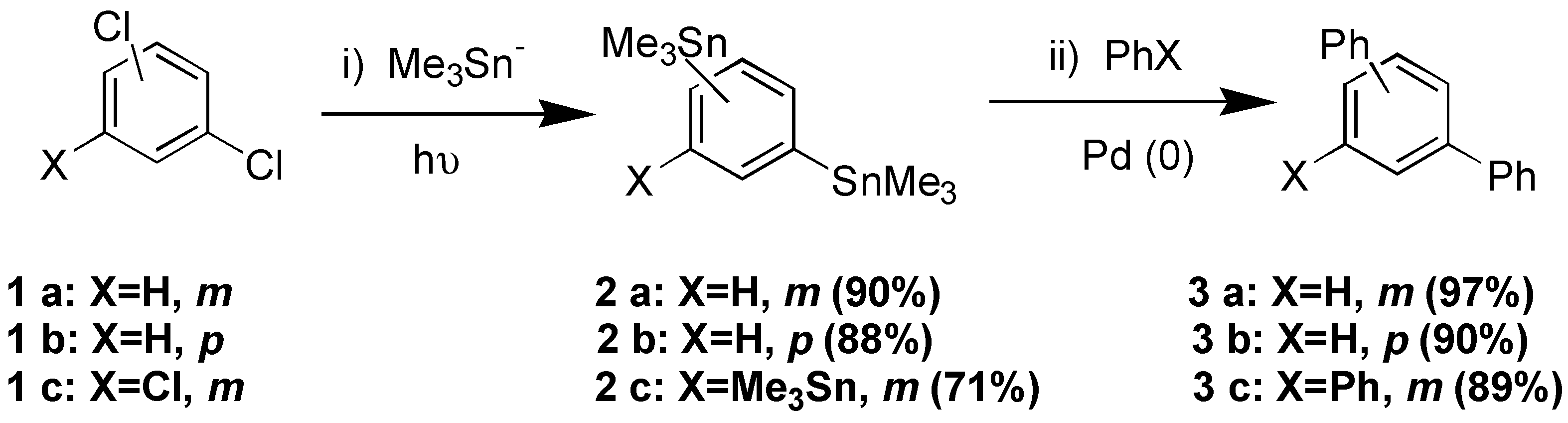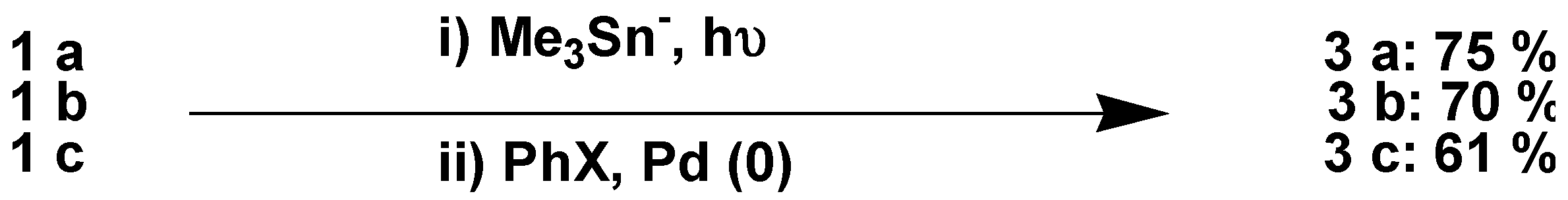Introduction
The radical nucleophilic substitution, or S
RN1 reaction, is a process through which a nucleophilic substitution is obtained [
1]. The scope of the process has considerably increased and nowadays it is an important synthetic possibility to achieve substitution of different substrates. Several nucleophiles can be used, such as carbanions and anions from compounds bearing heteroatoms, which react to form new C-C or C-heteroatom bonds in good yields. We thought that the photostimulated reaction of mono-, di-and trichloro- arenes with Me
3Sn
- ions in liquid ammonia to synthesize the trimethylarylstannanes followed by the Pd(0) cross coupling reaction with haloarenes (Stille Reaction) [
2,
3] would be an im-portant approach for the synthesis of arylated or polyarylated compounds [
4]. Thus, we undertook the study of the palladium catalyzed reaction of trimethylarylstannanes, synthesized by the S
RN1 mecha-nism, with mono-, di- and trichloro- arenes as a model reaction for this methodology. Also we per-formed both reactions in one-pot procedures.
Experimental
Organotin compounds were obtained by photostimulated reactions in liquid ammonia. Irradiation was conducted in a reactor equipped with two 250-W UV lamps emitting maximally at 350 nm. Cross coupling reactions were carried out with Pd(PPh3)2Cl2 as catalist (3-6%) and DMF as solvent.
Results and Discussion
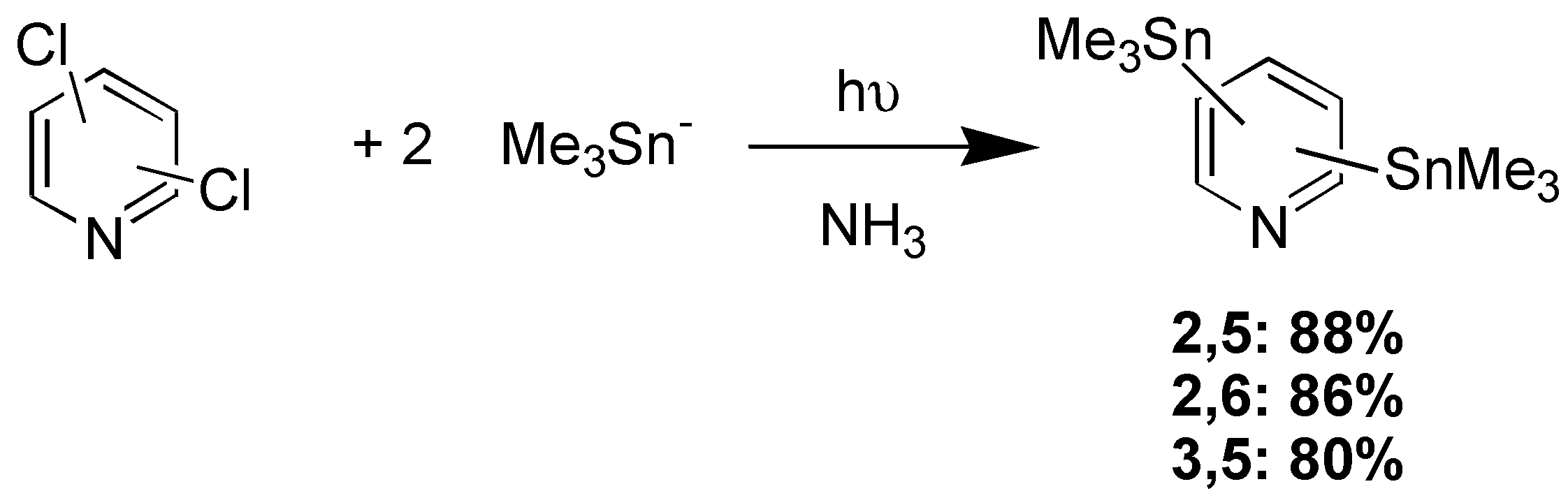
The photostimulated reactions of Me3Sn- with dichloropyridines, di- and trichlorobenzenes afford di and tritin compounds in very goods yields:
The palladium catalized cross coupling reactions of stannanes and aryl halides (PhBr or PhI) gave the respective arylated compounds:
We also studied the possibility of performing the synthesis of the stannane and the Stille reaction in a one-pot procedure:
All these results indicated that the SRN1 mechanism is an excellent method to obtain stannanes by the photostimulated reactions of mono-, di- and trichloro arenes with Me3Sn- in liquid ammonia. The stannanes thus obtained can be arylated by further reaction with bromo or iodoarenes through the pal-ladium catalyzed reactions (or to perform other palladium-catalyzed reactions). Further work is in pro-gress to examine reactions in a stepwise or one-pot conditions.