Introduction
According to the reports of
D. C. Rees, homocitrate (IUPAC Name: 4-carboxy-4-carboxymethylene-4-butanolide) is thought to exist in the form of a ligand with a Mo atom at the active center of nitrogenase in nitrogen-fixing bacteria [
1]. Furthermore,
Rees pointed out that homeocitrate is an essential component of Fe Mo-cofactor to their nitrogen fixation. Without the ligand of homocitrate the cofactor could not fix nitrogen [
2].
Our present work was initiated firstly by our aim to synthesize Mo-S complexes and Mo-Fe-S clusters, in which a Mo atom is coordinated by a homocitrate ligand, and to secondly test their nitrogen fixation. Obviously such syntheses are significant in simulating studies of the structure of the active center of nitrogenase. For these syntheses pure homocitrate is necessary. However, homocitrate is very expensive and not commercially readily available. The purity of commercial homocitrate is only 95%. Attempted repetition of the best of the literature procedures has led, in our hands, to disappointing results, and for this reason we must modify the literature procedures to synthesize and to isolate this product chemically. We now wish to report the preparation of homocitrate by our modified method as well as the results on the structural characterisation of the products. Our modified method (see reaction
Scheme 1,
Scheme 2 and
Scheme 3) is based on the preparation of homocitrate introduced by
Tucci et al. [
3].
Results and Discussions
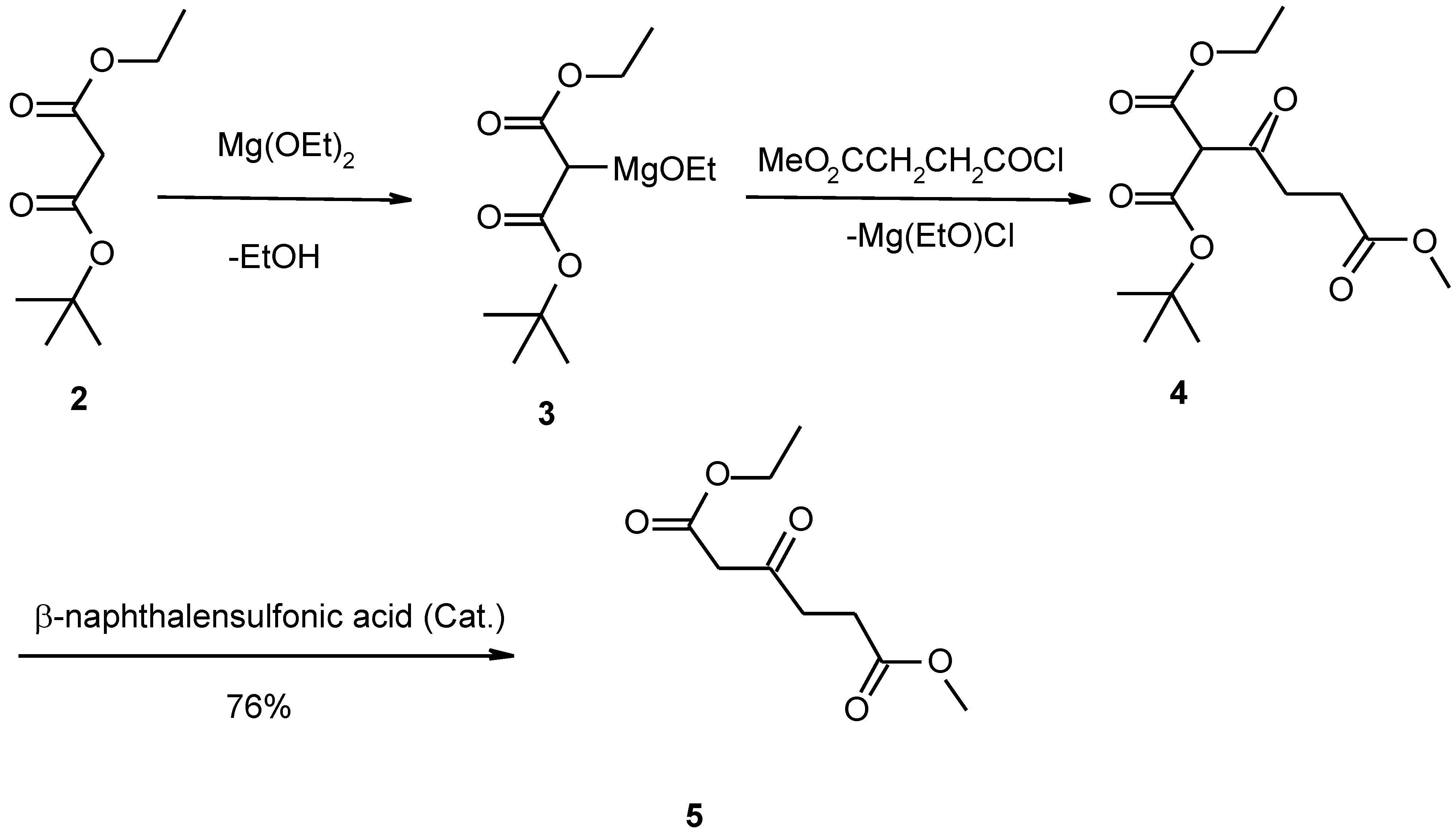
Our modified method has provided three improvements in obtaining this compound compared with the previously described methods. Firstly, in preparation of ethyl methyl β-ketoadipate (
5) we used ethyl t-butyl malonate instead of diethyl malonate to obtain the pure intermediate
5 so as to purify the end product easily (
Scheme 1 and
Scheme 2) [
4]. Previously described procedures for the preparation of this intermediate give such an impure product that the purification of homocitrate became one much more difficult.
Secondly, in our method monoethyl malonate and t-butyl alcohol are esterified at room temperature by the action of dicyclohexylcarbodiimide (DCC) in the presence of 4-dimethylaminopyridine (DMAP), which has been widely used as an efficient acylating catalyst in recent years, to yield ethyl t-butyl malonate [
5]. This is a new synthetic method of ethyl t-butyl malonate [
6].
Thirdly, the crude homocitric acid obtained is a pale yellow concentrated solution, and hardly able to be purified. In order to prepare available pure compound, we converted the crude
6 into homocitrate
7, which is a crystal lactone and can be easily isolated and purified. After
6 was treated with concentrated sulfuric acid, the residue from the ether extract was treated with ethyl acetate and benzene by codistillation, then, it was recrystallized (
Scheme 3). This method is more efficient than that of
Taccis [
2].
There are singlets at 3.11 ppm and at 3.03 ppm in the 1H-NMR spectra of 7, respectively, J=16.4 Hz, which is assigned to the geminal coupling of the methylene that is not part of the ring of 7. Thus, it shows the presence of a five-membered ring as the lactone 7. A strong and sharp characteristic absorption peak arising from the five-membered lactone falls in the region 1770 cm-1 in the IR spectra of 7. Other data showed that the structure of 7 is the five-membered lactone, also.
Homocitrate was also prepared enzymatically as described by
Schmidt et al. [
7,
8], however, this kind of method is not convenient for most chemical laboratories. Last but not least, we have provided the complete data determined with
1H-NMR, IR and MS of homocitrate, and these have never been seen in the previous related literature.
Experimental Section
General
M.p. was uncorrected. IR spectra were recorded on a Nicolet 5DXFT IR spectrometer with KBr discs.
1H-NMR spectra were determined with a FT80A spectrometer in CDCl
3 or CD
3COCD
3 with TMS as the internal standard for
1H-NMR. J values were given in Hz. Mass spectra were run on a HP5989A spectrometer. Elemental analysis were carried out with a PE-2740 elemental automatic analyzer. Monoethyl malonate, β-carbomethoxypropionyl chloride and 4-dimethylaminopyridine were prepared according to the literature [
6,
9,
10] respectively.
Ethyl t-butyl malonate (2)
To 13.2 g (0.10 mol) monoethyl malonate in 100 ml dried dichloromethane 0.6g DMAP (4-dimethylaminopyridine) and 14.9g (0.20 mol) t-butyl alcohol were added with stirring. After the mixture had heen cooled to 0°C with an ice bath, 22.7 g (0.11 mol) of DCC (dicyclohexylcarbodiimide) was added. Stirring was continued at 0°C for 0.5 h, then at room temperature for 6 h. The mixture was filtered to remove the resultant solid. The filtrate was washed with 0.5 mol/L HCI and saturated NaHCO3 solution, dried over anhydrous magnesium sulfate. Most of the solvent was then removed by distillation at atmospheric pressure and the liquid residue was distilled at reduced pressure through a 15 cm Vigreux column. The fraction at 75-77°C/93 Pa was collected yielding a colorless liquid, 15.3 g (81%). Analysis Found (%): C, 57.26; H, 8.61, C9H14O4 requires C, 57.43; H,8.57. 1H-NMR (CDCl3) 1.23 (3H, t, J=7, CH2CH3), 1.33 (9H, s, C (CH3)3), 3.48 (2H, s, CH2), 4.08 (2H, q, J=7, CH2CH3).
Ethyl methyl β-ketoadipate (5)
To 14.0 g (0.12 mol) freshly prepared magnesium ethoxide was added 80ml ether dried with Na. While excluding moisture and stirring, 18.8 g (0.10 mol) ethyl t-butyl malonate was added all at once, and the resulting mixture was stirred under reflux for 0.5 h. To the cooled solution was then added as rapidly as possible 18.6 g (0.12 mol) β-carbomethoxypropionyl chloride in anhydrous ether. After the vigorous exothermic reaction had subsided, the solution was stirred and refluxed for a further 3 h. The cooled solution was then acidified with 2 mol/L H2SO4 to pH=4, the ether layer was separated, the aqueous layer was extracted with ether (3×20 ml ), and the combined extracts were dried over MgSO4. After removal of the solvent, the residue was heated under vacuum, whereupon 8 ml liquid was distilled out at 34-58°C/400 Pa. The residue was slightly cooled and about 0.3 g β-naphthalenesulfonic acid was added. The oil was then refluxed under 133 Pa for 4-6 h until the vigorous evolution of gas had subsided. Distillation of the residue then gave 15.4 g colourless liquid b.p.118-120°C / 133 Pa (76%). Analysis Found (%): C, 52.94; H, 7.25. C9H14O5 requires C, 53.48; H,6.93. 1H-NMR (CD3COCD3): 1.27 (3H, t, CH3), 2.66 (2H, m, CH2CH2), 2.88 (2H, m, CH2CH2), 3.48 (2H, s, CH2), 3.67 (3H, s, CH3O), 4.15 (2H, q, CH2CH3).
Homocitric acid (6)
We used ethyl methyl β−ketoadipate obtained above to replace diethyl β-ketoadipate, the other procedures were the same as that described by
Tuccis [
3].
Isolation of homocitrate (7)
The homocitric acid (5.8 g) obtained above was dissolved in 2 ml of water, the pH was adjusted to 1 with concentrated sulfuric acid and then the solution was continuously extracted with ether for 3 days. The ether extract was evaporated to dryness and the residue was dissolved in a solution containing 50 ml of ethyl acetate and 150 ml of benzene, and was evaporated. The product was recrystallized twice from hot ethyl acetate and a yield of 5.2 g of white crystals was obtained (89%). M.p.158-160°C. Analysis Found (%): C, 44.22; H, 4.32, C7H8O6 requires C, 44.67; H,4.29. IR ν max/cm 2920, 1770, 1730, 1710, 1690, 1420, 1380, 1250, 1210, 1190, 1160, 1050 and 920. 1H-NMR(CD3COCD3): 2.49-2.63 (4H, m, CH2CH2), 3.30 (1H, s, JAB=16.4, CH2), 3.11 (1H, s, JAB= 16.4, CH2). MS (m/z) 189 (4.8%), 171 (6), 152 (21), 143 (85), 126 (23), 115 (100) and 98 (82).