrins Substituted in the meso-Position from Dipyrrolomethanes. Molecules. 2000; 5(3):531-532. https://doi.org/10.3390/50300531
Chicago/Turabian StyleMilanesio, E., S. M. Chiacchiera, J. J. Silber, and E. N. Durantini. 2000. "Synthesis of Asymmetrical Porphyrins Substituted in the meso-Position from Dipyrrolomethanes" Molecules 5, no. 3: 531-532. https://doi.org/10.3390/50300531
APA StyleMilanesio, E., Chiacchiera, S. M., Silber, J. J., & Durantini, E. N. (2000). Synthesis of Asymmetrical Porphyrins Substituted in the meso-Position from Dipyrrolomethanes. Molecules, 5(3), 531-532. https://doi.org/10.3390/50300531



