Tailoring the Composition of BaxBO3 (B = Fe, Mn) Mixed Oxides as CO or Soot Oxidation Catalysts in Simulated GDI Engine Exhaust Conditions
Abstract
:1. Introduction
2. Results and Discussion
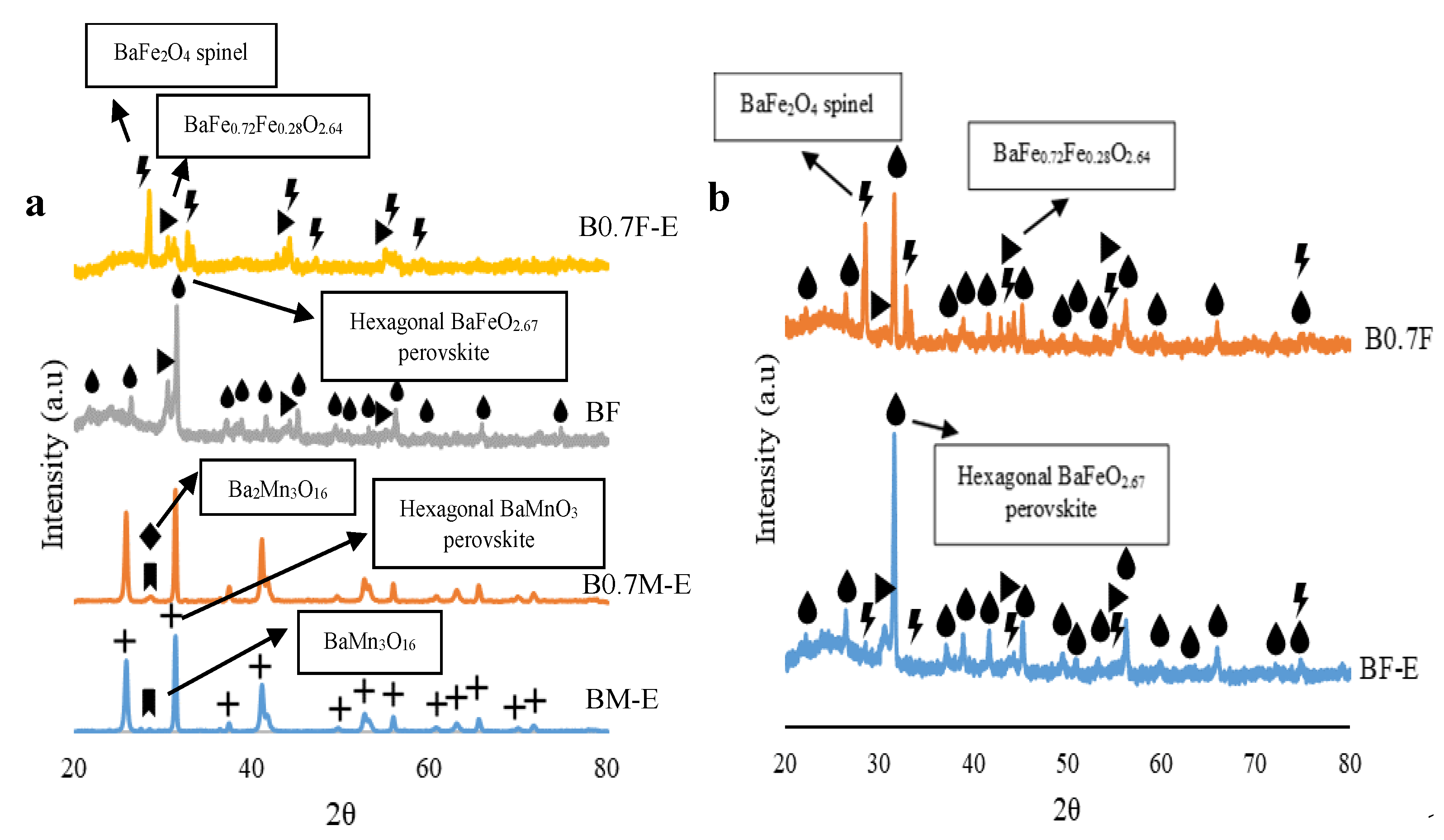
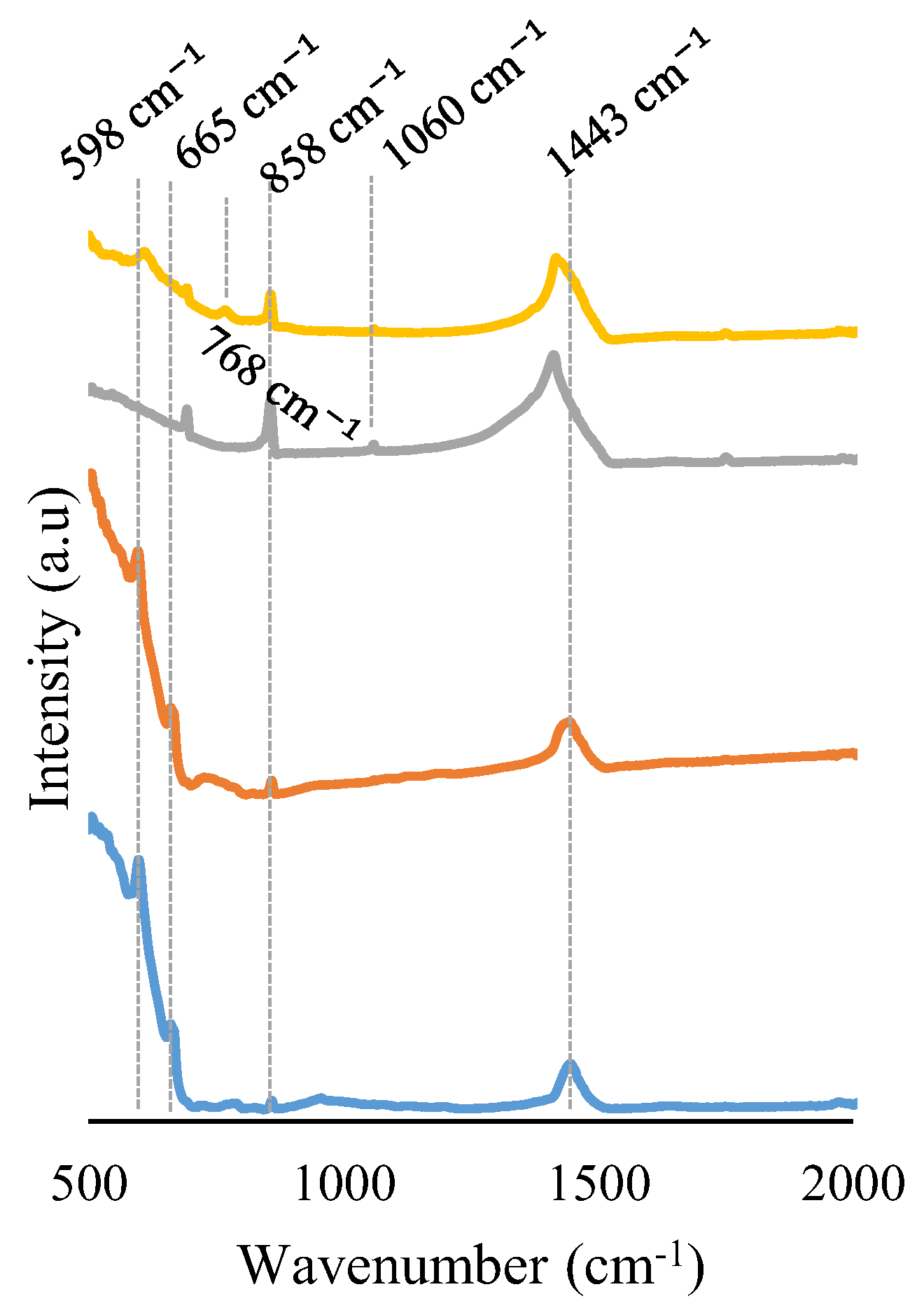
2.1. Chemical, Morphological, and Structural Characterization
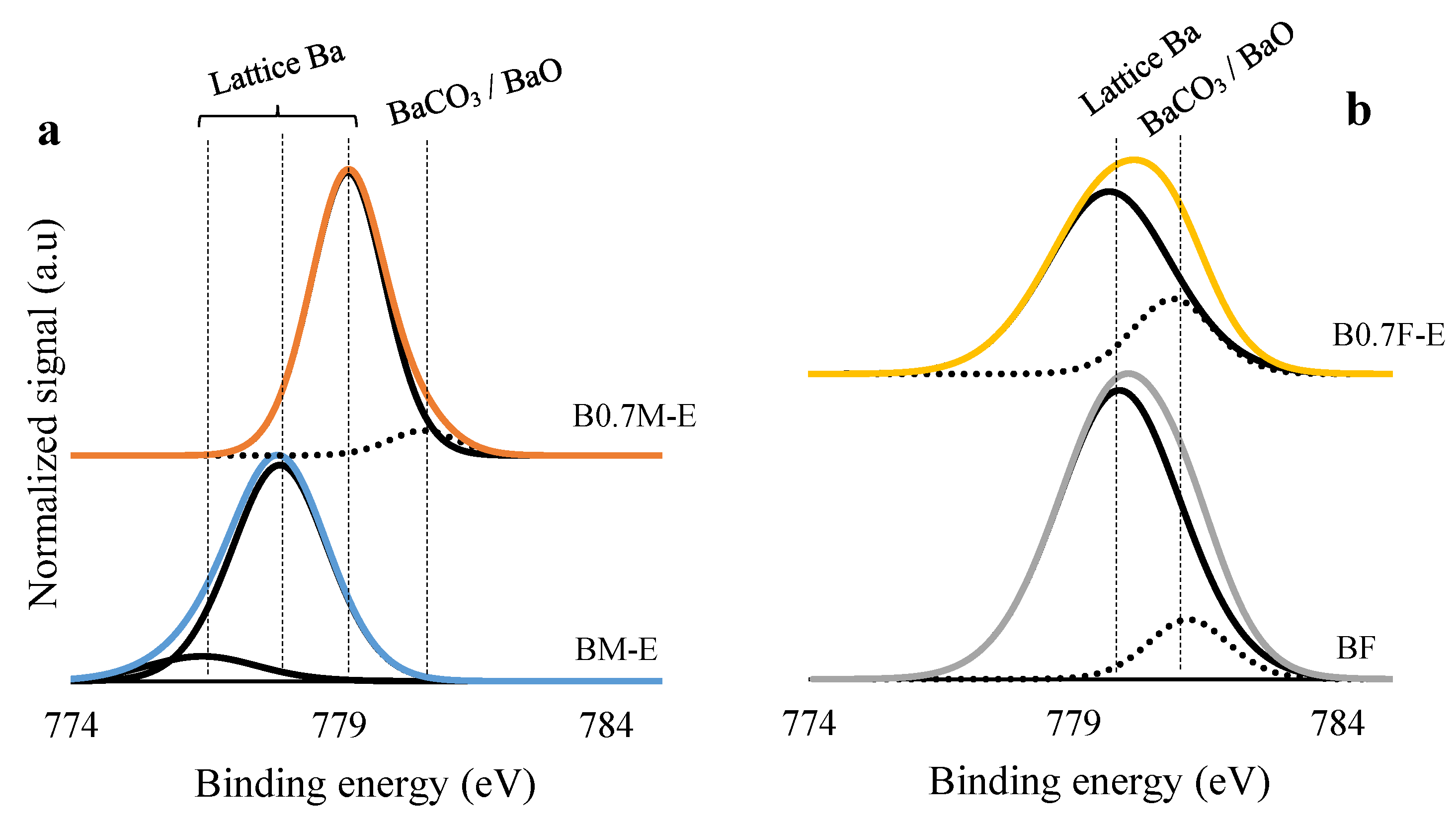
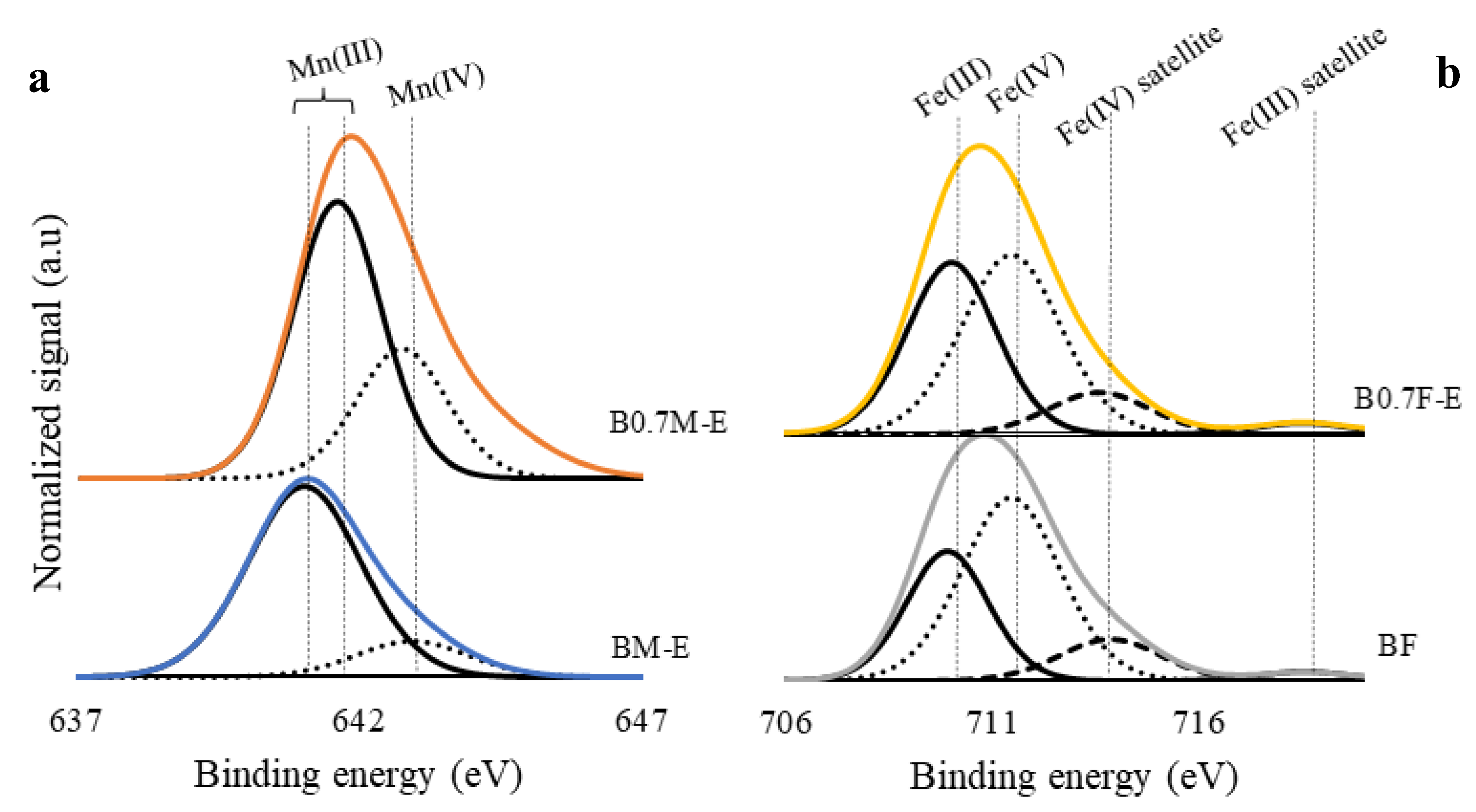
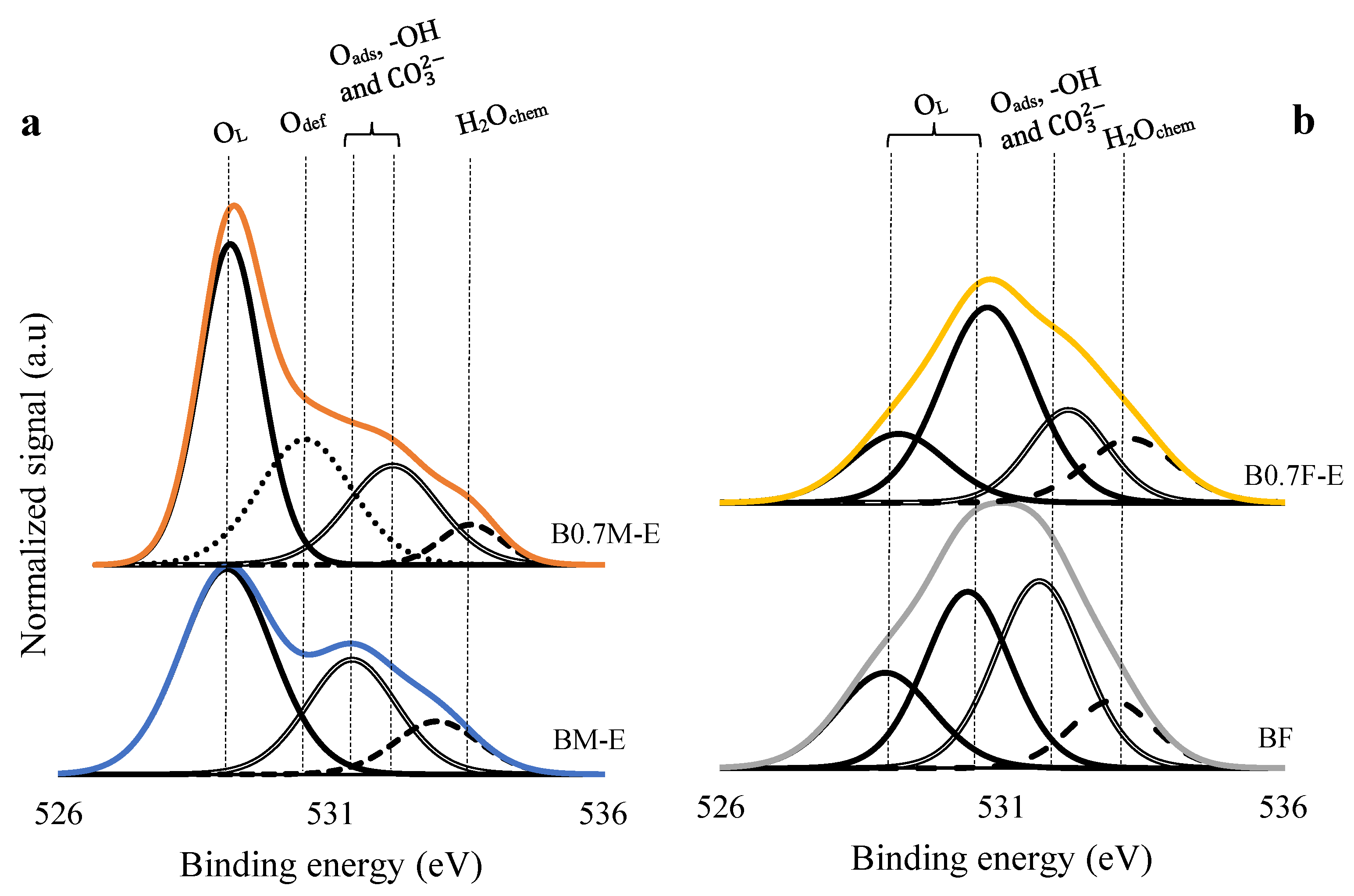
2.2. Surface Properties
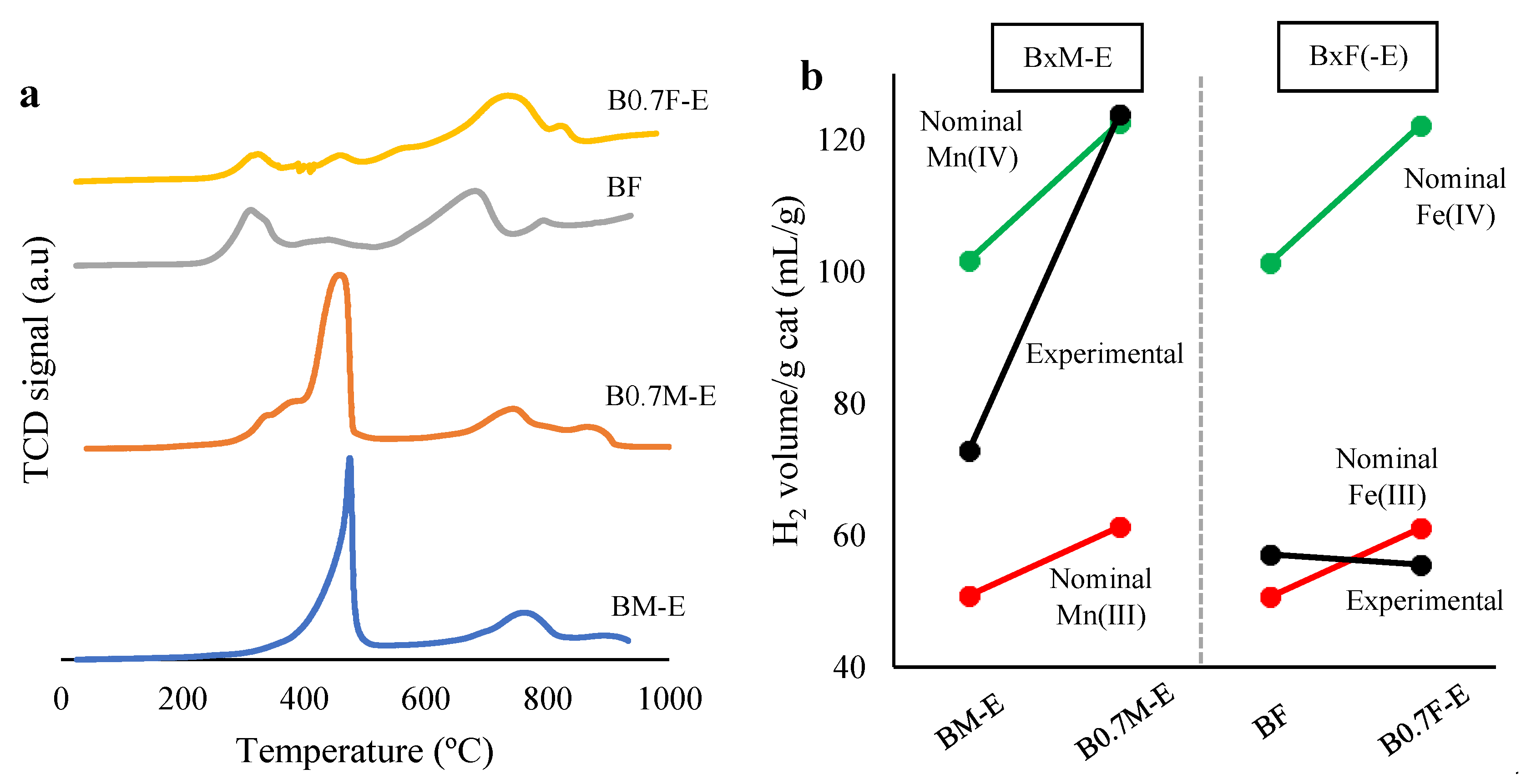
2.3. Redox Properties
2.4. O2 Release during Temperature-Programmed Desorption in He (O2-TPD)
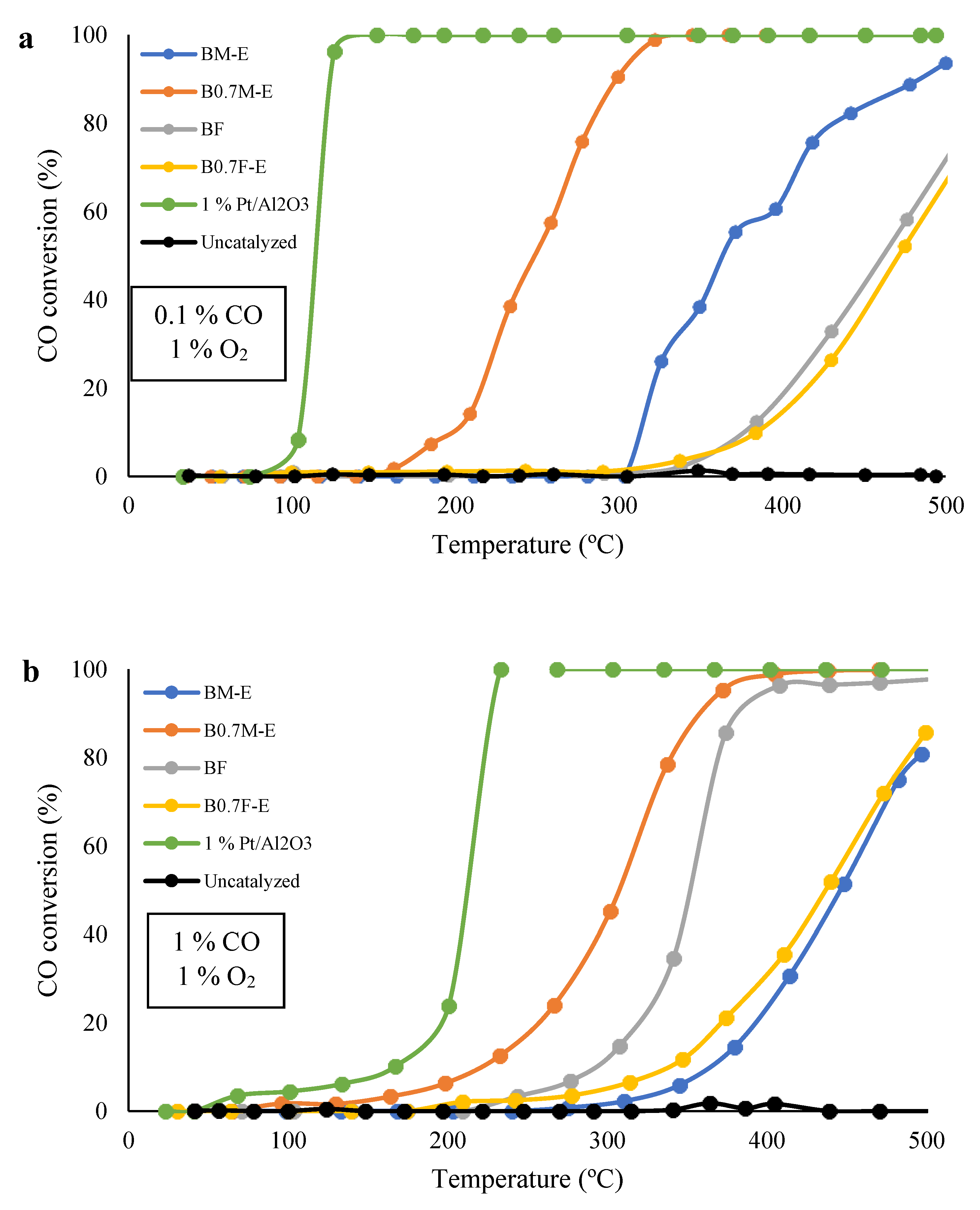
2.5. Catalytic Activity
3. Materials and Methods
3.1. Synthesis and Characterization of Catalysts
3.2. Activity Tests
4. Conclusions
- (1)
- Manganese-based samples present a BaMnO3 hexagonal perovskite structure, as the decrease in the amount of Ba does not significantly modify the crystalline structure. For iron-based samples, the decrease in the Ba content promotes the transition from a BaFeO2.67 hexagonal perovskite structure to a BaFe2O4 spinel structure.
- (2)
- To compensate for the Ba deficiency in the manganese-based samples, the amount of Mn(IV) and the oxygen vacancies increases, allowing a higher reducibility and oxygen mobility. In iron-based samples, the Ba deficiency only causes a change in the structure from perovskite to spinel.
- (3)
- Manganese-based perovskites (BM-E and B0.7M-E) show a better catalytic performance than iron-based perovskite (BF) for CO oxidation reactions due to the higher generation of actives sites.
- (4)
- The decrease in the Ba content improves the catalytic performance of both catalysts, as B0.7M-E is more active than BM-E for CO oxidation, and B0.7F-E presents a higher activity for soot conversion than BF under simulated GDI engine exhaust conditions. This shows that the composition of BaBO3 mixed oxides can be tailored as function of the oxidation reaction to be catalyzed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [Green Version]
- VijayaVenkataRaman, S.; Iniyan, S.; Goic, R. A review of climate change, mitigation and adaptation. Renew. Sustain. Energy Rev. 2012, 16, 878–897. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Huang, A. LEAP-based greenhouse gases emissions peak and low carbon pathways in China’s tourist industry. Int. J. Environ. Res. Public Health 2021, 18, 1218. [Google Scholar] [CrossRef] [PubMed]
- Massar, M.; Reza, I.; Rahman, S.M.; Abdullah, S.M.H.; Jamal, A.; Al-Ismail, F.S. Impacts of autonomous vehicles on greenhouse gas emissions—Positive or negative? Int. J. Environ. Res. Public Health 2021, 18, 5567. [Google Scholar] [CrossRef]
- Rajper, S.Z.; Albrecht, J. Prospects of electric vehicles in the developing countries: A literature review. Sustainability 2020, 12, 1906. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.B. Electric vehicles and the electric grid: A review of modelling approaches, impacts, and renewable energy integration. Renew. Sustain. Energy Rev. 2013, 19, 247–254. [Google Scholar] [CrossRef]
- Dolganova, I.; Rödl, A.; Bach, V.; Kaltschmitt, M.; Finkbeiner, M. A review of life cycle assessment studies of electric vehicles with a focus on resource use. Resources 2020, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Tran, M.K.; Bhatti, A.; Vrolyk, R.; Wong, D.; Panchal, S.; Fowler, M.; Fraser, R. A review of range extenders in battery electric vehicles: Current progress and future perspectives. World Electr. Veh. J. 2021, 12, 54. [Google Scholar] [CrossRef]
- Liang, H.; Jin, B.; Li, M.; Yuan, X.; Wan, J.; Liu, W.; Wu, X.; Liu, S. Highly reactive and thermally stable Ag/YSZ catalysts with microporous fiber-like morphology for soot combustion. Appl. Catal. B Environ. 2021, 294, 120271. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, J.; Li, M.; Liu, W.; Wu, X.; Liu, S. Model Ag/CeO2 catalysts for soot combustion: Roles of silver species and catalyst stability. Chem. Eng. J. 2022, 430, 132802. [Google Scholar] [CrossRef]
- Matarrese, R. Catalytic materials for gasoline particulate filters soot oxidation. Catalysts 2021, 11, 890. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Ag/CeO2 composites for catalytic abatement of CO, soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.A.; González, G.; Chen, L.; Valenzuela, M.A.; Moran-Pineda, M.; Vázquez, A.; Castillo, S. Templated synthesis and catalytic properties of an Rh/ceria-zirconia catalyst. React. Kinet. Catal. Lett. 2007, 90, 381–387. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Cauqui, M.A.; Corchado, P.; Pintado, J.M.; Rodríguez-Izquierdo, J.M.; Vidal, H. Fundamental properties of a new cerium-based mixed oxide alternative as TWC component. Stud. Surf. Sci. Catal. 1998, 116, 611–618. [Google Scholar]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, S.; Atri, S.; Tomar, R. A brief review on key role of perovskite oxides as catalyst. Chem. Sel. 2021, 6, 12947–12959. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite oxide catalysts for advanced oxidation reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Peron, G.; Glisenti, A. Perovskites as alternatives to noble metals in automotive exhaust abatement: Activation of oxygen on LaCrO3 and LaMnO3. Top. Catal. 2018, 62, 244–251. [Google Scholar] [CrossRef]
- Barbero, B.P.; Gamboa, J.A.; Cadús, L.E. Synthesis and characterization of La1-xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2006, 65, 21–30. [Google Scholar] [CrossRef]
- Cant, N.W.; Angove, D.E. The origin of apparent deactivation during the oxidation of carbon monoxide over silica-supported platinum at moderate temperatures. J. Catal. 1986, 97, 36–42. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.W.; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F.; Hagerman, M. Shriver & Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2010; p. 541. [Google Scholar]
- Voorhoeve, R.J.H. Perovskite-Related Oxides as Oxidation-Reduction Catalysts, 1st ed.; Academic Press Inc.: Cambridge, MA, USA, 1977; pp. 129–180. [Google Scholar]
- Tascón, J.M.D.; González-Tejuca, L. Catalytic activity of perovskite-type oxides LaMeO3. React. Kinet. Catal. Lett. 1980, 15, 185–191. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A review on CO oxidation over copper chromite catalyst. Catal. Rev. Sci. Eng. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Pinto, D.; Glisenti, A. Pulsed reactivity on LaCoO3-based perovskites: A comprehensive approach to elucidate the CO oxidation mechanism and the effect of dopants. Catal. Sci. Technol. 2019, 9, 2749–2757. [Google Scholar] [CrossRef]
- Ouyang, X.; Scott, S.L. Mechanism for CO oxidation catalyzed by Pd-substituted BaCeO3, and the local structure of active sites. J. Catal. 2010, 273, 83–91. [Google Scholar] [CrossRef]
- Najjar, H.; Lamonier, J.F.; Mentré, O.; Giraudon, J.M. Optimization of the combustion synthesis towards efficient LaMnO3+y catalysts in methane oxidation. Appl. Catal. B Environ. 2011, 106, 149–159. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between catalytic deactivation and physicochemical properties of LaMnO3 perovskite catalyst during catalytic oxidation of vinyl chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1-xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Zsako, J. TPR-study of substitution effects on reducibility and oxidative non-stoichiometry of La0.8A´0.2MnO3+δ perovskites. J. Therm. Anal. Calorim. 2000, 61, 819–825. [Google Scholar] [CrossRef]
- Patcas, F.; Buciuman, F.C.; Zsako, J. Oxygen non-stoichiometry and reducibility of B-site substituted lanthanum manganites. Thermochim. Acta 2000, 360, 71–76. [Google Scholar] [CrossRef]
- Irusta, S.; Pina, M.P.; Menéndez, M.; Santamaría, J. Catalytic combustion of volatile organic compounds over La-based perovskites. J. Catal. 1998, 179, 400–412. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Modified BaMnO3-based catalysts for gasoline particle filters (GPF): A preliminary study. Catalysts 2022, 12, 1325. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Exploring the effect of using carbon black in the sol-gel synthesis of BaMnO3 and BaMn0.7Cu0.3O3 perovskite catalysts for CO oxidation. Catal. Today 2023. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. In Catalizadores basados en BaxMnO3 para la oxidación de CO. In Proceedings of the IV Encuentro de Jóvenes Investigadores de la SECAT, Bilbao, Spain, 21–23 September 2020. [Google Scholar]
- Li, S.; Bergman, B. Doping effect on secondary phases, microstructure and electrical conductivities of LaGaO3 based perovskites. J. Eur. Ceram. Soc. 2009, 29, 1139–1146. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, Y.; Li, F.; Xia, M.; Xue, B.; Liu, D. Structure, dye degradation activity and stability of oxygen defective BaFeO3-x. Mater. Trans. 2010, 51, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.A.U.; Ikram, M. Structural stability improvement, Williamson Hall analysis and band-gap tailoring through A-site Sr doping in rare earth based double perovskite La2NiMnO6. Rare Met. 2019, 38, 805–813. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; De Rossi, S.; Faticanti, M.; Porta, P. Methane combustion and CO oxidation on LaAl1-xMnxO3 perovskite-type oxide solid solutions. Appl. Catal. B 2003, 43, 397–406. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Moreno-Marcos, C.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaFe1-xCuxO3 perovskites as active phase for diesel (DPF) and gasoline particle filters (GPF). Nanomaterials 2019, 9, 1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Han, X.; Zhao, Q.; Du, J.; Cheng, F.; Chen, J. Porous perovskite calcium-manganese oxide microspheres as an efficient catalyst for rechargeable sodium-oxygen batteries. J. Mater. Chem. A. 2015, 3, 3320–3324. [Google Scholar] [CrossRef]
- Xu, H.; Gao, L.; Guo, J. Preparation and characterizations of tetragonal barium titanate powders by hydrothermal method. J. Eur. Ceram. Soc. 2002, 22, 1163–1170. [Google Scholar] [CrossRef]
- Balamurugan, S.; Asha, M.K.S.; Gokul, R.T.S.; Parthiban, P. Mechano-thermal synthesis and characterization of BaMnO3 nano-needles. J. Nanosci. Nanotechnol. 2015, 15, 5978–5986. [Google Scholar] [CrossRef]
- Gao, F.; Lewis, R.A.; Wang, X.L.; Dou, S.X. Far-infrared reflection and transmission of La1-xCaxMnO3. J. Alloys Compd. 2002, 347, 314–318. [Google Scholar] [CrossRef]
- Roy, C.; Budhani, R.C. Raman- and infrared-active phonons in hexagonal BaMnO3. Phys. Rev. B 1998, 58, 8174–8177. [Google Scholar] [CrossRef]
- Xian, H.; Zhang, X.; Li, X.; Zou, H.; Meng, M.; Zou, Z.; Guo, L.; Tsubaki, N. Effect of the calcination conditions on the NOx storage behavior of the perovskite BaFeO3-x catalysts. Catal. Today 2010, 158, 215–219. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Wang, Y.; Sun, Y. Photoinduced decomposition of BaFeO3 during photodegradation of methyl orange. J. Mol. Catal. A Chem. 2007, 270, 56–60. [Google Scholar] [CrossRef]
- Singh, H.; Rajput, J.K. Novel perovskite nanocatalyst (BiFeO3) for the photodegradation of rhodamine B/tartrazine and swift reduction of nitro compounds. J. Iran. Chem. Soc. 2019, 16, 2409–2432. [Google Scholar] [CrossRef]
- Singh, H.; Garg, N.; Arora, P.; Rajput, J.K.; Jigyasa. Sucrose chelated auto combustion synthesis of BiFeO3 nanoparticles: Magnetically recoverable catalyst for the one-pot synthesis of polyhydroquinoline. Appl. Organomet. Chem. 2018, 32, e4357. [Google Scholar] [CrossRef]
- Fang, Z.; Jiang, H.; Gong, J.; Zhang, H.; Hu, X.; Ouyang, K.; Guo, Y.; Hu, X.; Wang, H.; Wang, P. Removal of tetracycline hydrochloride from water by visible-light photocatalysis using BiFeO3/BC materials. Catalysts 2022, 12, 1461. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy Learning Center. Available online: https://www.thermofisher.com/es/es/home/materials-science/learning-center/surface-analysis.html (accessed on 10 December 2022).
- Quiñonez-Ortiz, J.L.; García-González, L.; Cancino-Gordillo, F.E.; Pal, U. Particle dispersion and lattice distortion induced magnetic behavior of La1-xSrxMnO3 perovskite nanoparticles grown by salt-assisted solid-state synthesis. Mater. Chem. Phys. 2020, 246, 122834. [Google Scholar] [CrossRef]
- Ghaffari, M.; Shannon, M.; Hui, H.; Tan, O.K.; Irannejad, A. Preparation, surface state and band structure studies of SrTi(1-x)Fe(x)O(3-δ) (x = 0–1) perovskite-type nano structure by X-ray and ultraviolet photoelectron spectroscopy. Surf. Sci. 2012, 606, 670–677. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Disparity in electrical and magnetic properties of isostructural oxygen-deficient perovskites BaSrCo2O6-δ and BaSrCoFeO6-δ. J. Mater. Sci. Mater. Electron. 2018, 29, 13464–13473. [Google Scholar] [CrossRef]
- Tabata, K.; Hirano, Y.; Suzuki, E. XPS studies on the oxygen species of LaMn1-xCuxO3+λ. Appl. Catal. A Gen. 1998, 170, 245–254. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- LaSurface Database. Available online: http://www.lasurface.com/accueil/ (accessed on 13 December 2022).
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Trawczynski, J. Potassium-copper perovskite catalysts for mild temperature diesel soot combustion. Appl. Catal. A Gen. 2014, 485, 214–221. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.E.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Tailoring the properties of BaTi0.8Cu0.2O3 catalyst selecting the synthesis method. Appl. Catal. A Gen. 2016, 519, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yao, L.; Wang, M.; Wu, W. XPS and voltammetric studies on La1-xSrxCoO3-δ perovskite oxide electrodes. J. Alloys Compd. 2000, 311, 53–56. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Jia, J.; Sun, T. Catalytic oxidation of VOCs over SmMnO3 perovskites: Catalyst synthesis, change mechanism of active species, and degradation path of toluene. Inorg. Chem. 2019, 58, 14275–14283. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V. BaMnO3 Perovskite-Based Catalysts for Pollution Control Generated by Highly Efficient Automotive Engines. Doctoral Thesis, University of Alicante, San Vicente del Raspeig, Spain, 2021. [Google Scholar]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z. Lysine-aided PMMA-templating preparation and high performance of three dimensionally ordered microporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene. Appl. Catal. B Environ. 2012, 119–120, 20–31. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kaliaguine, S. Reduction kinetics of perovskite-based oxygen carriers for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 6946–6955. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kleitz, F.; Kaliaguine, S. Novel oxygen carriers for chemical looping combustion: La1-xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ. Sci. 2011, 4, 4258–4269. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Ma, H.; Li, Z. Preparation and characterization of the non-stoichiometric La-Mn perovskites. J. Alloys Compd. 2015, 646, 73–79. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Mortazavi, Y.; Khodadadi, A.A. Cumene cracking activity and enhanced regeneration of FCC catalysts comprising HY-zeolite and LaBO3 (B = Co, Mn, and Fe) perovskites. Appl. Catal. A Gen. 2014, 487, 26–35. [Google Scholar] [CrossRef]
- Gan, R.; Nishida, Y.; Haneda, M. Effect of B-site substitution on the catalytic activity of La-based perovskite for oxidative coupling of methane. Phys. Status Solidi B 2022, 259, 2100544. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Copper doped BaMnO3 perovskite catalysts for NO oxidation and NO2-assisted diesel soot removal. RSC Adv. 2017, 7, 35228–35238. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Sun, Y.; Niu, X.; Yuan, F.; Fu, H. Preparation of La-Mn-O perovskite catalyst by microwave irradiation method and its application to methane combustion. Catal. Lett. 2010, 135, 152–158. [Google Scholar] [CrossRef]
- Shibata, S.; Kamata, K.; Hara, M. Stability enhancement of iron-based perovskite catalysts by A-site substitution for oxidative transposition of α-bromostyrene to phenacyl bromide. ChemCatChem 2022, 14, e202200395. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Anderson, J.A.; Illán-Gómez, M.J. NOx storage on BaTi0.8Cu0.2O3 perovskite catalysts: Addressing a feasible mechanism. Nanomaterials 2021, 11, 2133. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nii, H.; Kagawa, S.; Jansson, K.; Nygren, M. Influence of the simultaneous substitution of Cu and Ru in the perovskite-type (La, Sr) MO3 (M = Al, Mn, Fe, Co) on the catalytic activity for CO oxidation and CO-NO reactions. Appl. Catal. A Gen. 2000, 194–195, 35–41. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. A review of synthesis, structure and applications in hopcalite catalysts for carbon monoxide oxidation. Aerosol Sci. Eng. 2019, 3, 97–131. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Campagnoli, E.; Tavares, A.; Fabbrini, L.; Rossetti, I.; Dubitsky, Y.A.; Zaopo, A.; Forni, L. Effect of preparation method on activity and stability of LaMnO3 and LaCoO3 catalysts for the flameless combustion of methane. Appl. Catal. B Environ. 2005, 55, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Marcos, C.; Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaFe1-xCuxO3 perovskites as soot oxidation catalysts for gasoline particulate filters (GPF): A preliminary study. Top. Catal. 2019, 62, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Martinovic, F.; Galletti, C.; Bensaid, S.; Pirone, R.; Deorsola, A. Soot oxidation in low-O2 and O2-free environments by lanthanum-based perovskites: Structural changes and the effect of Ag doping. Catal. Sci. Technol. 2022, 12, 5453–5464. [Google Scholar] [CrossRef]
- Rao, Y.K. Stoichiometry and Thermodynamics of Metallurgical Processes; Cambridge University Press: Cambridge, UK, 1985; p. 377. [Google Scholar]
- Wan, H.; Wang, Z.; Zhu, J.; Li, X.; Liu, B.; Gao, F.; Dong, L.; Chen, Y. Influence of CO pretreatment on the activities of CuO/γ-Al2O3 catalysts in CO + O2 reaction. Appl. Catal. B Environ. 2008, 79, 254–261. [Google Scholar] [CrossRef]
- Zhou, J.; Pan, J.; Jin, Y.; Peng, Z.; Xu, Z.; Chen, Q.; Ren, P.; Zhou, X.; Wu, K. Single-cation catalyst: Ni cation in monolayered CuO for CO oxidation. J. Am. Chem. Soc. 2022, 144, 8430–8433. [Google Scholar] [CrossRef]
- Fu, M.; Yue, X.; Ye, D.; Ouyang, J.; Huang, B.; Wu, J.; Liang, H. Soot oxidation via CuO doped CeO2 catalysts prepared using coprecipitation and citrate acid complex-combustion synthesis. Catal. Today 2010, 153, 125–132. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef] [Green Version]


| Catalyst | Average Crystal Size (nm) 1 | |||
|---|---|---|---|---|
| a | b | c | ||
| BM-E | 24.76 | 5.69 | 5.69 | 4.81 |
| B0.7M-E | 18.24 | 5.69 | 5.69 | 4.81 |
| BF | 49.52 | 5.67 | 5.67 | 13.96 |
| B0.7F-E | 23.09 | 19.02 | 5.38 | 8.48 |
| Catalyst | BET Surface Area (m2/g) | Chemical Composition (wt %) | |||||
|---|---|---|---|---|---|---|---|
| Experimental | Nominal | ||||||
| Ba | Mn or Fe | O | Ba | Mn or Fe | O | ||
| BM-E | 9 | 62 | 24 | 14 | 57 | 23 | 20 |
| B0.7M-E | 11 | 55 | 30 | 15 | 48 | 28 | 24 |
| BF | 9 | 59 | 24 | 17 | 58 | 24 | 18 |
| B0.7F-E | 10 | 51 | 30 | 19 | 44 | 36 | 20 |
| Catalyst | Mn(III)/Mn(IV) | Fe(III)/Fe(IV) | XPS OL/(Ba + Mn/Fe) (Nominal) |
|---|---|---|---|
| BM-E | 5.3 | - | 1.2 (1.5) |
| B0.7M-E | 2.1 | - | 1.2 (1.8) |
| BF | - | 0.6 | 1.1 (1.5) |
| B0.7F-E | - | 0.9 | 1.4 (1.3 1) |
| Catalyst | T50% (0.1% CO, 1% O2) (°C) | T50% (1% CO, 1% O2) (°C) | ΔT50% (°C) |
|---|---|---|---|
| BM-E | 364 | 446 | 82 |
| B0.7M-E | 249 | 307 | 58 |
| BF | 461 | 352 | −109 |
| B0.7F-E | 471 | 437 | −34 |
| 1% Pt/Al2O3 | 114 | 212 | 98 |
| Catalyst | 1% O2/He | 100% He | ||||
|---|---|---|---|---|---|---|
| T25% (°C) | T50% (°C) | SCO2 (%) | T25% (°C) | T50% (°C) | SCO2 (%) | |
| BM-E | 666 | 701 | 67 | 816 | 847 | 8 |
| B0.7M-E | 665 | 702 | 78 | 830 | 855 | 11 |
| BF | 674 | 710 | 71 | 794 | - | 42 |
| B0.7F-E | 653 | 686 | 63 | 892 | - | 33 |
| Uncatalyzed | 679 | 718 | 42 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Verde, Á.; Montilla-Verdú, S.; Torregrosa-Rivero, V.; Illán-Gómez, M.-J. Tailoring the Composition of BaxBO3 (B = Fe, Mn) Mixed Oxides as CO or Soot Oxidation Catalysts in Simulated GDI Engine Exhaust Conditions. Molecules 2023, 28, 3327. https://doi.org/10.3390/molecules28083327
Díaz-Verde Á, Montilla-Verdú S, Torregrosa-Rivero V, Illán-Gómez M-J. Tailoring the Composition of BaxBO3 (B = Fe, Mn) Mixed Oxides as CO or Soot Oxidation Catalysts in Simulated GDI Engine Exhaust Conditions. Molecules. 2023; 28(8):3327. https://doi.org/10.3390/molecules28083327
Chicago/Turabian StyleDíaz-Verde, Álvaro, Salvador Montilla-Verdú, Verónica Torregrosa-Rivero, and María-José Illán-Gómez. 2023. "Tailoring the Composition of BaxBO3 (B = Fe, Mn) Mixed Oxides as CO or Soot Oxidation Catalysts in Simulated GDI Engine Exhaust Conditions" Molecules 28, no. 8: 3327. https://doi.org/10.3390/molecules28083327






