The Reaction of Trimethylsilylethynyl(phenyl)iodonium Triflate with Some Phenolates: Formation of Substitution and sp2 C-H Insertion Products
Abstract
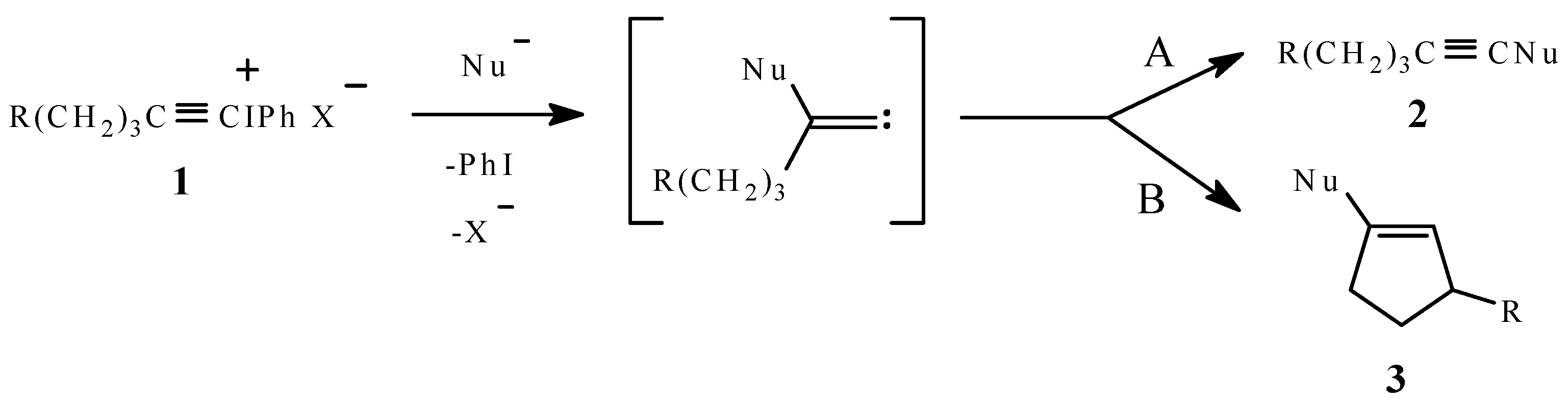
:Introduction
Results and Discussion
Experimental
General
Typical Procedure for the preparation of 6, 7 and 9b
References
- Zhdankin, V. V.; Stang, P. J. Alkynyliodonium salts in organic synthesis. Tetrahedron 1998, 54, 10927. [Google Scholar] [CrossRef]
- Tykwinski, R. R.; Whiteford, J. A.; Stang, P. J. Alkylidenecarbene insertions into aromatic C-H bond in solution. J. Chem. SoC., Chem. Commun. 1993, 1800. [Google Scholar] [CrossRef]
- Kitamura, T.; Geeing, L.; Taniguchi, H.; Sakurai, M.; Tanaka, R. Novel cyclisation of benzofurans in the reaction of alkynyl(p-phenylene)bis iodonium ditriflates with phenoxide anion. Tetrahedron. Lett. 1993, 34, 4055. [Google Scholar] [CrossRef]
- Kitamura, T.; Stud, K.; Fujiwara, Y. Novel heteroaromatic C-H insertion of alkylidenecarbenes. A new entry to furopyridine synthesis. Tetrahedron. Lett. 1998, 39, 5375. [Google Scholar] [CrossRef]
- Shu, T.; Chen, D.W.; Ochiai, M. Direct synthesis of 2-substituted furotropones from tropones utilizing alkynyl(phenyl)iodonium salts. Tetrahedron. Lett. 1996, 37, 5539. [Google Scholar] [CrossRef]
- Filippova, A. K.; Kashik, T.V.; Lyashenko, G. S.; Ponomareva, S. M.; Kalikhman, I. D.; Vyazankin, N.S. Quaternary ammonium bases and salts from aromatic acetylene ethers. Zh. Obshch. Khim. 1983, 53, 1141, CA, 99, 104884. [Google Scholar]
- Margida, A; Koser, F.G. Exchange of carbon ligands at iodine in iodonium salts. A direct synthesis of aryl(2-furyl)iodonium tosylates from aryl(tert-butylethynyl)iodonium tosylates. J. Org. Chem. 1984, 49, 4703. [Google Scholar]
- Mooradian, A.; Dupont, P.E. The preparation of O-aryl oximes and their conversion to benzo-furans. J. Heterocyclic Chem. 1967, 4, 441. [Google Scholar] [CrossRef]
- Spyroudis, S. P. Photochemical reactions of 2,4-dinitro-6-phenyliodonium phenolate with alkenes, al- kynes and aromatic compounds. J. Org. Chem. 1986, 51, 3453. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Horaguchi, T. The reaction of 2,3-dichloro-5,6-dicyano-p-benzoquinone with benzofurans and indoles. Bull. Chem. SoC. Jpn. 1993, 66, 1235. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
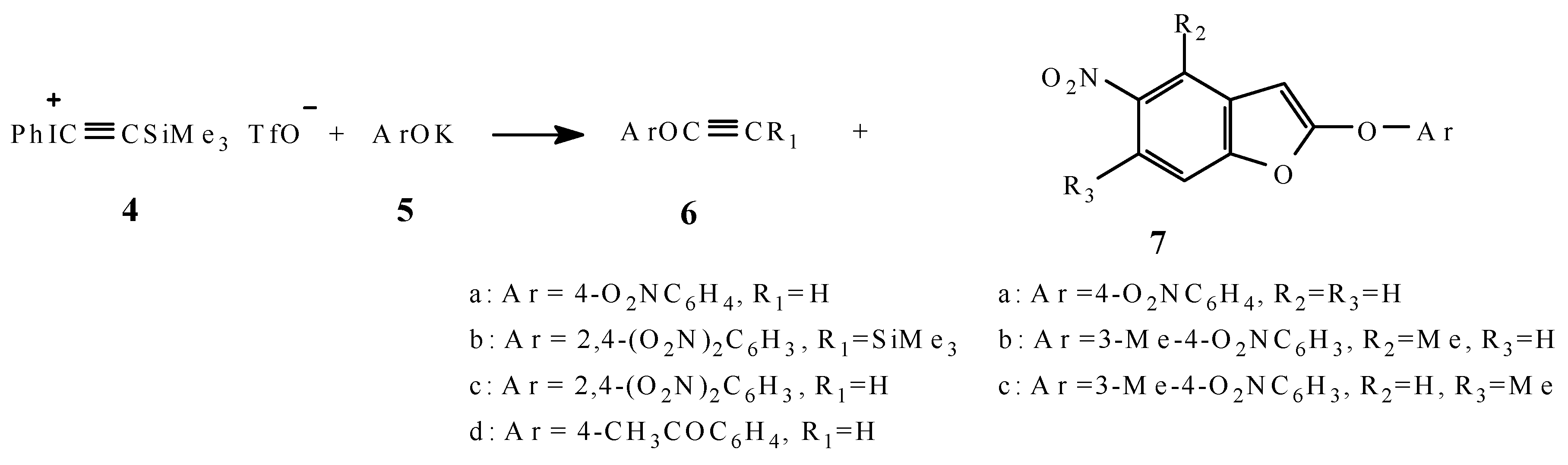

© MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Nikas, S.; Rodios, N.; Varvoglis, A. The Reaction of Trimethylsilylethynyl(phenyl)iodonium Triflate with Some Phenolates: Formation of Substitution and sp2 C-H Insertion Products. Molecules 2000, 5, 1182-1186. https://doi.org/10.3390/51101182
Nikas S, Rodios N, Varvoglis A. The Reaction of Trimethylsilylethynyl(phenyl)iodonium Triflate with Some Phenolates: Formation of Substitution and sp2 C-H Insertion Products. Molecules. 2000; 5(11):1182-1186. https://doi.org/10.3390/51101182
Chicago/Turabian StyleNikas, Spyros, Nestor Rodios, and Anastasios Varvoglis. 2000. "The Reaction of Trimethylsilylethynyl(phenyl)iodonium Triflate with Some Phenolates: Formation of Substitution and sp2 C-H Insertion Products" Molecules 5, no. 11: 1182-1186. https://doi.org/10.3390/51101182



