Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum moldavica L. Cultivars
Abstract
:1. Introduction
2. Results
2.1. Total Polyphenolic, Flavonoid, and Phenolic Acid Content
2.2. LC–MS Analysis
2.3. Antioxidant Capacity Assays
2.4. Antibacterial Assays
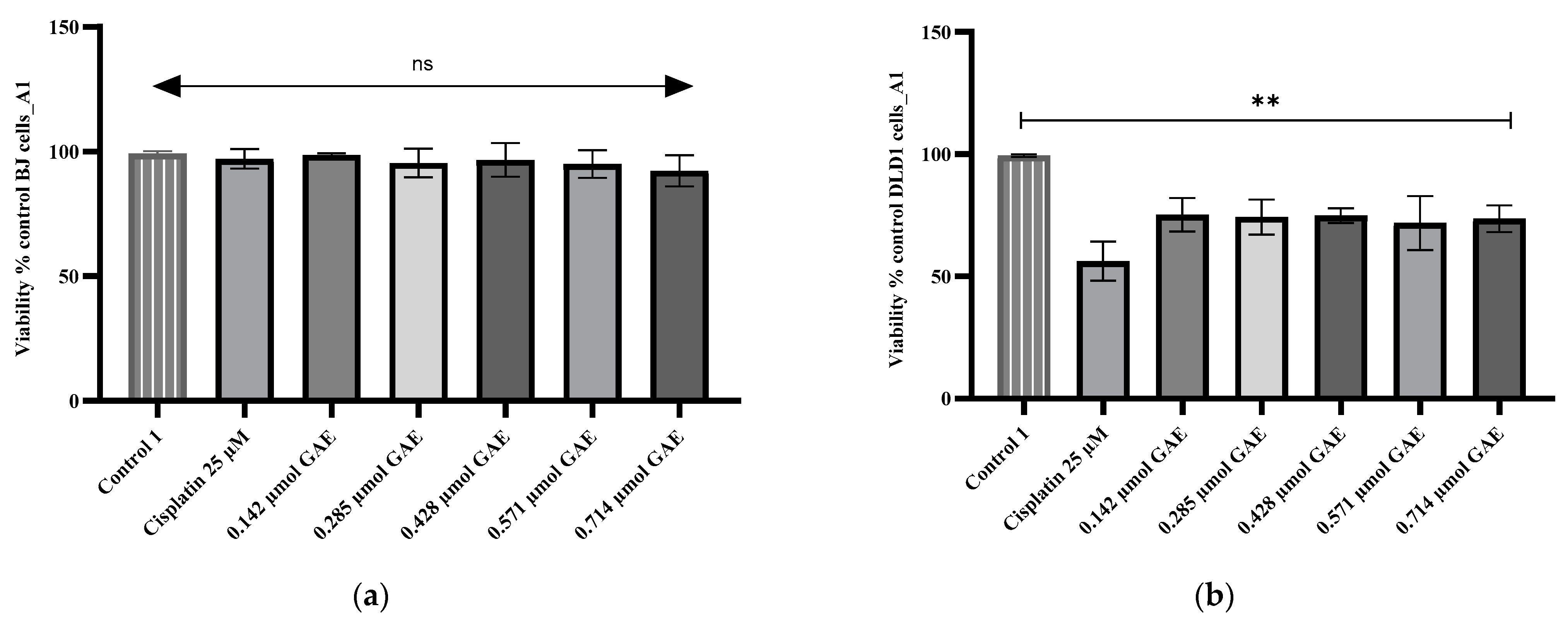
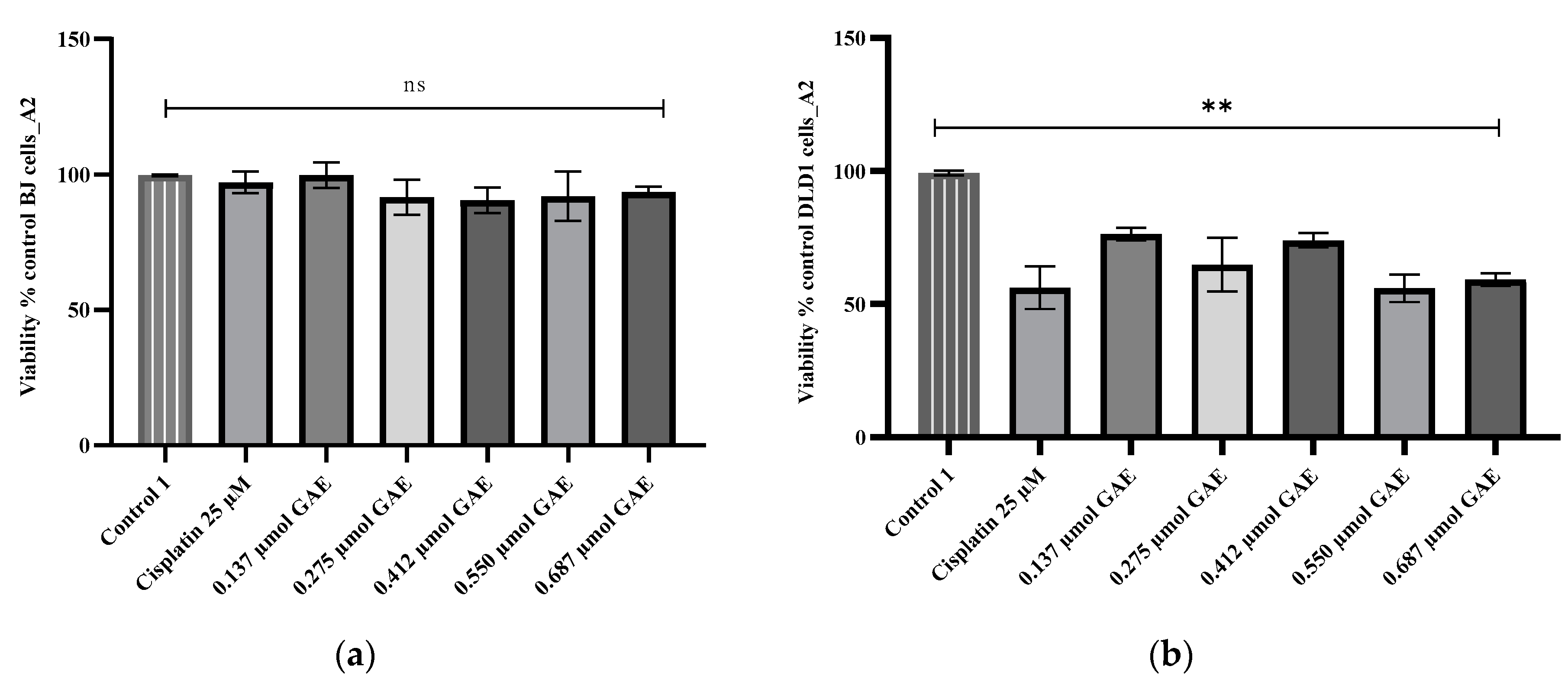
2.5. Cytotoxicity Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cultivation Conditions
- A1—Aroma 1, created in 1991 at the Institute of Genetics, Physiology, and Plant Protection, Chisinau (Republic of Moldova) (https://igfpp.md/certificate-soi-planta, accessed 17 November 2019), with blue flowers;
- A2—Aroma 2, created at the previously mentioned institution, with white flowers;
- B2—Buzău 2, created at the Vegetable Research–Development Station, Buzău‚ with blue flowers (Figure 4).
4.3. Preparation of Extracts
4.4. Total Polyphenolic, Flavonoid, and Phenolic Acid Content
4.5. LC–MS Analysis
LC–MS Apparatus
4.6. Antioxidant Capacity Assays
4.6.1. DPPH Radical Scavenging Activity
4.6.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
4.7. Antimicrobial Assays
4.8. Cytotoxicity Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aćimović, M.; Šovljanski, O.; Šeregelj, V.; Pezo, L.; Zheljazkov, V.D.; Ljujić, J.; Tomić, A.; Ćetković, G.; Čanadanović-Brunet, J.; Miljković, A.; et al. Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum Moldavica L. Essential Oil and Hydrolate. Plants 2022, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Said-Al Ahl, H.A.; Sabra, A.S.; El Gendy, A.N.G.; Aziz, E.E.; Tkachenko, K.G. Changes in Content and Chemical Composition of Dracocephalum Moldavica L. Essential Oil at Different Harvest Dates. J. Med. Plants Stud. 2015, 3, 61–64. [Google Scholar]
- Moldovan, C.; Nițu, S.; Hermeziu, M.; Vidican, R.; Sandor, M.; Gâdea, Ș.; David, A.; Stoian, V.A.; Vâtcă, S.D.; Stoian, V. Growth Characteristics of Dracocephalum Moldavica L. in Relation to Density for Sustainable Cropping Technology Development. Agriculture 2022, 12, 789. [Google Scholar] [CrossRef]
- Dmitruk, M.; Sulborska, A.; Żuraw, B.; Stawiarz, E.; Weryszko-Chmielewska, E. Sites of Secretion of Bioactive Compounds in Leaves of Dracocephalum Moldavica L.: Anatomical, Histochemical, and Essential Oil Study. Rev. Bras. Bot. 2019, 42, 701–715. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Rezaei-Chiyaneh, E.; Javanmard, A.; Maggi, F.; Morshedloo, M.R. Evaluation of Common Bean (Phaseolus Vulgaris L.) Seed Yield and Quali-Quantitative Production of the Essential Oils from Fennel (Foeniculum Vulgare Mill.) and Dragonhead (Dracocephalum Moldavica L.) in Intercropping System under Humic Acid Application. J. Clean. Prod. 2019, 235, 112–122. [Google Scholar] [CrossRef]
- Aslanipour, B.; Heidari, R.; Farnad, N. Phenolic Combination and Comparison of Antioxidant Activity in Three Different Alcoholic Extracts of Dracocephalum Moldavica L. Turkish J. Agric. Food Sci. Technol. 2017, 5, 199. [Google Scholar] [CrossRef]
- Faridvand, S.; Rezaei-Chiyaneh, E.; Battaglia, M.L.; Gitari, H.I.; Raza, M.A.; Siddique, K.H.M. Application of Bio and Chemical Fertilizers Improves Yield, and Essential Oil Quantity and Quality of Moldavian Balm (Dracocephalum Moldavica L.) Intercropped with Mung Bean (Vigna Radiata L.). Food Energy Secur. 2022, 11, e319. [Google Scholar] [CrossRef]
- Golparvar, A.R.; Hadipanah, A.; Gheisari, M.M.; Khaliliazar, R. Chemical Constituents of Essential Oil of Dracocephalum Moldavica L. And Dracocephalum Kotschyi Boiss. from Iran. Acta Agric. Slov. 2016, 107, 25–31. [Google Scholar] [CrossRef]
- Alaei, S. Essential Oil Content and Composition of Dracocephalum Moldavica under Different Irrigation Regimes. Int. J. Hortic. Sci. Technol. 2019, 6, 167–175. [Google Scholar] [CrossRef]
- Alaei, S.; Mahna, N. Comparison of Essential Oil Composition in Dracocephalum Moldavica in Greenhouse and Field. J. Essent. Oil-Bear. Plants 2013, 16, 346–351. [Google Scholar] [CrossRef]
- Fattahi, A.; Shakeri, A.; Tayarani-Najaran, Z.; Kharbach, M.; Segers, K.; Heyden, Y.V.; Taghizadeh, S.F.; Rahmani, H.; Asili, J. UPLC–PDA-ESI–QTOF–MS/MS and GC-MS Analysis of Iranian Dracocephalum Moldavica L. Food Sci. Nutr. 2021, 9, 4278–4286. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Rotinberg, P.; Trifan, A.; Luca, S.V.; Petreus, T.; Gille, E.; Miron, A. Antigenotoxic and Antioxidant Activities of a Polyphenolic Extract from European Dracocephalum Moldavica L. Ind. Crops Prod. 2016, 79, 248–257. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.J.D.; Oinonen, P.P.; Darwis, Y.; Laaksi, I.; Hiltunen, R. Chemical Composition and in Vitro Antioxidative Activity of a Lemon Balm (Melissa Officinalis L.) Extract. LWT Food Sci. Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Jöhrer, K.; Galarza Pérez, M.; Kircher, B.; Çiçek, S.S. Flavones, Flavonols, Lignans, and Caffeic Acid Derivatives from Dracocephalum Moldavica and Their In Vitro Effects on Multiple Myeloma and Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 14219. [Google Scholar] [CrossRef] [PubMed]
- Khattulanuar, F.S.; Sekar, M.; Fuloria, S.; Gan, S.H.; Mat Rani, N.N.I.; Ravi, S.; Chidambaram, K.; Begum, M.Y.; Azad, A.K.; Jeyabalan, S.; et al. Tilianin: A Potential Natural Lead Molecule for New Drug Design and Development for the Treatment of Cardiovascular Disorders. Molecules 2022, 27, 673. [Google Scholar] [CrossRef]
- Li, J.; Xu, S. Tilianin Attenuates MPP + -induced Oxidative Stress and Apoptosis of Dopaminergic Neurons in a Cellular Model of Parkinson’s Disease. Exp. Ther. Med. 2022, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yang, J.; Yu, P. Tilianin Promotes the Proliferation and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Curr. Top. Nutraceutical Res. 2022, 20, 259–264. [Google Scholar] [CrossRef]
- Ehsani, A.; Alizadeh, O.; Hashemi, M.; Afshari, A.; Aminzare, M. Phytochemical, Antioxidant and Antibacterial Properties of Melissa Officinalis and Dracocephalum Moldavica Essential Oils. Vet. Res. Forum. Int. Q. J. 2017, 8, 223–229. [Google Scholar]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Moradshahi, A. Evaluation of the Growth, Essential Oil Composition and Antioxidant Activity of Dracocephalum Moldavica under Water Deficit Stress and Symbiosis with Claroideoglomus Etunicatum and Micrococcus Yunnanensis. Sci. Hortic. 2019, 256, 108652. [Google Scholar] [CrossRef]
- Povilaityte, V.; Venskutonis, P.R. Antioxidative Activity of Purple Peril (Perilla Frutescens L.), Moldavian Dragonhead (Dracocephalum Moldavica L.), and Roman Chamomile (Anthemis Nobilis L.) Extracts in Rapeseed Oil. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 951–956. [Google Scholar] [CrossRef]
- Rezaie Keikhaie, K.; Jahantigh, H.R.; Bagheri, R.; Rezaie Kehkhaie, A. The Effects of the Ethanol Extract of Dracocephalum Moldavica (Badrashbu) Against Strains of Antibiotic-Resistant Escherichia Coli and Klebsiella Pneumonia. Int. J. Infect. 2017, 5. [Google Scholar] [CrossRef]
- Yu, H.; Liu, M.; Liu, Y.; Qin, L.; Jin, M.; Wang, Z. Antimicrobial Activity and Mechanism of Action of Dracocephalum Moldavica L.Extracts against Clinical Isolates of Staphylococcus Aureus. Front. Microbiol. 2019, 10, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, C.; Jiang, W.; Yang, X.; He, C.; Wang, W.; Xing, J. Pretreatment with Total Flavonoid Extract from Dracocephalum Moldavica L. Attenuates Ischemia Reperfusion-Induced Apoptosis. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yu, H.; Jin, X.; Yan, L.; Wang, J.; Wang, Z. Dracocephalum Moldavica L. Extracts Protect H9c2 Cardiomyocytes against H2O2-Induced Apoptosis and Oxidative Stress. Biomed Res. Int. 2020, 2020, 8379358. [Google Scholar] [CrossRef] [PubMed]
- Dawuti, A.; Sun, S.; Wang, R.; Gong, D.; Yuan, T.; Zhang, L.; Yang, S.; Xing, J.; Zheng, R.; Lu, Y.; et al. Systems Pharmacology-Based Strategy to Investigate Pharmacological Mechanisms of Total Flavonoids in Dracocephalum Moldavica on Chronic Heart Failure. Int. J. Mol. Sci. 2022, 23, 8409. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-X.; Zhang, Y.; Wang, Z.-L.; Yan, X.-S.; Jin, M.; Huo, D.-S.; Wang, H.; Yang, Z.-J. The Inhibitory Effects of Dracocephalum Moldavica L. (DML) on Rat Cerebral Ischemia Reperfusion Injury. J. Toxicol. Environ. Heal. Part A Curr. Issues 2017, 80, 1206–1211. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.; Wang, T.; Chen, H.; Zhao, H.; Yan, X.; Wang, Z.; Sun, X.; Zheng, Q. Antioxidative and Cardioprotective Effects of Total Flavonoids Extracted from Dracocephalum Moldavica L. against Acute Ischemia/Reperfusion-Induced Myocardial Injury in Isolated Rat Heart. Cardiovasc. Toxicol. 2014, 14, 74–82. [Google Scholar] [CrossRef]
- Nie, L.; Li, R.; Huang, J.; Wang, L.; Ma, M.; Huang, C.; Wu, T.; Yan, R.; Hu, X. Abietane Diterpenoids from Dracocephalum Moldavica L. and Their Anti-Inflammatory Activities in Vitro. Phytochemistry 2021, 184, 112680. [Google Scholar] [CrossRef]
- Maham, M.; Akbari, H.; Delazar, A. Chemical Composition and Antinociceptive Effect of the Essential Oil of Dracocephalum Moldavica L. Pharm. Sci. 2013, 18, 187–192. [Google Scholar]
- Deepa, P.; Bae, H.J.; Park, H.B.; Kim, S.Y.; Choi, J.W.; Kim, D.H.; Liu, X.Q.; Ryu, J.H.; Park, S.J. Dracocephalum Moldavica Attenuates Scopolamine-Induced Cognitive Impairment through Activation of Hippocampal ERK-CREB Signaling in Mice. J. Ethnopharmacol. 2020, 253, 112651. [Google Scholar] [CrossRef]
- Barchuk, O.; Pryshlyak, A.; Shanaida, M. Chemical Compositions and Sedative Activities of the Dracocephalum Moldavica L. and Ocimum Americanum L. Essential Oils. Pharmacologyonline 2021, 2, 179–187. [Google Scholar]
- Wang, J.; Jin, M.; Sun, J.; Qi, Y.; Cui, L.; Wang, M.; Zhou, W.; Li, G. Triterpenes from Dracocephalum Moldavica with Cytotoxic Activities. Chem. Nat. Compd. 2022, 58, 581–583. [Google Scholar] [CrossRef]
- Zúñiga, M.I.J.; Mariles, A.J.H.; Flores, J.L.C.; Herrera, J.A.M.; Sotelo, M.G.R.; Montes, G.I.C.; De Las Mercedes Gómez Y Gómez, Y. Antidepressant-like Effects of Dracocephalum Moldavica L. In Mouse Models of Immobility Tests. Pharmacogn. J. 2019, 11, 976–983. [Google Scholar] [CrossRef]
- Nadeem, A.; Khubaib, H.M.; Samee, A.; Ali, A.; Kamal, S.; Ansari, N.; Rehman, M.; Roohi, A.; Ali, M.F. Novel Development of Nutritive, Enhanced Bioactivities, Antioxidant, Antimicrobial, Antiparasitic, DNA Protection and New Insights. Saudi J. Pathol. Microbiol. 2022, 7, 228–232. [Google Scholar] [CrossRef]
- Abidullah, S.; Rauf, A.; Zaman, W.; Ullah, F.; Ayaz, A.; Batool, F.; Saqib, S. Consumption of Wild Food Plants among Tribal Communities of Pak-Afghan Border, near Bajaur, Pakistan. Acta Ecol. Sin. 2022. [Google Scholar] [CrossRef]
- Saquib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Vârban, R.; Vidican, R.; Ona, A.D.; Vârban, D.; Stoie, A.; Gâdea, Ş.; Vâtcă, S.; Stoian, V.; Crisan, I.; Stoian, V. Modelling Plant Morphometric Parameters as Predictors for Successful Cultivation of Some Medicinal Agastache Species. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12638. [Google Scholar] [CrossRef]
- Abidullah, S.; Rauf, A.; Khan, S.W.; Ayaz, A.; Liaquat, F.; Saqib, S. A Comprehensive Review on Distribution, Paharmacological Uses and Biological Activities of Argyrolobium Roseum (Cambess.) Jaub. & Spach. Acta Ecol. Sin. 2022, 42, 198–205. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Daryai, F.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Yousefzadeh, T.; Mohammadi, K. Morphological, Essential Oil and Biochemical Variation of Dracocephalum Moldavica L. Populations. J. Appl. Res. Med. Aromat. Plants 2018, 10, 59–66. [Google Scholar] [CrossRef]
- Nasiri, Y. Crop Productivity and Chemical Compositions of Dragonhead (Dracocephalum Moldavica L.) Essential Oil under Different Cropping Patterns and Fertilization. Ind. Crops Prod. 2021, 171, 113920. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Amirnia, R.; Fotohi Chiyaneh, S.; Maggi, F.; Barin, M.; Razavi, B.S. Improvement of Dragonhead (Dracocephalum Moldavica L.) Yield Quality through a Coupled Intercropping System and Vermicompost Application along with Maintenance of Soil Microbial Activity. L. Degrad. Dev. 2021, 32, 2833–2848. [Google Scholar] [CrossRef]
- Simea, S.A.; Duda, M.; Ghete, A.; Muresan, C.; Crışan, I. The Importance and Use of the Species Dracocepahlum Moldavica. Hop Med. Plants 2018, 26, 39–43. [Google Scholar]
- Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.C.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants 2022, 11, 2524. [Google Scholar] [CrossRef]
- Toma, C.C.; Simu, G.M.; Hanganu, D.; Olah, N.; Vata, F.M.G.; Hammami, C.; Hammami, M. Chemical Composition of the Tunisian Nigella Sativa. Note I. Profile on Essential Oil. Farmacia 2010, 58, 458–464. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B. Interactive Effects of Abscisic Acid and Temperature on Rosmarinic Acid, Total Phenolic Compounds, Anthocyanin, Carotenoid and Flavonoid Content of Dragonhead (Dracocephalum Moldavica L.). Sci. Hortic. 2019, 250, 302–309. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-Dependent Effect of Exogenous Abscisic Acid on Photosynthesis, Growth and Phenolic Content of Dracocephalum Moldavica L. under Drought Stress. Planta 2021, 253, 1–18. [Google Scholar] [CrossRef]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Zarei, M. Mitigation of Water Deficit Stress in Dracocephalum Moldavica by Symbiotic Association with Soil Microorganisms. Sci. Hortic. 2020, 272, 109549. [Google Scholar] [CrossRef]
- Naseri, A.; Alirezalu, A.; Noruzi, P.; Alirezalu, K. The Effect of Different Ammonium to Nitrate Ratios on Antioxidant Activity, Morpho-Physiological and Phytochemical Traits of Moldavian Balm (Dracocephalum Moldavica). Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the Enzymatic and Non-Enzymatic Antioxidant Defense by Applying Iron Oxide Nanoparticles in Dracocephalum Moldavica L. Plant under Salinity Stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.; Gwon, H.; Lee, K.Y.; Choi, S.G.; Atiqual Islam, M.; Chun, J.; Hwang, J. Phytochemical Profile and Antioxidant Activity of Dracocephalum Moldavica L. Seed Extracts Using Different Extraction Methods. Food Chem. 2021, 350, 128531. [Google Scholar] [CrossRef]
- Xing, J.; Peng, K.; Cao, W.; Lian, X.; Wang, Q.; Wang, X. Effects of Total Flavonoids from Dracocephalum Moldavica on the Proliferation, Migration, and Adhesion Molecule Expression of Rat Vascular Smooth Muscle Cells Induced by TNF-α. Pharm. Biol. 2013, 51, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Aisa, H.A.; Aisa, H.A.; Eshbakova, K.A. Flavonoids from Dracocephalum Moldavica. Chem. Nat. Compd. 2008, 44, 366–367. [Google Scholar] [CrossRef]
- Wu, C.; Liu, H.; Rong, X.; Liu, J.; Ding, W.; Cheng, X.; Xing, J.; Wang, C. Phytochemical Composition Profile and Space–Time Accumulation of Secondary Metabolites for Dracocephalum Moldavica Linn. via UPLC–Q/TOF–MS and HPLC–DAD Method. Biomed. Chromatogr. 2020, 34, e4865. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeil, M.; Sabzi-Nojadeh, M.; Movafeghi, A.; Aghbash, B.N.; Kosari-Nasab, M.; Zengin, G.; Maggi, F. Phytotoxic Activity of Moldavian Dragonhead (Dracocephalum Moldavica L.) Essential Oil and Its Possible Use as Bio-Herbicide. Process Biochem. 2022, 114, 86–92. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, L.; Dong, X.; Guo, S.; Xing, J.; Li, Z.; Liu, R. Tilianin Extracted From Dracocephalum Moldavica L. Induces Intrinsic Apoptosis and Drives Inflammatory Microenvironment Response on Pharyngeal Squamous Carcinoma Cells via Regulating TLR4 Signaling Pathways. Front. Pharmacol. 2020, 11, 205. [Google Scholar] [CrossRef]
- Crisan, I.; Vidican, R.; Stole, A.; Simea, S.A. Spring-Autumn Arbuscular Mycorrhiza Colonization Dynamic in Iris Germanica L. from Urban Microclimate. Agrolife Sci. J. 2020, 9, 82–90. [Google Scholar]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ştefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa Vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Mihart, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-KB Pathways in Rats With Experimentally Induced Hyperglycemia by Administration of Thymus Marshallianus Willd. Extracts. Front. Pharmacol. 2020, 11, 581470. [Google Scholar] [CrossRef]
- Ielciu, I.; Filip, G.A.; Oniga, I.; Olah, N.K.; Bâldea, I.; Olteanu, D.; Burtescu, R.F.; Turcuș, V.; Sevastre-Berghian, A.C.; Benedec, D.; et al. Oxidative Stress and Dna Lesion Reduction of a Polyphenolic Enriched Extract of Thymus Marschallianus Willd. In Endothelial Vascular Cells Exposed to Hyperglycemia. Plants 2021, 10, 2810. [Google Scholar] [CrossRef]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Cioc, N. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus Marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines & Health Care: Strasbourg, France, 2022.
- Ielciu, I.; Frederich, M.; Hanganu, D.; Angenot, L.; Olah, N.-K.; Ledoux, A.; Crisan, G.; Păltinean, R. Flavonoid Analysis and Antioxidant Activities of the Bryonia Alba L. Aerial Parts. Antioxidants 2019, 8, 108. [Google Scholar] [CrossRef]
- Ielciu, I.; Vlase, L.; Frédérich, M.; Hanganu, D.; Păltinean, R.; Cieckiewicz, E.; Olah, N.-K.; Gheldiu, A.-M.; Crişan, G. Polyphenolic Profile and Biological Activities of the Leaves and Aerial Parts of Echinocystis Lobata (Michx.) Torr. et A. Gray (Cucurbitaceae). Farmacia 2017, 65, 179–183. [Google Scholar]
- Ielciu, I.; Hanganu, D.; Păltinean, R.; Vlase, L.; Frédérich, M.; Gheldiu, A.-M.; Benedec, D.; Crişan, G. Antioxidant Capacity and Polyphenolic Content of the Echinocystis Lobata (Michx.) Torr. et A.Gray Flowers. Pak. J. Pharm. Sci. 2018, 31 (Suppl. S2), 677–683. [Google Scholar]
- Ielciu, I.; Niculae, M.; Pall, E.; Barbălată, C.; Tomuţă, I.; Olah, N.K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and Antimicrobial Effects of Rosmarinus Officinalis L. Loaded Liposomes. Molecules 2022, 27, 3988. [Google Scholar] [CrossRef]
- Buza, V.; Niculae, M.; Hanganu, D.; Pall, E.; Burtescu, R.F.; Olah, N.K.; Matei-Lațiu, M.C.; Vlasiuc, I.; Iozon, I.; Szakacs, A.R.; et al. Biological Activities and Chemical Profile of Gentiana Asclepiadea and Inula Helenium Ethanolic Extracts. Molecules 2022, 27, 3560. [Google Scholar] [CrossRef]
- Mot, A.C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. Redox Reactivity in Propolis: Direct Detection of Free Radicals in Basic Medium and Interaction with Hemoglobin. Redox Rep. 2009, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Filip, L.; Oniga, I.; Tiperciuc, B.; Olah, N.-K.; Gheldiu, A.-M.; Raita, O.; Vlase, L. Chemical, Antioxidant and Antibacterial Studies of Romanian Heracleum Sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chişe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus Officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing, (EUCAST). Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method; 2020; Available online: https://www.eucast.org/ (accessed on 30 December 2022).
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria Schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Faryad, S.; Afridi, M.I.; Arshad, B.; Younas, M.; Naeem, M.; Zaman, W.; Ullah, F.; Nisar, M.; Ali, S.; et al. Bimetallic Assembled Silver Nanoparticles Impregnated in Aspergillus Fumigatus Extract Damage the Bacterial Membrane Surface and Release Cellular Contents. Coatings 2022, 12, 1505. [Google Scholar] [CrossRef]
- Sevastre, B.; Sárpataki, O.; Stan, R.L.; Taulescu, M.; Sevastre-Berghian, A.C.; Olah, N.K.; Furtuna, F.; Hanganu, D.; Hangan, A.C.; Cenariu, M.; et al. Anticancer Activity of Euonymus Europaeus Fruits Extract on Human Melanoma Cells. Farmacia 2017, 65, 56–62. [Google Scholar]


| Sample | TPC (g RAE/100 g Dry Plant Material) | TPC (g GAE/100 g Dry Plant Material) | TFC (g RE/100 g Dry Plant Material) | TPAC (g RAE/100 g Dry Plant Material) |
|---|---|---|---|---|
| A1 | 4.644 ± 0.135 | 4.862 ± 0.163 | 1.218 ± 0.096 | 2.407 ± 0.261 * |
| A2 | 4.474 ± 0.115 | 4.620 ± 0.151 | 1.100 ± 0.063 | 2.116 ± 0.176 |
| B2 | 5.363 ± 0.157 ** | 5.631 ± 0.175 ** | 1.255 ± 0.167 | 2.711 ± 0.414 ** |
| Name of Reference or Separated Compound | Reference | Separated Compound | Samples | ||||
|---|---|---|---|---|---|---|---|
| Retention Time (min) | Main MS Transition | Retention Time (min) | Main MS Transition | A1 Content (mg/mL) | A2 Content (mg/mL) | B2 Content (mg/mL) | |
| Caffeic acid | 13.5 | 179.0 > 135.0 | 13.7 | 179.0 > 135.0 | 0.500 ± 0.0163 | 0.477 ± 0.0262 | 0.527 ± 0.0330 |
| Chlorogenic acid | 11.9 | 353.0 > 191.0 | 12.0 | 353.0 > 191.0 | 0.287 ± 0.0287 | 0.237 ± 0.0249 | 0.273 ± 0.0125 |
| trans-p-coumaric acid | 17.4 | 163.0 > 119.0 | 17.5 | 163.0 > 119.0 | 0.130 ± 0.0216 | 0.107 ± 0.0125 | 0.177 ± 0.0287 |
| Ellagic acid | 27.2 | 301.0 > 185.0 | 26.9 | 301.0 > 185.0 | 0.597 ± 0.0411 | 0.393 ± 0.0368 | 0.147 ± 0.0205 |
| Ferulic acid | 18.4 | 193.0 > 134.0 | 18.5 | 193.0 > 134.0 | 0.012 ± 0.0021 | 0.014 ± 0.0022 | 0.013 ± 0.0017 |
| Rosmarinic acid | 21.4 | 358.9 > 161.0 | 21.3 | 358.9 > 161.0 | 5.833 ± 0.0624 | 5.337 ± 0.0411 | 6.320 ± 0.0535 |
| Salicylic acid | 23.5 | 137.0 > 93.0 | 23.4 | 137.0 > 93.0 | 0.098 ± 0.0155 | 0.098 ± 0.0155 | 0.085 ± 0.0041 |
| Carnosic acid | 32.0 | 331.2 > 285.1 | 30.6 | 331.2 > 285.1 | 0.098 ± 0.0165 | 0.098 ± 0.0165 | 0.098 ± 0.0165 |
| Carnosol | 30.6 | 329.1 > 285.1 | 30.4 | 329.1 > 285.1 | 0.003 ± 0.0005 | 0.003 ± 0.0005 | 0.003 ± 0.0005 |
| Apigenin | 28.1 | 269.0 > 117.0 | 28.1 | 269.0 > 117.0 | 0.967 ± 0.0492 | 0.487 ± 0.0492 | 0.767 ± 0.0386 |
| Hyperoside | 20.3 | 463.1 > 300.0 | 20.6 | 463.1 > 300.0 | 0.068 ± 0.0040 | 0.370 ± 0.0327 | 0.038 ± 0.0033 |
| Luteolin | 26.8 | 287.0 > 153.0 | 26.8 | 287.0 > 153.0 | 0.019 ± 0.0017 | 0.010 ± 0.0012 | 0.020 ± 0.0041 |
| Luteolin-7-O-glucoside | 19.9 | 447.0 > 284.9 | 19.8 | 447.0 > 284.9 | 0.820 ± 0.0327 | 0.787 ± 0.0330 | 0.667 ± 0.0655 |
| Naringenin | 26.2 | 271.0 > 119.0 | 26.2 | 271.0 > 119.0 | 0.004 ± 0.0005 | 0.004 ± 0.0005 | 0.005 ± 0.0005 |
| Quercetin | 25.4 | 300.9 > 151.0 | 25.4 | 300.9 > 151.0 | 0.003 ± 0.0009 | 0.006 ± 0.0008 | 0.003 ± 0.0008 |
| Rutoside | 20.2 | 609.0 > 300.0 | 20.3 | 609.0 > 300.0 | 0.068 ± 0.0029 | 0.062 ± 0.0053 | 0.049 ± 0.0054 |
| Vitexin | 18.4 | 431.0 > 311.0 | 18.5 | 431.0 > 311.0 | 0.004 ± 0.0005 | 0.004 ± 0.0005 | 0.003 ± 0.0005 |
| Sample | DPPH IC50 (µg/mL) | FRAP µmol Trolox Equivalent/g Dry Plant Material |
|---|---|---|
| A1 | 40.901 ± 0.161 | 293.194 ± 0.213 |
| A2 | 35.542 ± 0.043 ** | 301.493 ± 0.115 |
| B2 | 35.650 ± 0.063 ** | 330.165 ± 0.754 ** |
| Ascorbic acid | 5.691 ± 0.123 | - |
| Zone of Inhibition (mm) | ||||
|---|---|---|---|---|
| Sample | MSSA | MRSA | Escherichia coli | Pseudomonas aeruginosa |
| A1 | 19.67 ± 0.52 | 17.33 ± 0.52 | 15.50 ± 0.55 | 0 |
| A2 | 20.17 ± 0.41 | 18.17 ± 0.41 | 15.83 ± 0.41 | 0 |
| B2 | 23.50 ± 0.55 a, b, d | 20.50 ± 0.55 a, b, d | 17.33 ± 0.52 | 0 |
| Amoxicillin–clavulanic acid | 29 ± 0.00 a, b, c | 28 ± 0.00 a, b, c | 19 ± 0.00 a, b, c | 0 |
| Gentamicin | 20 ± 0.00 | 17 ± 0.00 | 19 ± 0.00 | 18 ± 0.00 |
| MIC Index MBC (μmol GAE/100 μL)/MIC (μmol GAE/100 μL) | |||
|---|---|---|---|
| Sample | MSSA | MRSA | Escherichia coli |
| A1 | 1 0.356/0.356 | 2 0.712/0.356 | 1 0.712/0.712 |
| A2 | 2 0.343/0.171 | 4 0.687/0.171 | 4 2.750/0.687 |
| B2 | 2 0.825/0.412 | 4 0.825/0.206 | 4 3.300/0.825 |
| Parameters of Analysis | Analytical Methods | Values |
|---|---|---|
| pH | Potentiometric | 8.05 |
| Humus | Walkley–Black | 3.24% |
| Nitrogen | Kjeldahl | 0.129% |
| Phosphorus | Colorimetric | 224 ppm |
| Potassium | Flame photometry | 304 ppm |
| Basic cation saturation | Kappen | 20.96 m/100 g |
| CaCO3 | Scheibler | 10.4 m/100 g |
| Base saturation (V) | Calculation | 92 |
| Granulometric | Kacinscki | clay loam |
| Cultivars (Samples) | Average Mass of a Plant (g) | Drying Yield | |
|---|---|---|---|
| Fresh 27 July 2020 | Dry 8 August 2020 | ||
| A1 | 223.3 | 54.1 | 1/4.13 |
| A2 | 205.8 | 51.8 | 1/3.97 |
| B2 | 212.5 | 55.2 | 1/3.85 |
| Time (min) | % Methanol | % Water | % of 2% Formic Acid in Water |
|---|---|---|---|
| 0.00 | 5 | 90 | 5 |
| 3.00 | 15 | 70 | 15 |
| 6.00 | 15 | 70 | 15 |
| 9.00 | 21 | 58 | 21 |
| 13.00 | 21 | 58 | 21 |
| 18.00 | 30 | 41 | 29 |
| 22.00 | 30 | 41 | 29 |
| 26.00 | 50 | 0 | 50 |
| 29.00 | 50 | 0 | 50 |
| 29.01 | 5 | 90 | 5 |
| 35.00 | 5 | 90 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simea, Ș.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Burtescu, R.F.; Olah, N.-K.; Cenariu, M.; Oniga, I.; Benedec, D.; et al. Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum moldavica L. Cultivars. Molecules 2023, 28, 1604. https://doi.org/10.3390/molecules28041604
Simea Ș, Ielciu I, Hanganu D, Niculae M, Pall E, Burtescu RF, Olah N-K, Cenariu M, Oniga I, Benedec D, et al. Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum moldavica L. Cultivars. Molecules. 2023; 28(4):1604. https://doi.org/10.3390/molecules28041604
Chicago/Turabian StyleSimea, Ștefania, Irina Ielciu, Daniela Hanganu, Mihaela Niculae, Emoke Pall, Ramona Flavia Burtescu, Neli-Kinga Olah, Mihai Cenariu, Ilioara Oniga, Daniela Benedec, and et al. 2023. "Evaluation of the Cytotoxic, Antioxidative and Antimicrobial Effects of Dracocephalum moldavica L. Cultivars" Molecules 28, no. 4: 1604. https://doi.org/10.3390/molecules28041604





