An Asymmetric Synthetic Approach to the A-ring of the Taxol Family of Anti-Cancer Compounds
Abstract
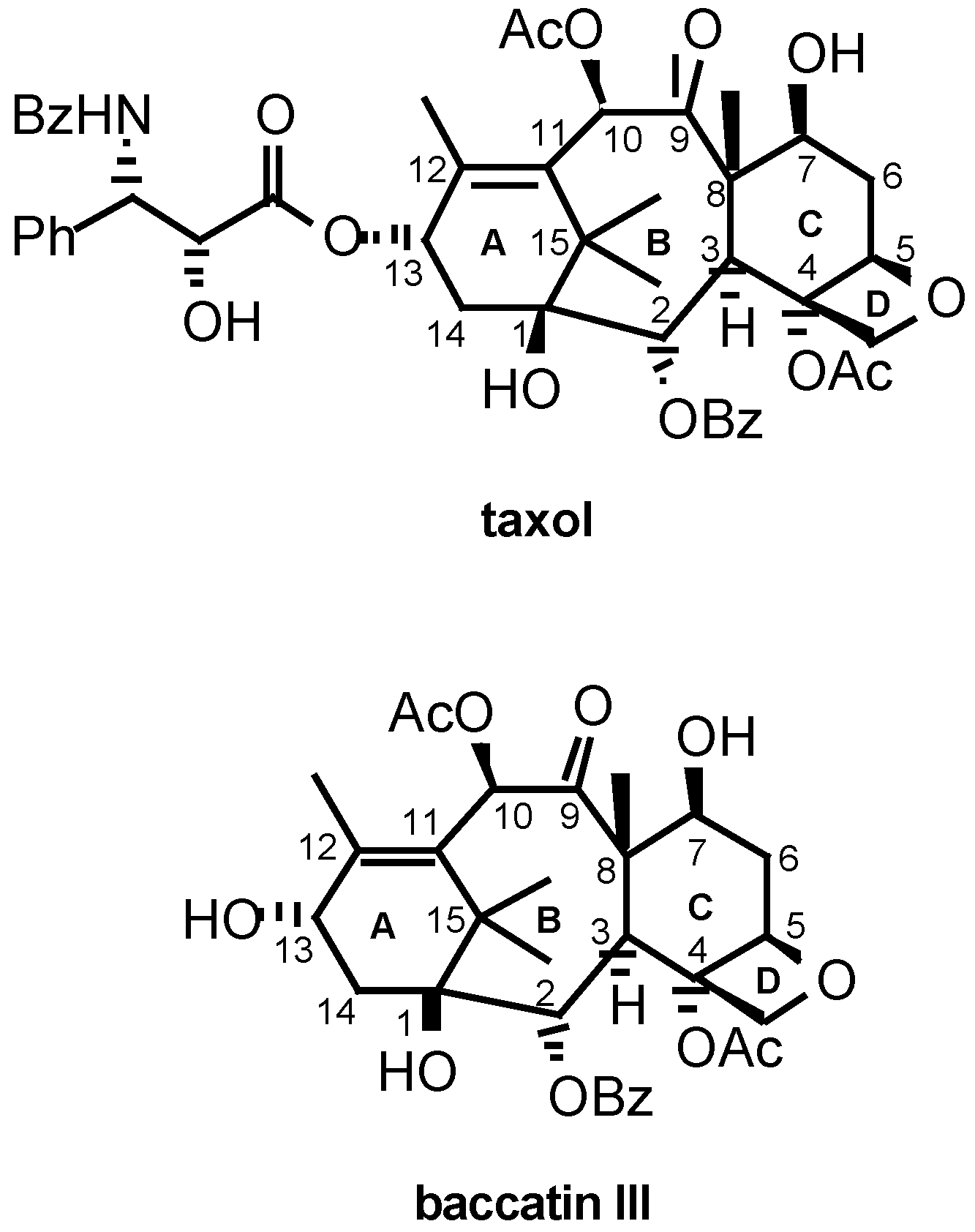
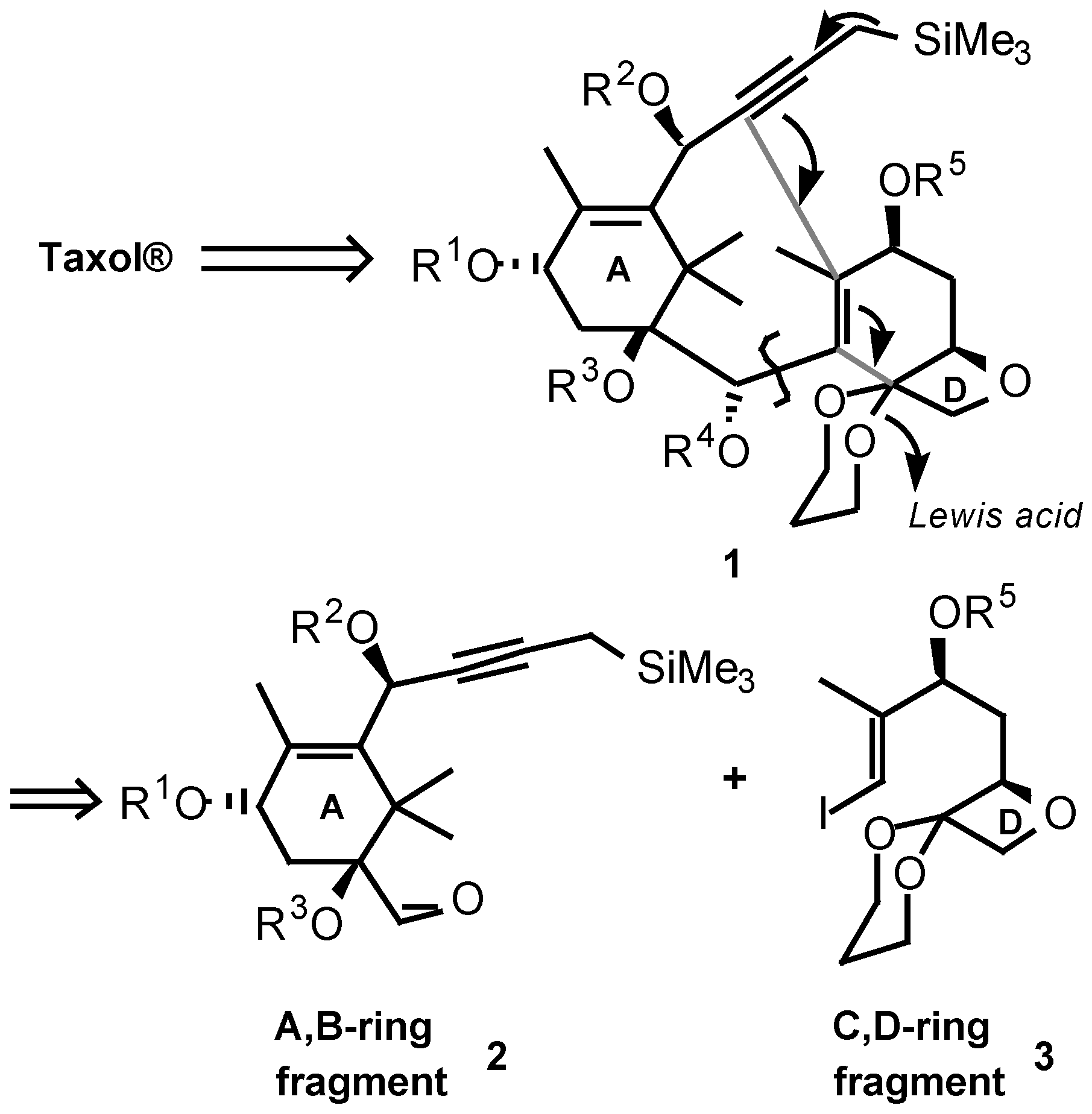
:Introduction
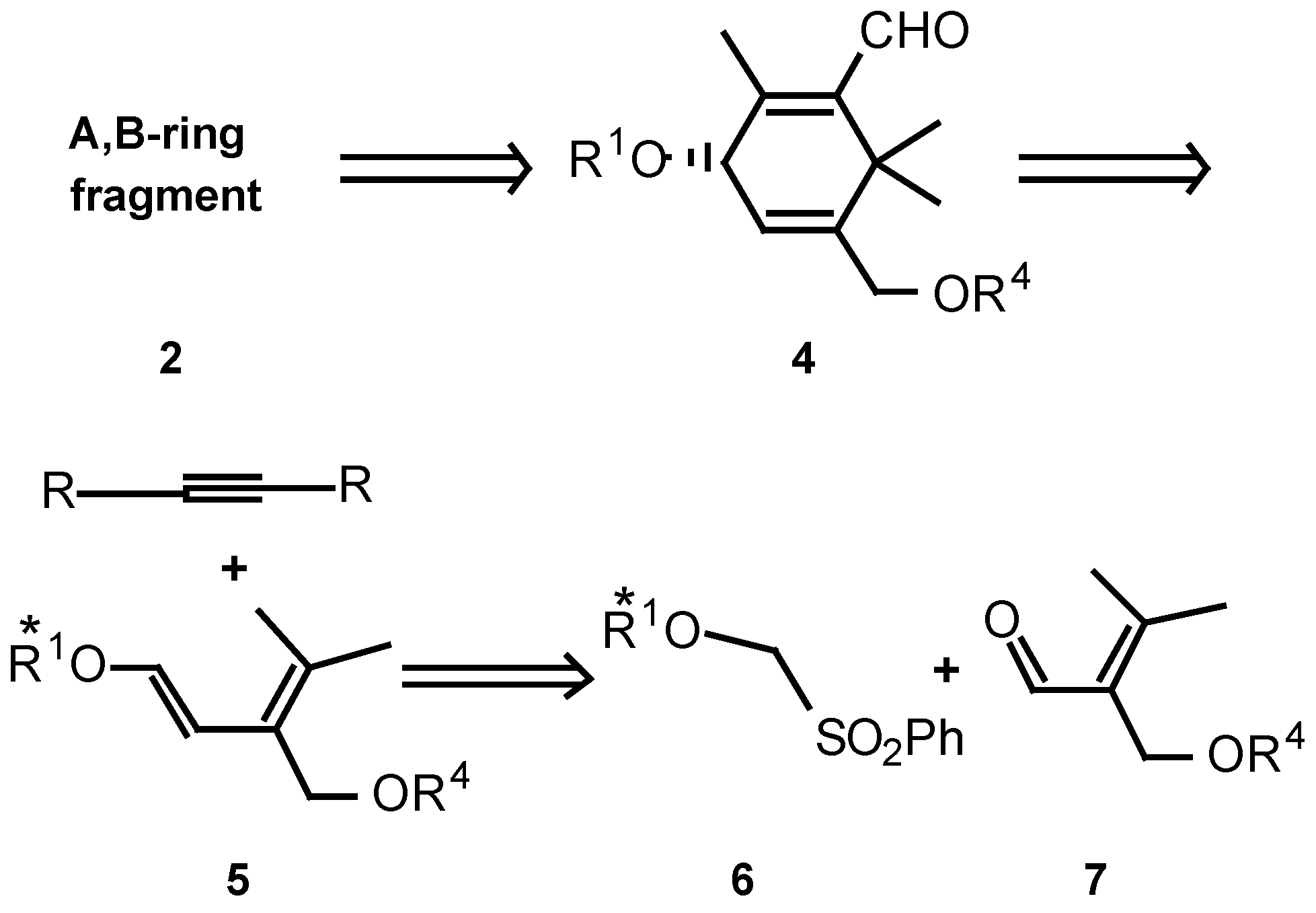
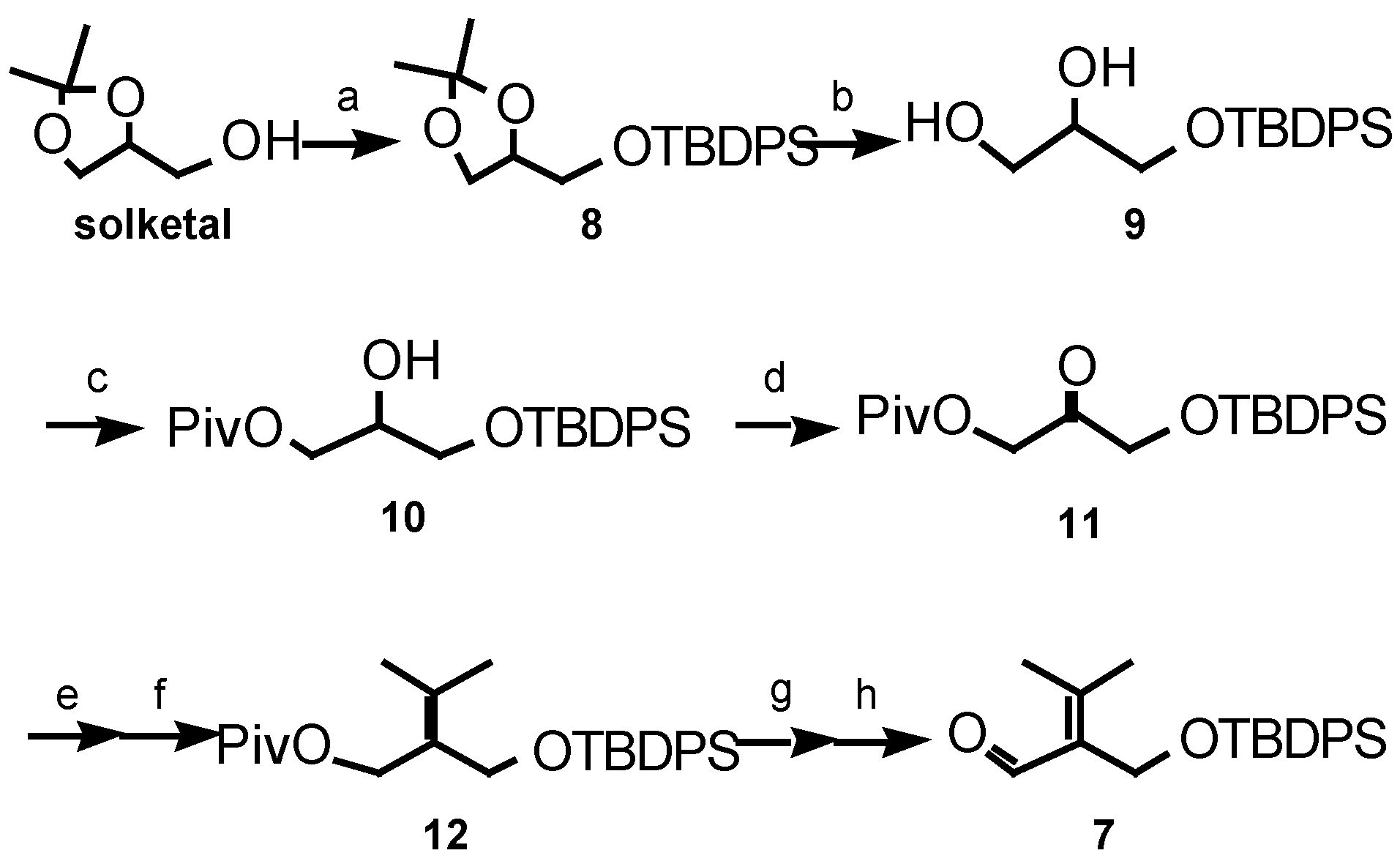
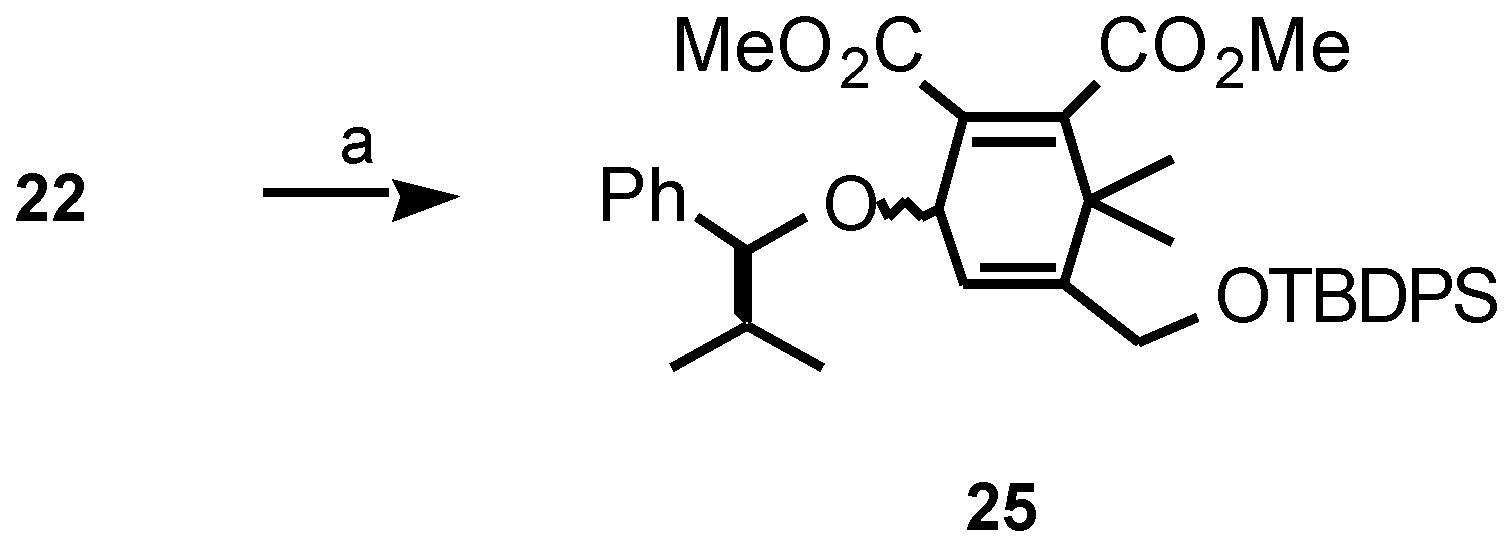
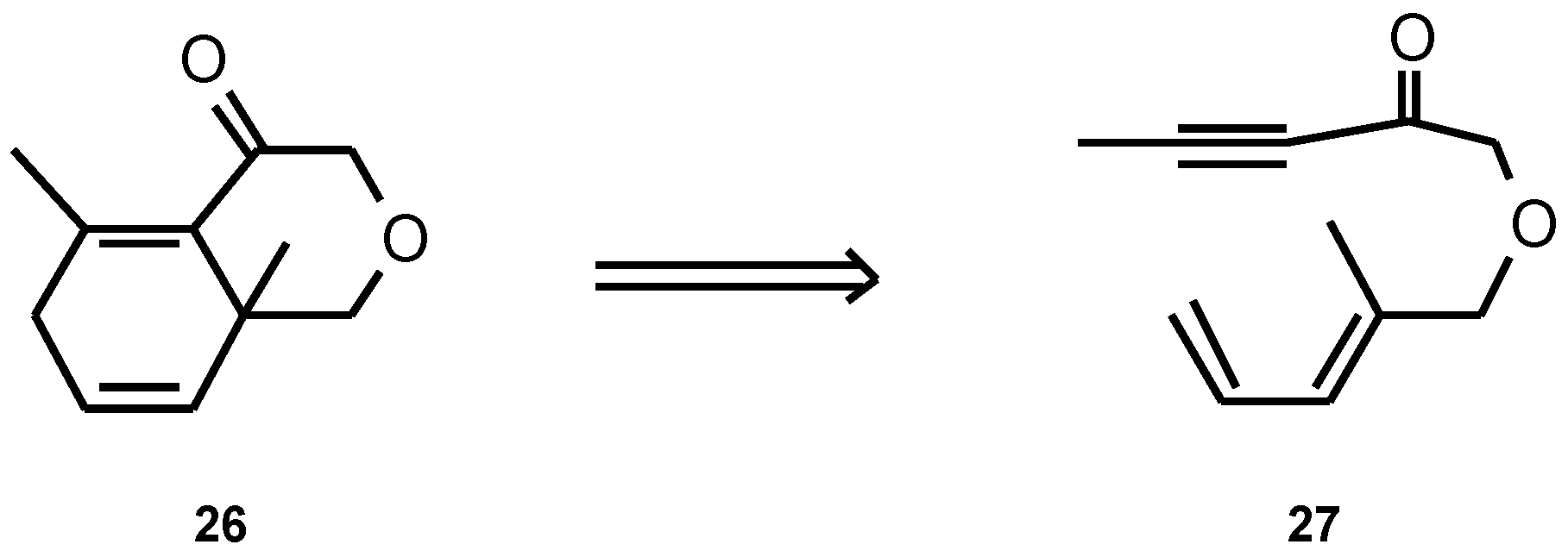
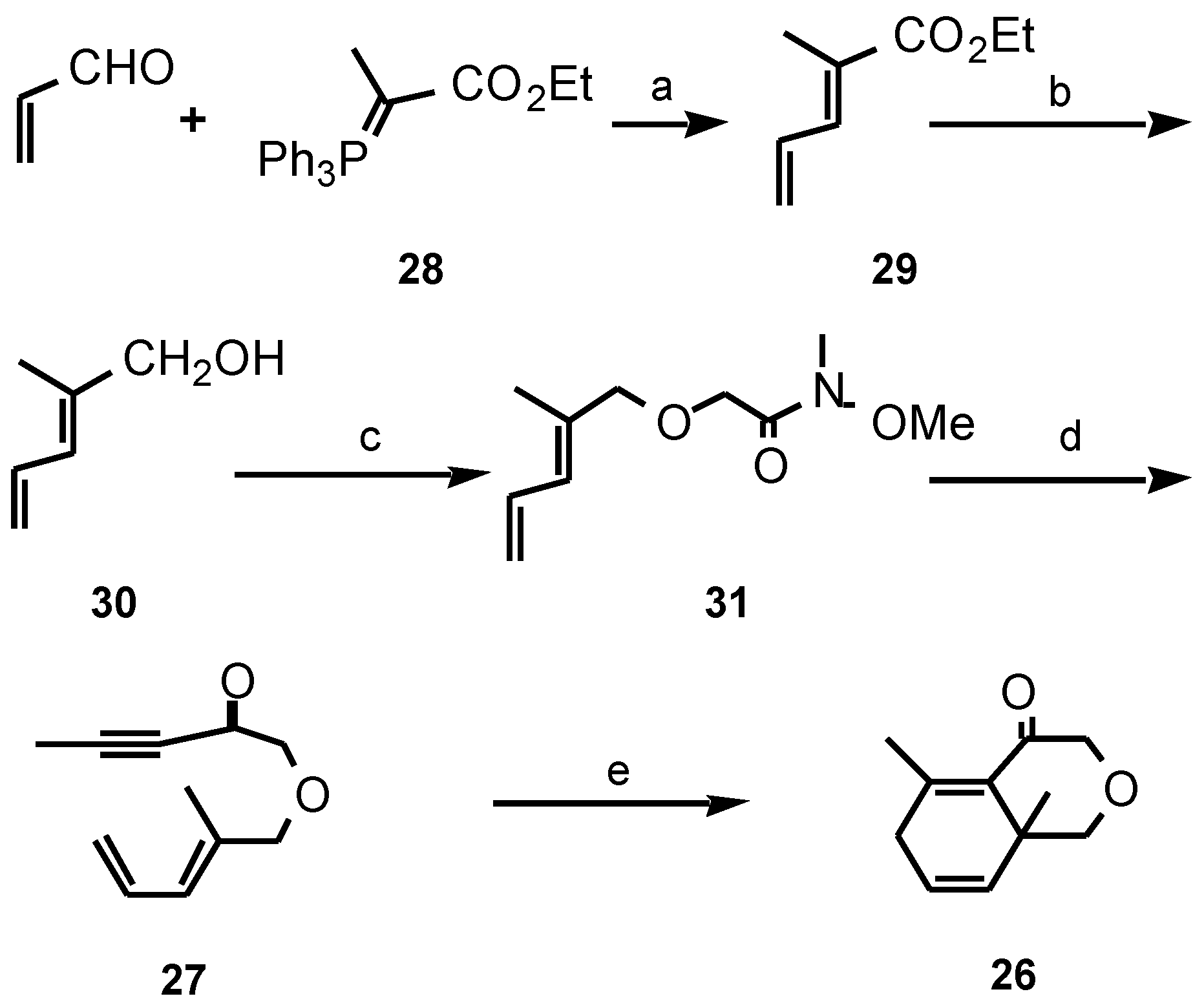
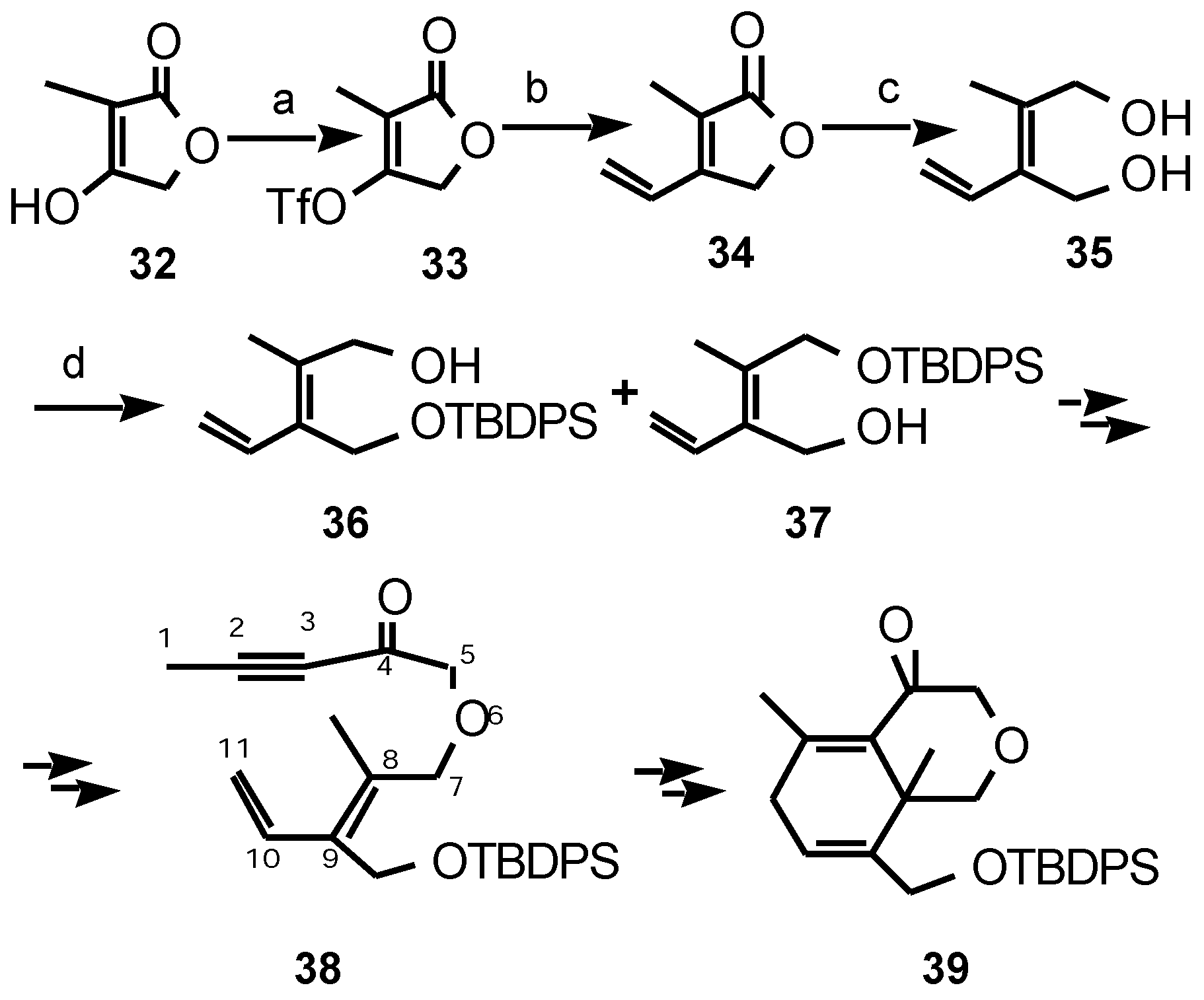
Results and Discussion
Acknowledgements
References and Notes
- Wani, W. C.; Taylor, H. L.; Wall, M. E.; Coggon, P.; McPhail, A. T. J. Am Chem. Soc. 1971, 93, 2325. [PubMed]
- (a) Nicolaou, K. C.; Yang, Z.; Liu, J. J.; Ueno, H; Nantermet, P. G.; Guy, R. K.; Clairborne, C. F.; Renaud, J.; Couladouros, E. A.; Paulvannan, K.; Sorensen, E. J. Nature 1994, 367, 630. [PubMed] Nicolaou, K. C.; Nantermet, P. G.; Ueno, H.; Guy, R. K.; Couladouros, E. A.; Sorensen, E. J. J. Am Chem. Soc. 1995, 117, 624. Nicolaou, K. C.; Liu, J. -J.; Yang, Z.; Ueno, H.; Sorensen, E. J.; Clairborne, C. F.; Guy, R. K.; Guy, R. K.; Hwang, C. K.; Nakada, M.; Nantermet, P. G. ibid. 1995, 117, 634. Nicolaou, K. C.; Yang, Z.; Liu, J. -J.; Nantermet, P. G.; Clairborne, C. F.; Renaud, J.; Guy, R. K.; Shibayama, K. ibid. 1995, 117, 645. (b) Nicolaou, K. C.; Ueno, H.; Liu, J. -J.; Nantermet, P. G.; Yang, Z.; Rebaud, J.; Paulvannan, K.; Chadha, R. ibid. 1995, 117, 653. Holton, R. A.; Somoza, C.; Kim, H. B.; Liang, F.; Biediger, R. J.; Boatman, R. D.; Shindo, M.; Smith, C. C.; Kim, S. C.; Nadizadeh, H.; Suzuki, Y.; Tao, C. L.; Vu, P.; Tang, S. H.; Zhang, P. S.; Murthi, K. K.; Gentile, L. N.; Liu, J. H. J. Am Chem. Soc 1994, 116, 1591. Holton, R. A.; Kim, H. B.; Somoza, C.; Liang, F.; Biediger, R. J.; Boatman, P. D.; Shindo, M.; Smith, C. C.; Kim, S. C.; Nadizadeh, H.; Suzuki, Y.; Tao, C. L.; Vu, P.; Tang, S. H.; Zhang, P. S.; Murthi, K. K.; Gentile, L. N.; Liu, J. H. ibid. 1994, 116, 1599. (c) Masters, J. J.; Link, J. T.; Synder, L. B.; Young, W. B.; Danishefsky, S. J. Angew. Chem. Int. Ed. Engl. 1995, 34, 1723. For recent comprehensive collections of references to work in the area, see: Nicolaou, K. C.; Dai, W. -M.; Guy, R. K. Angew. Chem. Int. Ed. Engl. 1994, 33, 45. Paquette, L. A.; Thomson, R. C. J. Org. Chem. 1993, 58, 4952.
- Craig, D.; Fischer, D. A.; Kemal, O.; Marsh, A.; Plessner, T.; Slawin, A. M. Z.; Williams, D. J. Tetrahedron 1991, 47, 3095. Craig, D.; Geach, N. J.; Pearson, C. J.; Slawin, A. M. Z.; White, A. J. P.; Williams, D. J. Tetrahedron 1995, 51, 6071. Clasby, M. C.; Craig, D. Tetrahedron Lett. 1992, 33, 3813. Clasby, M. C.; Craig, D.; Marsh, A. Angew. Chem. Int. Ed. Engl. 1993, 32, 1444.
- Ainsworth, P. J.; Craig, D.; Reader, J. C.; Slawin, A. M. Z.; White, A. J. P.; Williams, D. J. Tetrahedron 1995, 51, 11601. Ainsworth, P. J.; Craig, D.; Reader, J. C.; Slawin, A. M. Z.; White, A. J. P.; Williams, D. J. Tetrahedron 1996, 52, 695. Craig, D.; Ford, M. J.; Stones, J. A. Tetrahedron Lett. 1996, 37, 535.
- Craig, D.; Munasinghe, V. R. N. Tetrahedron Lett. 1992, 33, 663. Craig, D.; Munasinghe, V. R. N. J. Chem. Soc., Chem. Commun. 1993, 901. Craig, D.; Pennington, M. W.; Warner, P. Tetrahedron Lett. 1993, 34, 8539. Craig, D.; Pennington, M. W.; Warner, P. Tetrahedron Lett 1995, 36, 5815.
- Rubenstein, S. M.; Williams, R. M. J. Org. Chem. 1995, 60, 7215.
- Connor, D. S.; Klein, G. W.; Taylor, G. N.; Boeckman, R. K.; Nedwid, J. B. Org. Synth. Coll. Vol. VI, 101.
- Corey, E. J.; Gras, J. L.; Ulrich, P. Tetrahedron Lett. 1976, 11, 809.
- Noth, H.; Vahrenkamp, H. J. Organomet. Chem. 1968, 11, 399.
- Morton, H. E.; Guindon, Y. J. Org. Chem. 1985, 50, 5379. Guindon, Y.; Yoakim, C.; Morton, H. E. J. Org. Chem. 1984, 49, 3912.
- Ihara, N.; Suzuki, S.; Taniguchi, T.; Tokunaga, Y.; Fukumoto, K. Synlett 1994, 859.
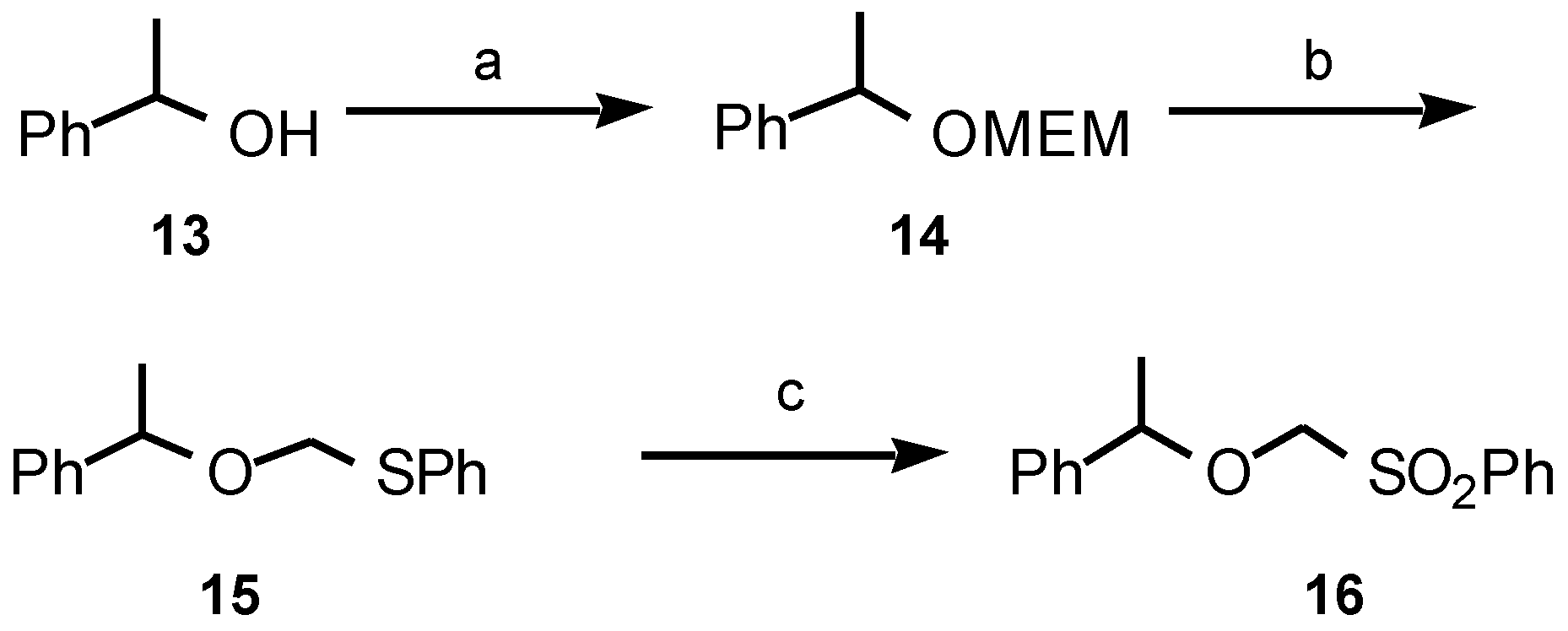
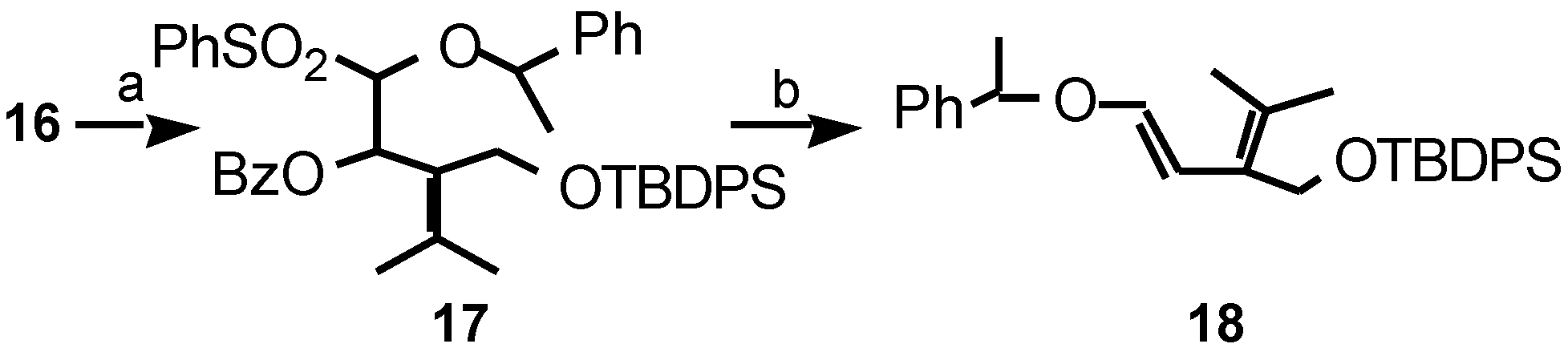
- Data of compound 18: νmax (film) 3069, 3050, 1648, 1623, 1588, 1492, 1181, 1142, 1111, 1069, 759, 739 cm-1; δH (300 MHz) 7.64-7.24 (15H, m, Ph+Ph-Si), 6.63 (1H, d, J 12.4 Hz, H-5maj), 5.96 (1H, d, J 12.4 Hz, H-4maj), 5.88 (1H, d, J 6.2 Hz, H-5min), 4.93 (1H, d, J 6.2 Hz, H-4min), 4.77 (1H, q, J 6.5 Hz, PhCH(CH3)Omaj), 4.70 (1H, q, J 6.5 Hz, PhCH(CH3)Omin), 4.52 (1H, d, J 11.5 Hz, H-3′min), 4.48 (1H, d, J 11.5 Hz, H-3′min), 4.25 (1H, d, J 11.6 Hz, H-3maj), 4.18 (1H, d, J 11.6 Hz, H-3′maj), 1.68, 1.59 (2 x 3H, 2s, H-1+H-2′min), 1.65, 1.44 (2 x 3H, 2s, H-1+H-2maj), 1.53 (3H, d, J 6.5 Hz, PhCH(CH3)Omaj), 1.40 (3H, d, J 6.5 Hz, PhCH(CH3)Omin), 1.04 (9H, s, SiC(CH3)3min), 0.95 (9H, s, SiC(CH3)3maj); δC (75MHz) (major isomer only) 146.4 (C-5), 143.0, 135.8, 133.9, 129.5, 129.1, 128.5, 127.5, 125.9 (Ph+Ph-Si+C-2+C-3), 107.2 (C-4), 78.9 (PhCH(CH3)O), 61.2 (C-3′), 26.9 (SiC(CH3)3), 23.7 (PhCH(CH3)O), 20.8 (C-1+C-2′), 19.2 (SiC(CH3)3; m/z (CI) 489 [M+NH4+H]+, 488 [M+NH4]+, 471 [M+H]+, 470 M+, 366, 309, 232, 215, 111, 105 (Found: [M+NH4]+, 488.301177. C31H38O2Si requires [M+NH4]+, 488.298483).
- Kim, S.; Ahn, K. H. J. Org. Chem. 1984, 49, 1717.
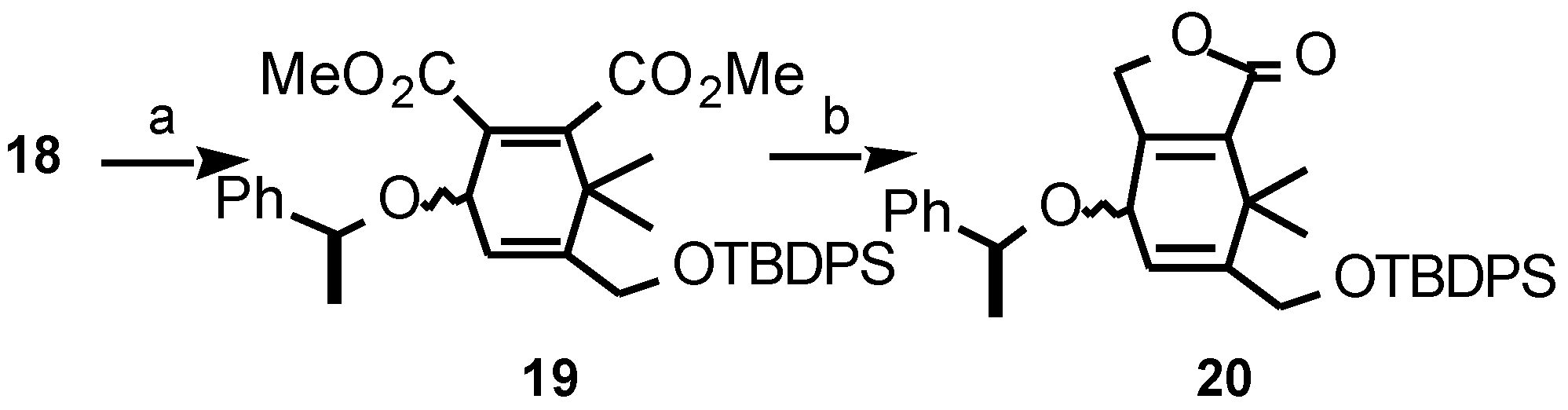
- Data of compound 20: νmax (film) 3048, 3030, 1750, 1472, 1461, 1280, 1250, 701 cm-1; δH (300 MHz) 7.52-7.30 (15H, m, Ph+Ph-Si), 6.05 (1H, broad s, H-5), 4.73 (1H, broad s, H-4), 4.68 (2H, s, H-3), 4.57 (1H, q, J 6.4 Hz, PhCH(CH3)O), 4.31 (2H, broad s, H-6′), 1.50 (3H, d, J 6.4 Hz, PhCH(CH3)O), 1.28, 1.14 (2 x 3H, 2s, H-7′+H-7″), 1.08 (9H, s, SiC(CH3)3); δC (75 MHz) 171.9 (C-1), 156.7, 147.5, 143.8, 135.9, 134.4, 133.6, 130.2, 129.1, 128.7, 128.4, 128.1, 126.9, 126.8 (Ph+Ph-Si+C-3a+C-7a+C-6), 118.1 (C-5), 77.9, 67.7 (C-4+PhCH(CH3)3), 69.7 (C-3), 61.9 (C-6′), 34.9 (C-7), 27.2 (SiC(CH3)3), 25.5, 24.7, 24.0 (C-7′+C-7″+PhCH(CH3)O), 19.7 (SiC(CH3)3); m/z (CI) 571 [M+H+NH4]+, 570 [M+NH4]+, 553 [M+H]+, 552 M+, 525, 512, 510, 508, 491, 465, 461, 274, 122 (Found [M+NH4]+, 570.304537. C35H40O4Si requires [M+NH4]+, 570.303963).
- Maugras, I.; Poncet, J.; Jouin, P. Tetrahedron 1990, 46, 2807.
- Data of compound 26: νmax (film) 3025, 1693, 1671, 1280, 1159, 1137, 1110, 966, 719 cm-1; δH (400 MHz) 5.73 (1H, ddd, J 9.9, 4.2, 2.6 Hz), 5.48 (1H, ddd, J 9.9, 2.6, 0.8 Hz) (H-5+H-6), 4.19 (1H, d, J 16.5 Hz, H-2), 3.98 (1H, d, J 16.5 Hz, H-2), 3.65 (1H, d, J 11.0 Hz, H-4), 3.63 (1H, d, J 11.0 Hz, H-4), 2.93 (1H, broad d, J 23.4 Hz, H-7), 2.73 (1H, broad d, J 23.4 Hz, H-7), 2.06 (3H, s), 1.21 (3H, s) (C-4a CH3+C-8 CH3); δC (75 MHz) 198.8 (C-1), 143.1, 133.1 (C-8+C-8a), 130.1, 123.5 (C-5+C-6), 75.3, 75.2 (C-2+C-4), 65.8 (C-4a), 35.1 (C-7), 26.3, 20.9 (C-4a CH3+C-8 CH3); m/z (CI) 197 [M+H+NH4]+, 196 [M+NH4]+, 179 [M+H]+, 178 M+, 177, 163, 150, 133, 105 (Found [M+H]+, 179.106865. C11H14O2 requires [M+H]+, 179.107205).
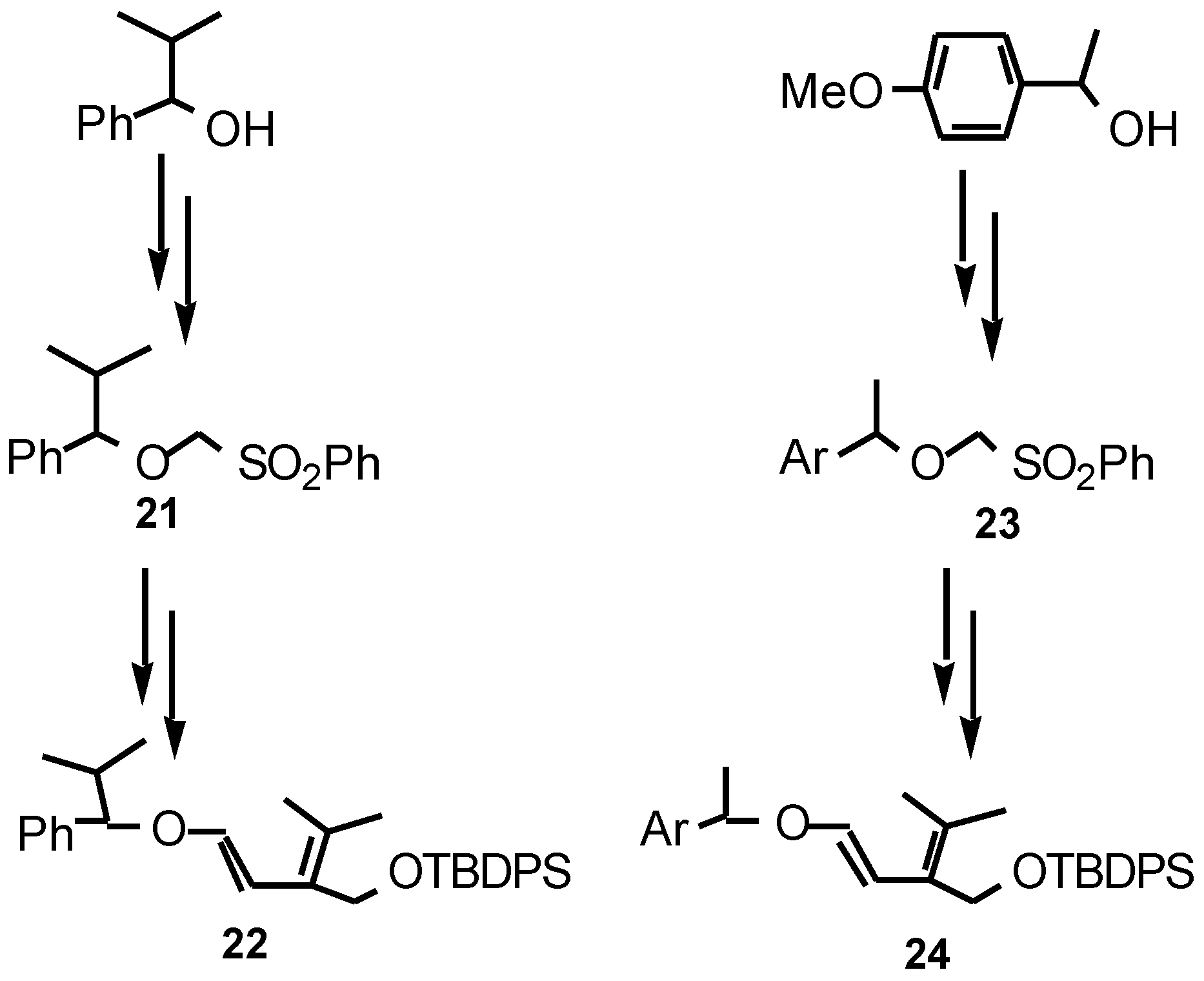
- The presence of a substituent at the diene C-3 presumably reduces the energy difference between the unreactive transoid and reactive cisoid conformations.
- Ramage, R.; Griffiths, G. J.; Shutt, F. E. J. Chem. Soc., Perkin Trans. I 1984, 1539. Ramage, R.; Griffiths, G. J.; Shutt, F. E. J. Chem. Soc. Perkin Trans. I 1984, 1531.
- Grigg, R.; Kennewell, P.; Savic, V. Tetrahedron 1994, 50, 5489.
- Houpis, I. N. Tetrahedron Lett. 1991, 32, 6675.
- Enas, J. D.; Shen, G.-Y.; Okamura, W. H. J. Am. Chem. Soc. 1991, 113, 3873.
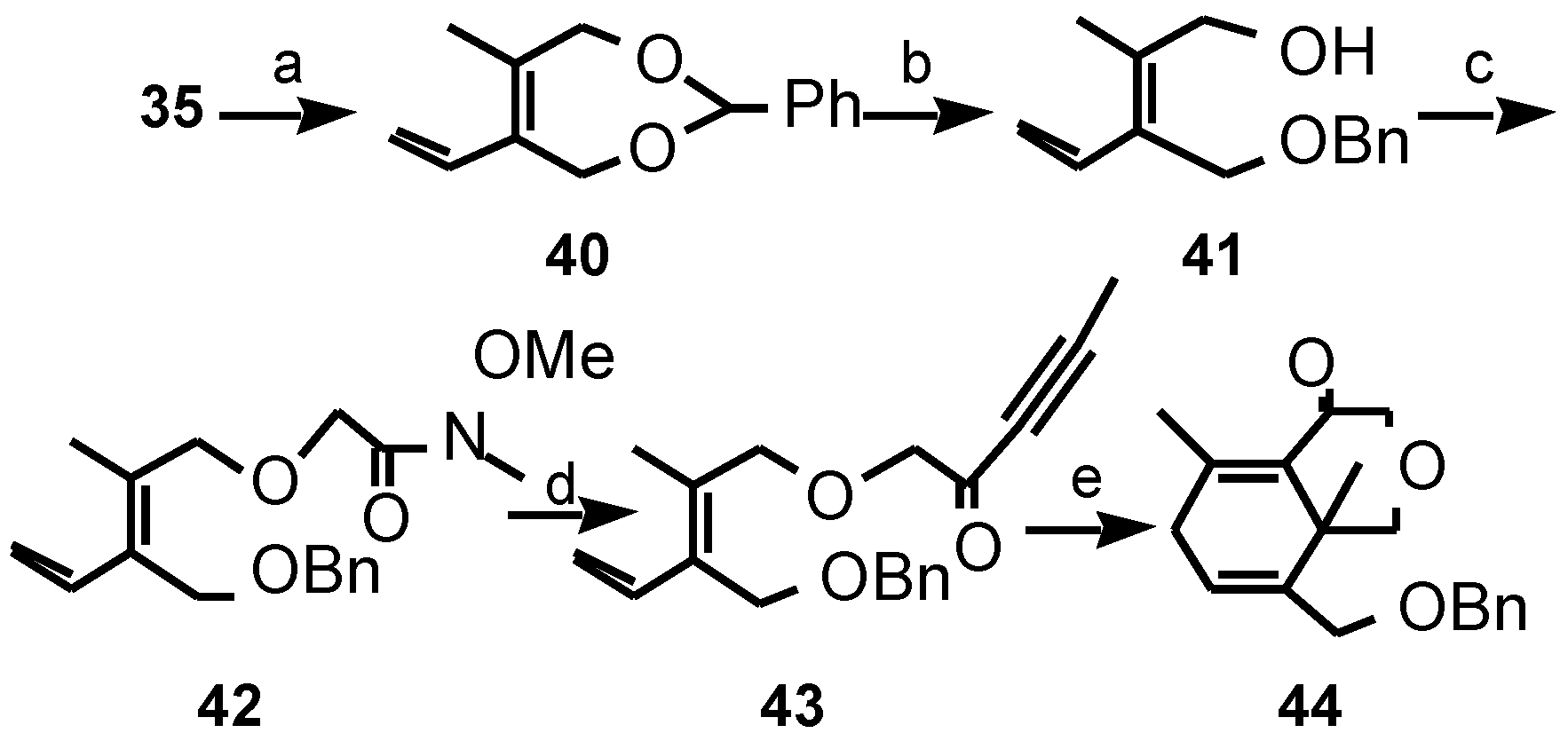
- Data of compound 44: δH (400 MHz) 7.40-7.30 (5H, m, Ph-H), 5.83 (1H, m, H-6), 4.51 (1H, d, J 10.7 Hz, PhCH2-O), 4.48 (1H, d, J 10.7 Hz, PhCH2-O), 4.18 (1H, d, J 16.6 Hz, H-2), 4.04 (1H, d, J 11.3 Hz), 3.99 (1H, d, J 16.6 Hz, H-2), 4.01 (1H, d, J 11.7 Hz), 3.97 (1H, d, J 11.7 Hz), 3.78 (1H, d, J 11.3 Hz) (H-4+H-5′), 2.99 (1H, d, J 23.7 Hz, H-7), 2.78 (1H, dd, J 23.7, 4.6 Hz, H-7), 2.09 (3H, s), 1.30 (3H, s) (C-4a CH3+ C-8 CH3); δC (75 MHz) 198.6 (C-1), 133.8 (ipso-Ph), 128.5, 127.9 (ortho +meta +para Ph), 127.9 (C-6), 74.1, 73.5, 72.2, 71.3 (C-2+C-4+C-5′+PhCH2-O), 35.3 (C-7), 25.6, 21.5 (C-4a CH3+C-8 CH3); m/z (CI) 316 [M+NH4]+, 299 [M+H] 283, 274, 257, 242, 222, 205, 108, 91 (Found [M+NH4]+, 316.191447. C19H22O3 requires [M+NH4]+, 316.191269).
- Sample Availability: Available from MDPI.

© 1998 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
Share and Cite
Craig, D.; Marin, M.L. An Asymmetric Synthetic Approach to the A-ring of the Taxol Family of Anti-Cancer Compounds. Molecules 1998, 3, 64-70. https://doi.org/10.3390/30300064
Craig D, Marin ML. An Asymmetric Synthetic Approach to the A-ring of the Taxol Family of Anti-Cancer Compounds. Molecules. 1998; 3(3):64-70. https://doi.org/10.3390/30300064
Chicago/Turabian StyleCraig, D., and M. L. Marin. 1998. "An Asymmetric Synthetic Approach to the A-ring of the Taxol Family of Anti-Cancer Compounds" Molecules 3, no. 3: 64-70. https://doi.org/10.3390/30300064



