Abstract
A new synthesis of 3,4-diphenyl-4-aryl-1,2,5-thiadiazolines 1,1-dioxide through the addition of aromatic derivatives to 1,2,5-thiadiazole 1,1-dioxide is presented. Anhydrous AlCl3 is used as catalyst.
Introduction
Compounds of the 1,2,5-thiadiazolidines 1,1-dioxide type (3) are interesting owing to a number of therapeutic and synthetic applications [1]. Their are almost exclusively obtained through the condensation reaction of vicinal diamines or amino-alcohols with sulfamide. The availability of the precursors limits the synthetic possibilities.
A recently reported new method [2] for the synthesis of 3 uses substituted thiadiazolines intermediates (2), obtained from thiadiazoles (1) by addition with Grignard reagents.
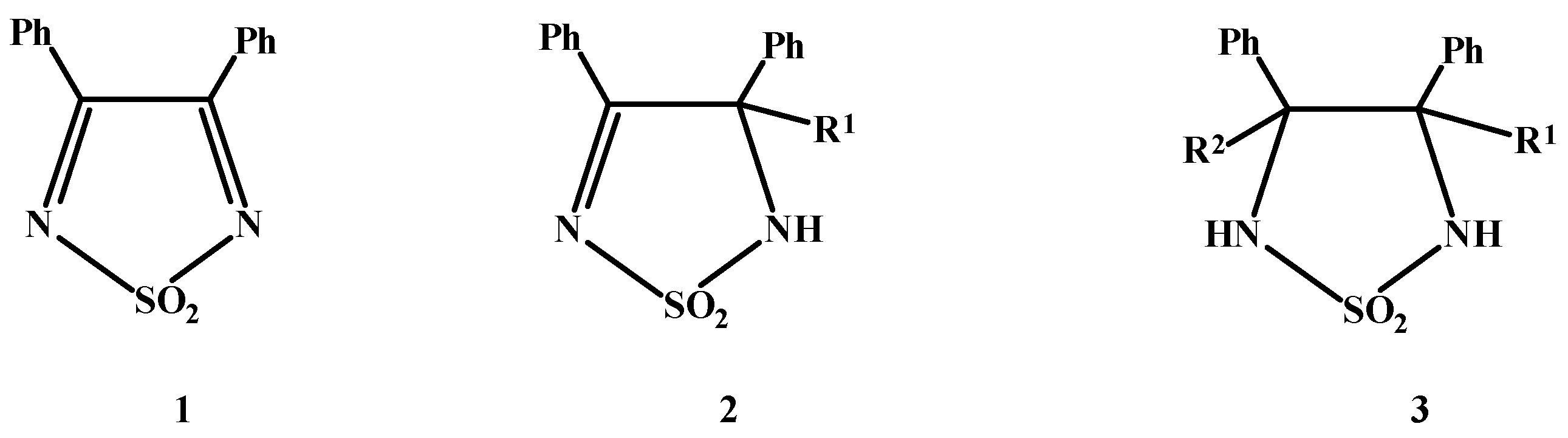

A new method for the addition of activated aryl nucleophiles to the C=N double bond of 1 is presented in this work. The addition is carried out in solution at room temperature and with adequate yields, using ACl3 as a catalyst.
Experimental
The synthesis were carried out in Cl2CH2 solution, except in the cases of toluene and anisole addition, were these reagents were also used as solvents.
Anhydrous AlCl3 was added at room temperature to a magnetically stirred solution of 1, in a molar ratio R = [AlCl3]/[1] ≅ 10. The course of the reaction was followed by TLC.
Results and Discussion
The nucleophiles used were anisole, toluene, phenol, N,N-dimethylaniline, resorcinol and benzene. The products and yields obtained were: 3,4-diphenyl-4-(4-methoxyphenyl)- (η= 64%), 3,4- diphenyl -4-(4-methylphenyl)-(η= 92%), 3,4- diphenyl -4-(4-hidroxyphenyl)- (η= 90%) and 3,4- diphenyl -4-(4- N,N-dimethylaminophenyl)-1,2,5-thiadiazoline 1,1-dioxide (η (unoptimized) = 38%).
The products were purified, their EA was obtained and crystals were grown for X-ray diffraction structure measurements. Spectroscopic (IR, 1H-RMN, 13C-RMN and EM) characterization was also performed.
In the case of benzene, a complex mixture of reaction products, containing mainly polymers derived from benzene, was obtained. Three as yet unidentified reaction products were obtained with resorcinol.
Acknowledgements
This work has been financially supported by the National University of La Plata (Argentine), the National University of São Carlos (Brazil), CNPQ, CONICET and CIC.Pcia of Bs. As. We are grateful with UMyMFOR for the performance of the NMR spectra.
References and Notes
- Castro, Matassa. J. Org. Chem 1994, 59, 2289.
- Pansare. Synlett 1998, ISS6, 623.