Unexpected Thorpe Reaction of an α-Alkoxynitrile
Abstract
:Introduction
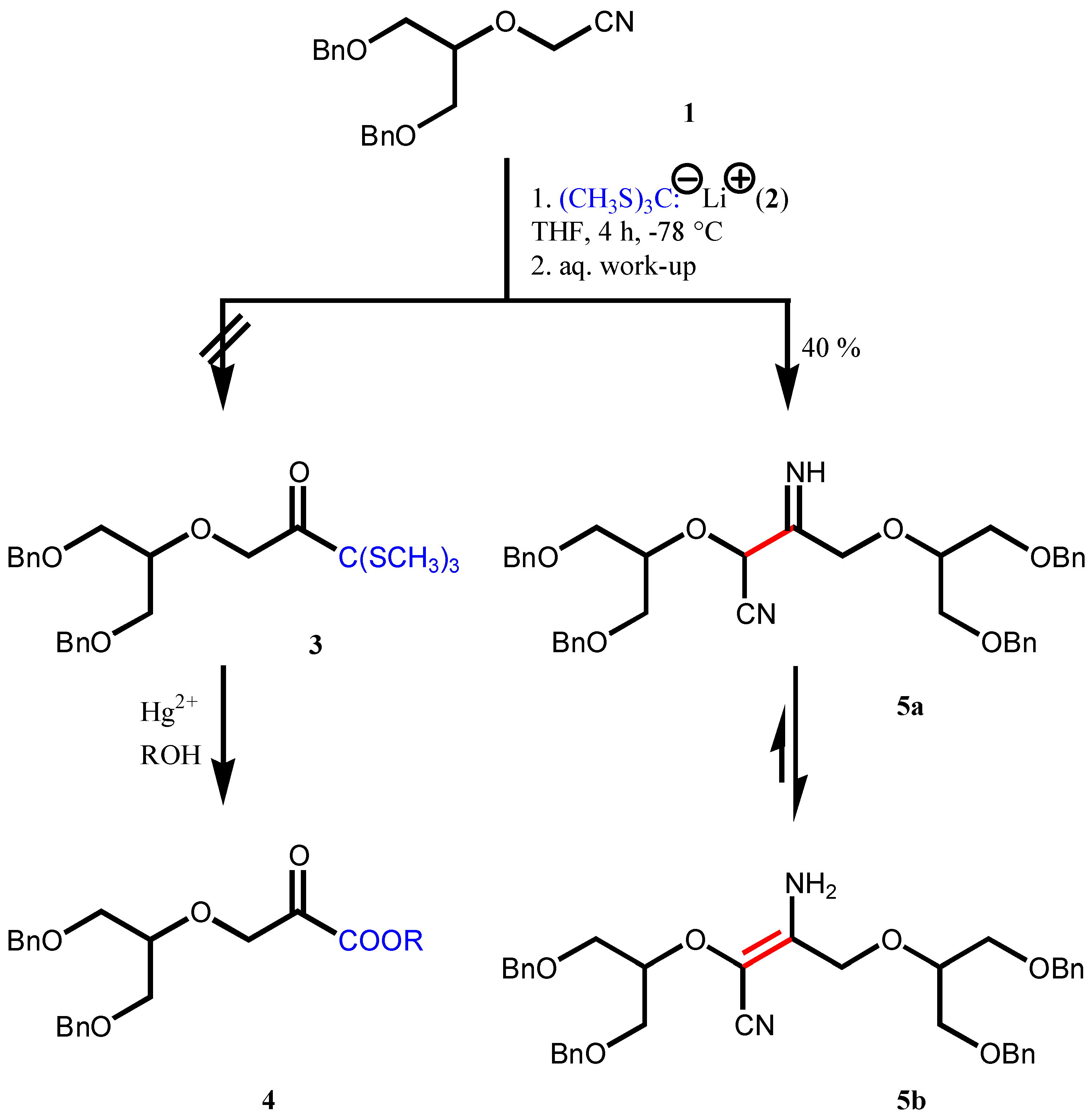
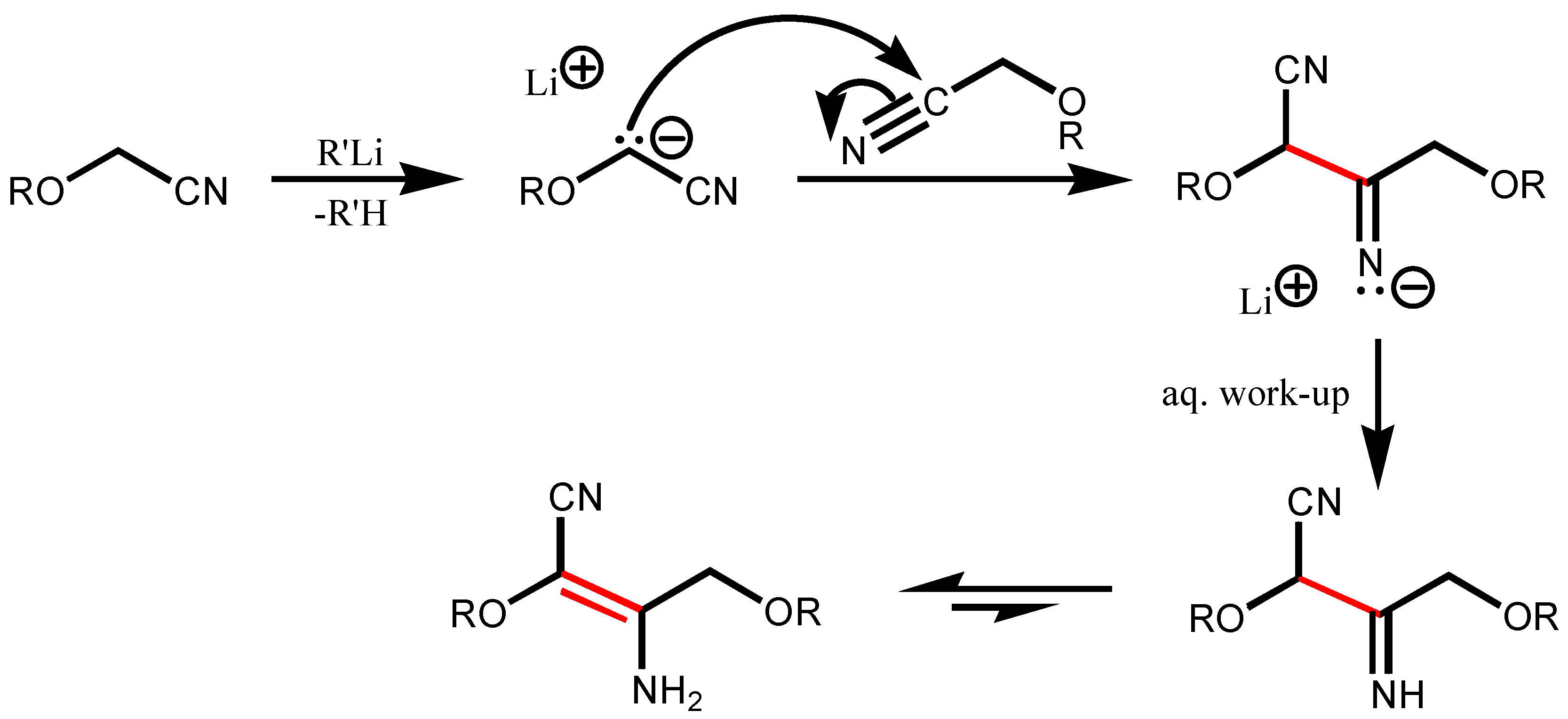
Results and Discussion
Experimental
General
Spectral Data
References and Notes
- Kovács, L.; Herczegh, P.; Batta, G.; Farkas, I. Two acyclic analogues of 2-β-D-ribofuranosyl-thiazole-4-carboxamide (tiazofurin). Heterocycles 1987, 26, 947–960. [Google Scholar]
- March, J. Advanced Organic Chemistry, 4th ed.; John Wiley and Sons: New York, 1992; pp. 935–936. [Google Scholar]
- Gaudemar, M. The Reformatsky reaction in the course of the last thirty years. Organometal. Chem. Rev. A 1972, 8, 183–233. [Google Scholar]
- Holmberg, B. On the esters of orthotrithioformic acid. Ber. Dtsch. Chem. Ges. 1907, 40, 1740–1743. [Google Scholar] [CrossRef]
- Dailey, O. D.; Fuchs, P. L. Synthesis of a model for the BCE ring system of bruceantin. A caveat on the cyclohexene→trans diaxial diol conversion. J. Org. Chem. 1980, 45, 216–236. [Google Scholar] [CrossRef]
- Hengeveld, J. E.; Grief, V.; Tadanier, J.; Lee, C.-M.; Riley, D.; Lartey, P. A. A general synthesis of methyl aldulosonates using tris(methylthio)methyl lithium as the ester anion equivalent. Tetra-hedron Lett. 1984, 25, 4075–4078. [Google Scholar] [CrossRef]
- Damon, R. E.; Schlessinger, R. H. An efficient and stereospecific total synthesis of DL-protoli-chesterinic acid. Tetrahedron Lett. 1976, 1561–1564. [Google Scholar] [CrossRef]
- Antia, M. B.; Kaushal, R. Reformatsky condensation of ketonic esters with halo esters. Agra Univ. J. Res., Sci. 1954, 3, 197–201, (Chem. Abstr. 1955, 49, 6121h). [Google Scholar]
- Clerici, A.; Clerici, L.; Porta, O. Acylation of a transient Ti(IV)-enolate by acyl halides and anhy-drides. Facile synthesis of α-hydroxy-β-ketones. Tetrahedron 1996, 52, 11037–11044. [Google Scholar] [CrossRef]
- Lingo, S. P.; Henze, H. R. Substituted ethers derived from ethylene chlorohydrin. J. Am. Chem. Soc. 1939, 61, 1574–1576. [Google Scholar] [CrossRef]
- Gauthier, R.; Axiotis, G. P.; Chastrette, M. Double addition of organometallic compounds to β-oxygenated nitriles RCN. Preparation of primary amines of RCR1R2NH2 type. J. Organomet. Chem. 1977, 140, 245–255. [Google Scholar] [CrossRef]
- Allen, C. F. H.; van Allan, J. A. Some macrocyclic oxalactones and related substances. J. Org. Chem. 1949, 14, 754–760. [Google Scholar] [CrossRef]
- Guibert, S.; Cariou, M.; Simonet, J. Research on novel organic solvents with potential use in lith-ium batteries 2. The properties of certain aliphatic nitriles substituted by donor groups. Bull. Soc. Chim. Fr. 1988, 924–929. [Google Scholar]
- March, J. Advanced Organic Chemistry, 4th ed.; John Wiley and Sons: New York, 1992; pp. 963–964. [Google Scholar]
- Jones, R. L.; Pearson, D. E.; Gordon, M. Studies on the reaction of phenylmagnesium bromides with acetonitrile. J. Org. Chem. 1972, 37, 3369–3370. [Google Scholar] [CrossRef]
- Samples Availability: not available.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kovács, L. Unexpected Thorpe Reaction of an α-Alkoxynitrile. Molecules 2000, 5, 127-131. https://doi.org/10.3390/50200127
Kovács L. Unexpected Thorpe Reaction of an α-Alkoxynitrile. Molecules. 2000; 5(2):127-131. https://doi.org/10.3390/50200127
Chicago/Turabian StyleKovács, Lajos. 2000. "Unexpected Thorpe Reaction of an α-Alkoxynitrile" Molecules 5, no. 2: 127-131. https://doi.org/10.3390/50200127



