Synthesis and Reactions of Substituted 3-amino-2-furyl(aryl)-thieno[2,3-b]pyridines
Abstract
:Introduction
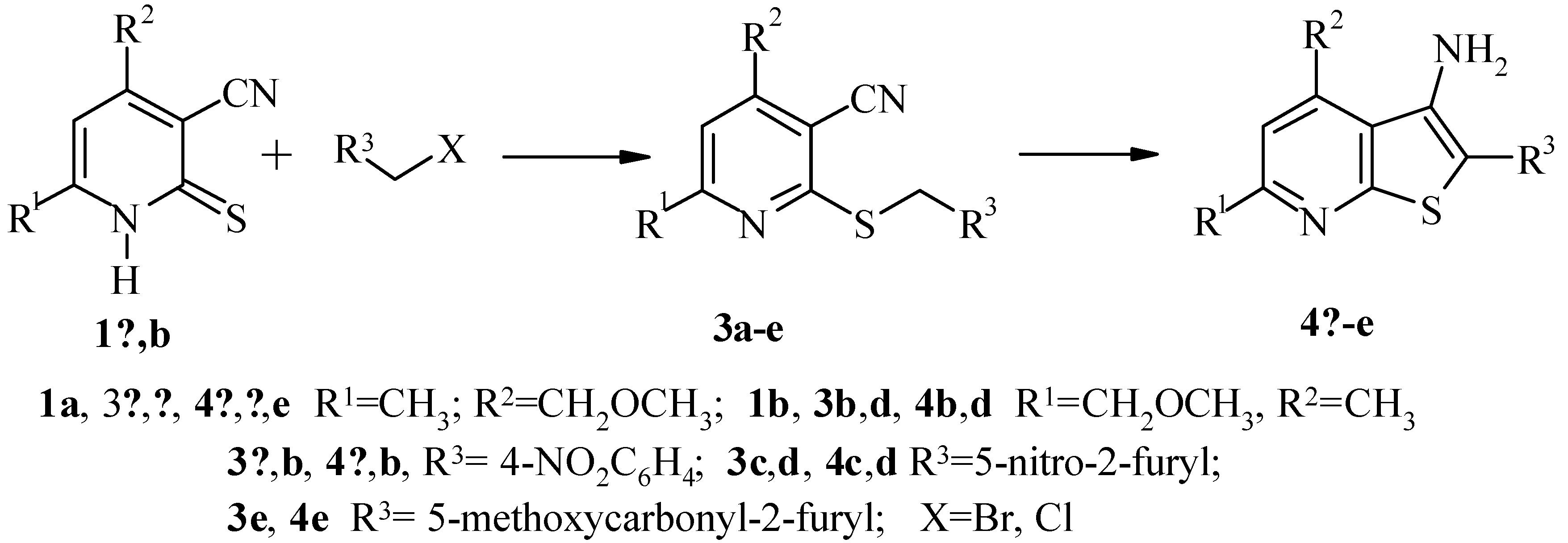
Results and Discussion
Experimental
General
6-Methyl-4-methoxymethyl-3-cyano-2-(4-nitrobenzyl)thiopyridine (3a).
3-Amino-2-(4-nitrophenyl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4a).
3-Amino-2-(4-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4c).
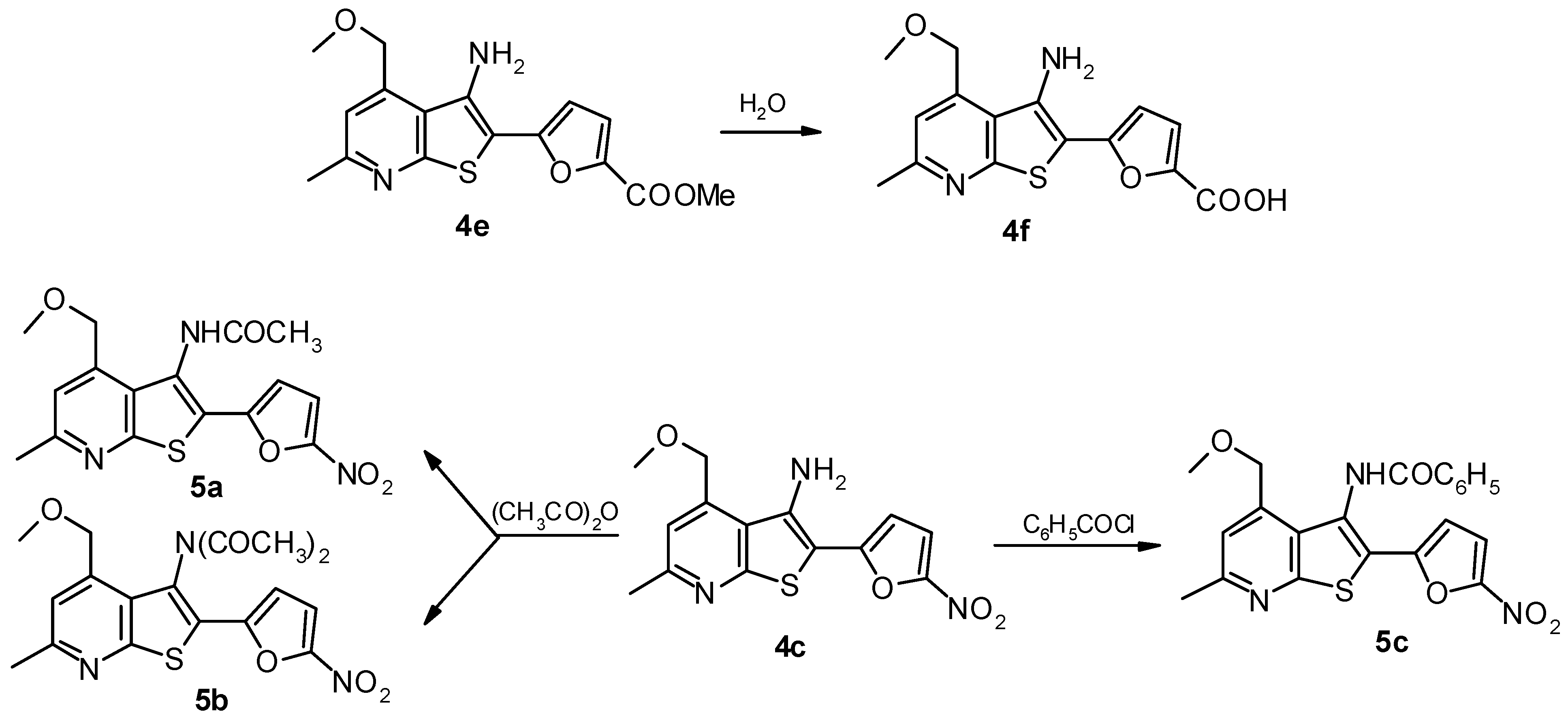
3-Amino-2-(5-carboxylfuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (4f).
3-N-Acetylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]-pyridine (5a).
3-N-Acetylamino- and 3-N,N-diacetylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno- [2,3-b]-pyridine (5a) and (5b).
3-N-Benzoylamino-2-(5-nitrofuran-2-yl)-6-methyl-4-methoxymethylthieno[2,3-b]pyridine (5c).
References
- Litvinov, V. P.; Krivokolysko, S. G.; Dyachenko, V. D. Khim. Geterotsikl. Soedin. 1999, 35, 579.
- Gewald, K.; Hentschel, M.; Illgen, U. J. Prakt. Chem. 1974, 316, 1030.
- Sharanin, Yu.A.; Shestopalov, A.M.; Promonenkov, V.K. Zh. Org. Khim. 1984, 20, 2012.
- Furukawa, N.; Kawai, T.; Oae, S.; Iwasaki, F. Synthesis 1984, 746.
- Kaigorodova, E. A.; Konyushkin, L. D.; Niyazymbetov, M. E.; Kvak, S.N.; Zaplishny, V.N.; Litvinov, V. P. Russ. Chem. Bull. 1994, 43, 2095.
- Kaigorodova, E.A.; Konyushkin, L.D.; Mikhailichenko, S.N.; Vasilin, V.K.; Kulnevich, V.G. Khim. Geterotsikl. Soedin. 1996, 32, 1432.
- Rodinovskaya, L.A.; Sharanin, Yu.A.; Litvinov, V.P.; Shestopalov, A.M.; Promonenkov, V.K.; Zolotarev, B.M.; Mortikov, V.Yu. Zh. Org. Khim. 1985, 21, 2439.
- Sample Availability: Samples are available from the authors.
| Compound | Empirical formula | Analysis, Found, %Calculated, % | M.p., °C | Yield, % | |||
| C | H | N | S | ||||
| 3a | C16H15N3O3S | 58.28 | 4.48 | 12.71 | 9.61 | 109-110 | 92 |
| 58.35 | 4.59 | 12.76 | 9.73 | ||||
| 3b | C16H15N3O3S | 58.27 | 4.42 | 12.69 | 9.65 | 151-152 | 90 |
| 58.35 | 4.59 | 12.76 | 9.73 | ||||
| 3c | C14H13N3O4S | 52.49 | 4.08 | 12.99 | 10.00 | 117-120 | 86 |
| 52.66 | 4.10 | 13.16 | 10.04 | ||||
| 3d | C14H13N3O4S | 52.54 | 3.98 | 13.08 | 9.90 | 112-114 | 86 |
| 52.66 | 4.10 | 13.16 | 10.04 | ||||
| 3e | C16H16N2O4S | 57.79 | 4.88 | 8.50 | 9.57 | 97-98 | 81 |
| 57.82 | 4.85 | 8.43 | 9.65 | ||||
| 4a | C16H15N3O3S | 58.29 | 4.58 | 12.69 | 9.72 | 204-205 | 93 |
| 58.35 | 4.59 | 12.76 | 9.73 | ||||
| 4b | C16H15N3O3S | 58.29 | 4.58 | 12.77 | 9.77 | 217-218 | 91 |
| 58.35 | 4.59 | 12.76 | 9.73 | ||||
| 4c | C14H13N3O4S | 52.50 | 4.05 | 13.02 | 9.96 | 202-203 | 75 |
| 52.66 | 4.10 | 13.16 | 10.04 | ||||
| 4d | C14H13N3O4S | 52.57 | 4.02 | 13.00 | 9.95 | 199-201 | 73 |
| 52.66 | 4.10 | 13.16 | 10.04 | ||||
| 4e | C16H16N2O4S | 57.79 | 4.82 | 8.41 | 9.63 | 130-131 | 73 |
| 57.82 | 4.85 | 8.43 | 9.65 | ||||
| 4f | C15H14N2O4S | 56.57 | 4.42 | 8.76 | 10.00 | 202-203 | 61 |
| 56.59 | 4.43 | 8.80 | 10.07 | ||||
| 5a | C16H15N3O5S | 53.10 | 4.19 | 11.66 | 8.85 | 238-239 | 73(12) |
| 53.18 | 4.18 | 11.63 | 8.87 | ||||
| 5b | C18H17N3O6S | 53.60 | 4.20 | 10.38 | 7.92 | >250 | 67 |
| 53.59 | 4.25 | 10.42 | 7.95 | decomposed | |||
| 5c | C21H17N3O5S | 59.54 | 4.00 | 9.87 | 7.55 | 108-110 | 92 |
| 59.57 | 4.05 | 9.92 | 7.57 | ||||
- For compounds 3a-d, 4c,d the eluent was 1:1 hexane -acetone; for compounds 4a,b,e,f, and 5a-c, 2:1 hexane -acetone was used.
| Compound | UV-VIS (EtOH) [?max (nm), log e (dm3mol-1cm-1)] | IR spectra, ν, cm-1 | |||||
| C≡N | C–O–C | C=C, C=N | C–HAr | NO2 | NH2 | ||
| 3a | 218(4.50), | 2205 | 1095, 1120 | 1580, 1560, | 3170 | 1495, | – |
| 269(4.48) | 1530 | 1220 | |||||
| 3b | 219(4.39), | 2210 | 1140, 1110 | 1598, 1588, | 3165 | 1505, | – |
| 268(4.27) | 1570 | 1240 | |||||
| 3c | 264(4.18), | 2205 | 1120, | 1560 | 3140 | 1530, | − |
| 317(4.23) | 1090 | 1220 | |||||
| 3d | 264(4.11), | 2207 | 1125, | 1570 | 3120 | 1530, | – |
| 312(4.17) | 1090 | 1220 | |||||
| 3e | 221(4.23), | 2230 | 1060 | 1600 | 3070 | – | – |
| 270(4.38), | 1030 | 1570 | 3040 | ||||
| 308(3.66) | 1010 | ||||||
| 4a | 209(4.29), | – | 1110, 1080 | 1640, 1570 | 3070, | 1490, | 3320, |
| 226(4.19), | 3050, | 1240, | 3400 | ||||
| 287(4.16), | 2730 | 1260 | |||||
| 320(3.94), | |||||||
| 423(4.05) | |||||||
| 4b | 208(4.28), | – | 1105, 1070 | 1640, 1570 | 3050, | 1490, | 3330, |
| 223(4.22), | 2720 | 1230 | 3400 | ||||
| 255(4.11), | |||||||
| 283(4.20), | |||||||
| 410(4.00) | |||||||
| 4c | 238(4.12), | – | 1100, 1090 | 1610, 1575, | 3130 | 1540, | 3220, |
| 284(4.13), | 1560 | 1230 | 3300 | ||||
| 347(3.94), | |||||||
| 478(4.20) | |||||||
| 4d | 235(4.11), | – | 1105, 1080 | 1610, 1570 | 3130 | 1570, | 3415 |
| 274(4.11), | 1220 | ||||||
| 340(3.99), | |||||||
| 464(4.13) | |||||||
| 4e | 220(4.12), | – | 1170, | 1640, | 3150, | – | 3460, |
| 253(3.81), | 1130, 1080 | 1590 | 3125 | 3360, | |||
| 317(4.13), | 3220 | ||||||
| 393(4.06) | |||||||
| 4f | 218(4.32), | – | 1110, 1040 | 1600, 1590 | 3030, | – | 3480, |
| 247(3.99), | 3010, | 3340 | |||||
| 312(4.37), | 3000 | ||||||
| 386(4.17) | |||||||
| 5a | 214(4.18), | – | 1140, | 1580 | 3150 | 1570, | 3240 |
| 232(4.28), | 1060, 1050 | 1210 | |||||
| 286(397), | |||||||
| 296(3.98), | |||||||
| 309(3.97), | |||||||
| 388(4.29) | |||||||
| 5b | 214(4.33), | – | 1110, | 1600 | 3150, | 1580, | 3230 |
| 228(4.33), | 1070, 1050 | 3120 | 1220 | ||||
| 283(3.97), | |||||||
| 293(3.98), | |||||||
| 307(3.96), | |||||||
| 380(4.23) | |||||||
| 5c | 211(4.28), | – | 1130, 1040 | 1600, 1580 | 3090 | 1540, | |
| 232(4.45), | 1210 | ||||||
| 387(4.25) | |||||||
| Compound | Signals, δ (ppm) | ||||
| CH3-Het, s | OCH3, s | OCH2, s | SCH2, s | Other protons (see R) | |
| 3a | 2.28 | 3.16 | 4.39 | 4.31 | 7.10 (s, 1H, HHet), 7.63 (d, J=9Hz, 2H, HAr), 8.21 (d, J=9Hz, 2H, HAr) |
| 3b | 2.45 | 3.45 | 3.46 | 3.40 | 7.11 (s, 1H, HHet), 7.53 (d, J=9Hz, 2H, HAr), 8.11 (d, J=9Hz, 2H, HAr) |
| 3c | 2.24 | 3.20 | 4.39 | 4.32 | 6.43 (d, J=3.6Hz, 1H, 3-HFur), 7.11 (d, J=3.6Hz, 1H, 4-HFur), 7.08 (s, 1H, HHet) |
| 3d | 2.53 | 3.42 | 4.59 | 4.36 | 6.45 (d, J=3.6Hz, 1H, 3-HFur), 7.10 (d, J=3.6Hz, 1H, 4-HFur), 7.19 (s, 1H, HHet) |
| 3e | 2.58 | 3.42 3.88 | 4.68 | 4.53 | 6.38 (d, J=4Hz, 1H, 3-HFur), 6.95 (d, J=4Hz, 1H, 4-HFur), 7.01 (s, 1H, HHet) |
| 4a | 2.63 | 3.40 | 4.78 | – | 6.95 (s, 1H, HHet), 7.72 (d, J=9.5Hz, 2H, HAr), 8.28 (d, J=9.5Hz, 2H, HAr), 4.75 (broad s, 1H, NH), 4.98 (broad s, 1H, NH) |
| 4b | 2.81 | 3.46 | 4.58 | – | 7.17 (s, 1H, HHet), 7.73 (d, J=9.5Hz, 2H, HAr), 8.30 (d, J=9.5Hz, 2H, HAr), 4.45 (broad s, 2H, NH2). |
| 4c | 2.58 | 3.43 | 4.86 | – | 6.28 (broad s, 2H, NH2), 7.07 (d, J=5.2Hz, 1H, 3-HFur), 7.26 (s, 1H, HHet), 7.85 (d, J=5.2Hz, 1H, 4-HFur) |
| 4d | 2.84 | 3.41 | 4.51 | – | 5.96 (broad s, 2H, NH2), 6.98 (d, J=6.2Hz, 1H, 3-HFur), 7.17 (s, 1H, HHet), 7.78 (d, J=5.2Hz, 1H, 4-HFur) |
| 4e | 2.10 | 3.29 | 4.65 | – | 3.78 (s, 3H, O-CH3), 5.85 (broad s, 2H, NH2), 6.38 (d, J=4.0Hz, 1H, 3-HFur), 6.88 (s, 1H, HHet), 7.45 (d, J=4.0Hz, 1H, 4-HFur) |
| 4f | 2.58 | 3.42 | 4.81 | − | 5.8 (broad s, 2H, NH2), 6.59 (d, J=4.4Hz, 1H, 3-HFur), 7.21 (d, J=4.4Hz, 1H, 4-HFur), 7.15 (s, 1H, HHet), |
| 5a | 2.27 | 2.69 3.42 | 4.74 | – | 6.74 (d, J=4.5Hz, 1H, 3-HFur), 7.16 (s, 1H, HHet), 7.18 (broad s, 1H, NH), 7.44(d, J=4.5Hz, 1H, 4-HFur) |
| 5b | 2.12 | 2.40 2.68 3.37 | 4.47 | – | 6.79 (d, J=4.0Hz, 1H, 3-HFur), 7.22(s, 1H, HHet), 7.25 (d, J=4.0Hz, 1H, 4-HFur) |
| 5c | 2.61 | 3.12 | 4.73 | − | 6.68 (d, J=4.0Hz, 1H, 3-HFur), 7.09 (d, J=4.0Hz, 1H, 4-HFur), 7.75 (m, 6H, ΣHHet, C6H5) |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Kaigorodova, Y.A.; Vasilin, V.K.; Konyushkin, L.D.; Usova, Y.B.; Krapivin, G.D. Synthesis and Reactions of Substituted 3-amino-2-furyl(aryl)-thieno[2,3-b]pyridines. Molecules 2000, 5, 1085-1093. https://doi.org/10.3390/51001085
Kaigorodova YA, Vasilin VK, Konyushkin LD, Usova YB, Krapivin GD. Synthesis and Reactions of Substituted 3-amino-2-furyl(aryl)-thieno[2,3-b]pyridines. Molecules. 2000; 5(10):1085-1093. https://doi.org/10.3390/51001085
Chicago/Turabian StyleKaigorodova, Ye. A., V. K. Vasilin, L. D. Konyushkin, Ye. B. Usova, and G. D. Krapivin. 2000. "Synthesis and Reactions of Substituted 3-amino-2-furyl(aryl)-thieno[2,3-b]pyridines" Molecules 5, no. 10: 1085-1093. https://doi.org/10.3390/51001085




