The Last Decade of Optically Active α-Aminophosphonates
Abstract
:1. Introduction
2. Synthetic Approaches for Optically Active α-Aminophosphonates
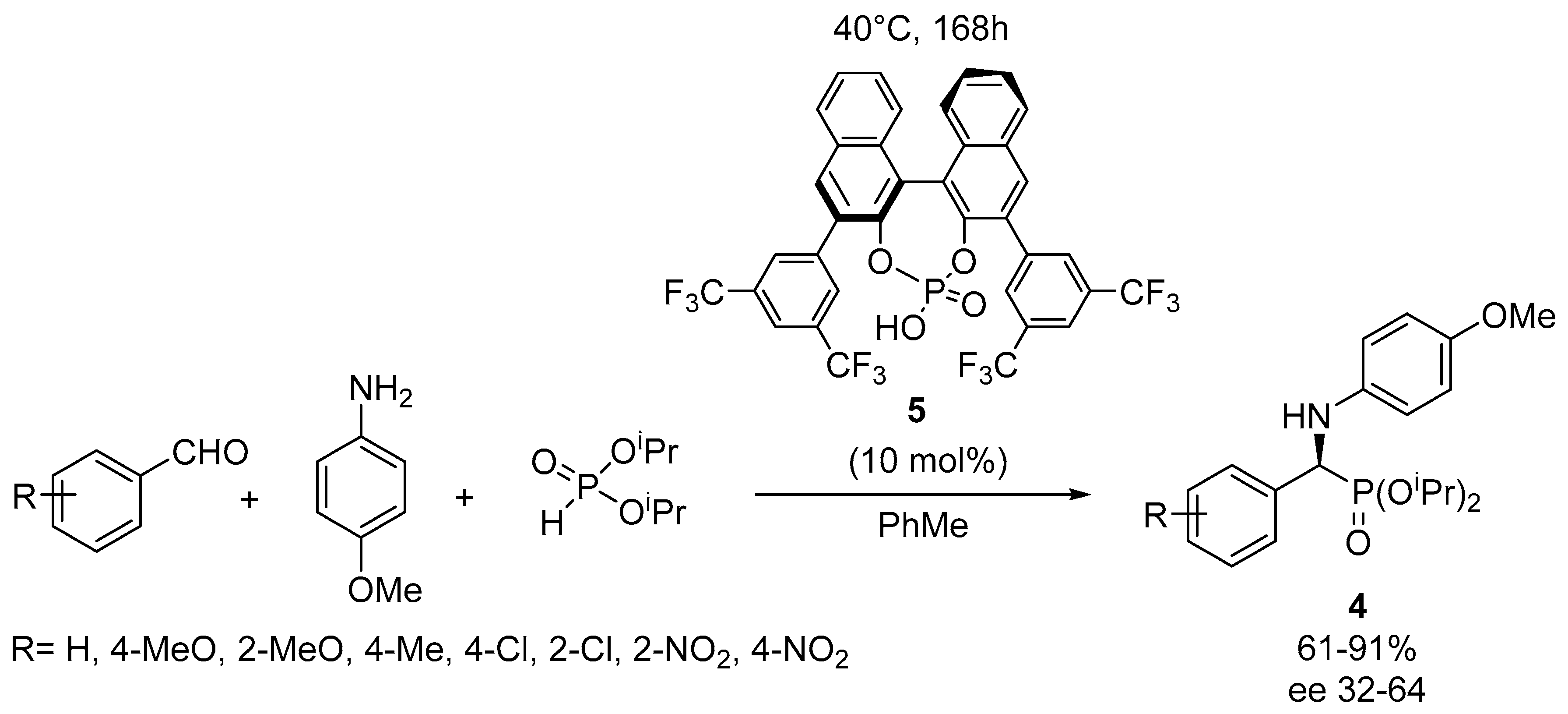
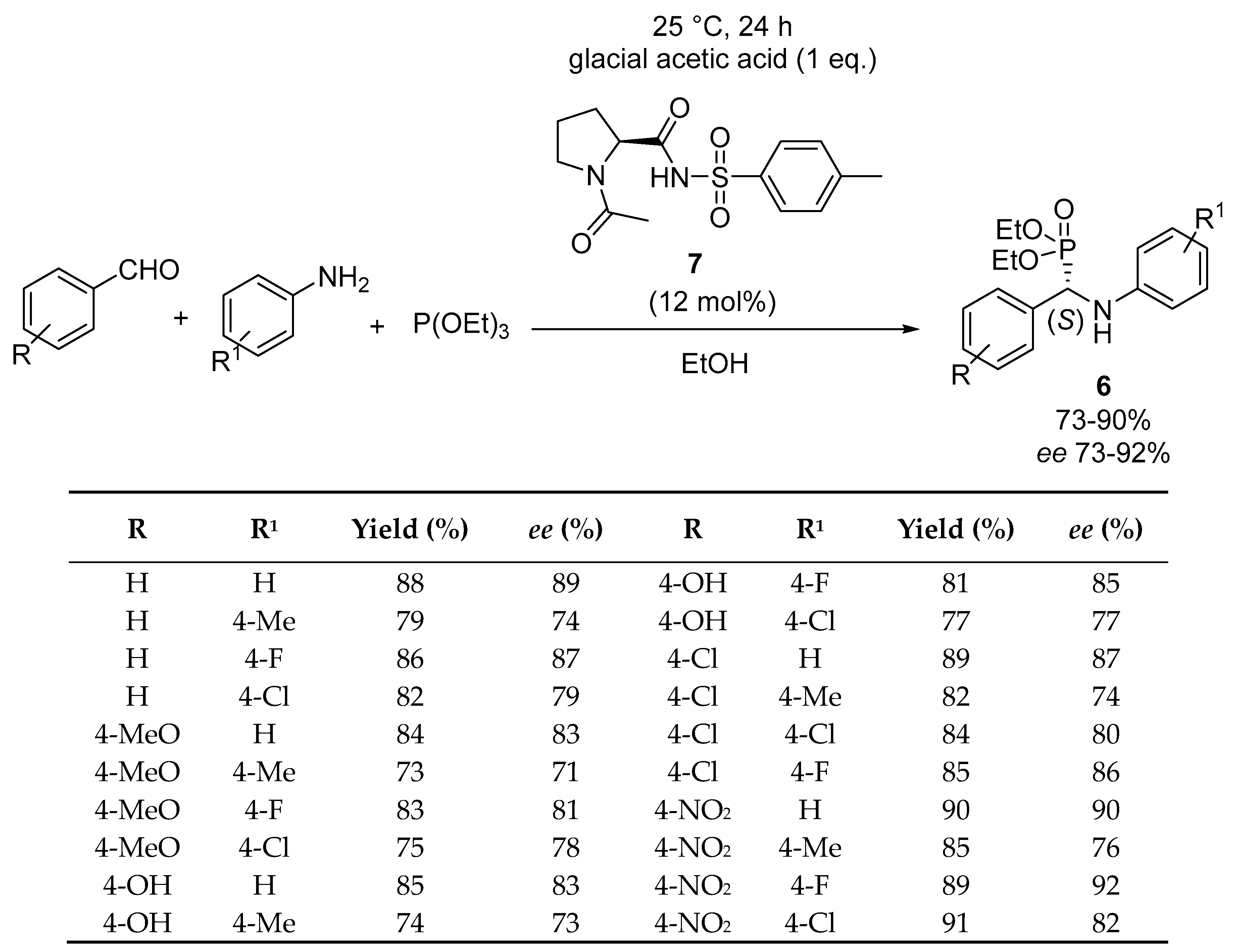
2.1. Enantioselective Kabachnik–Fields Reactions
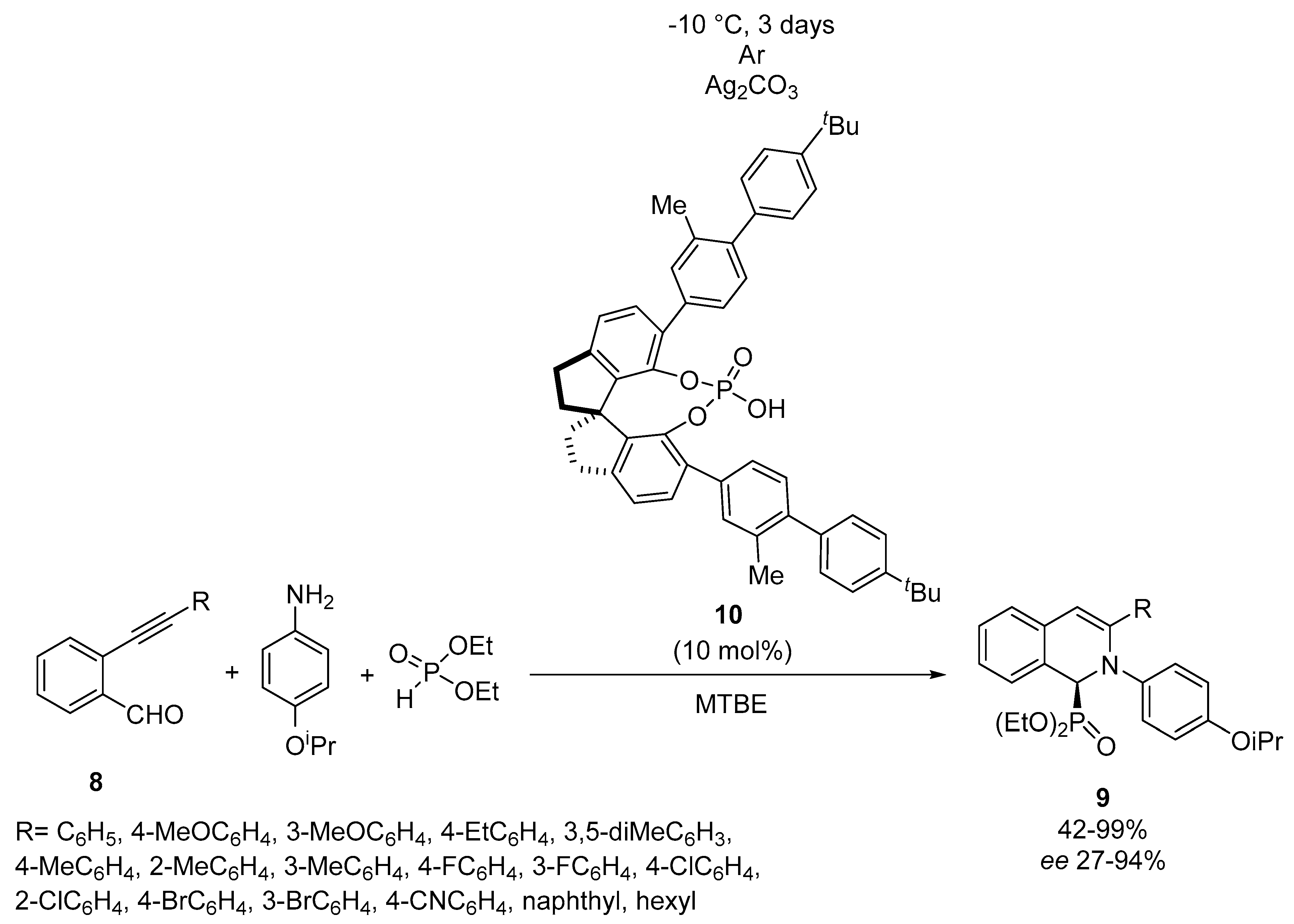
2.2. Optically Active α-Aminophosphonate Derivatives Provided by the Aza-Pudovik Reaction
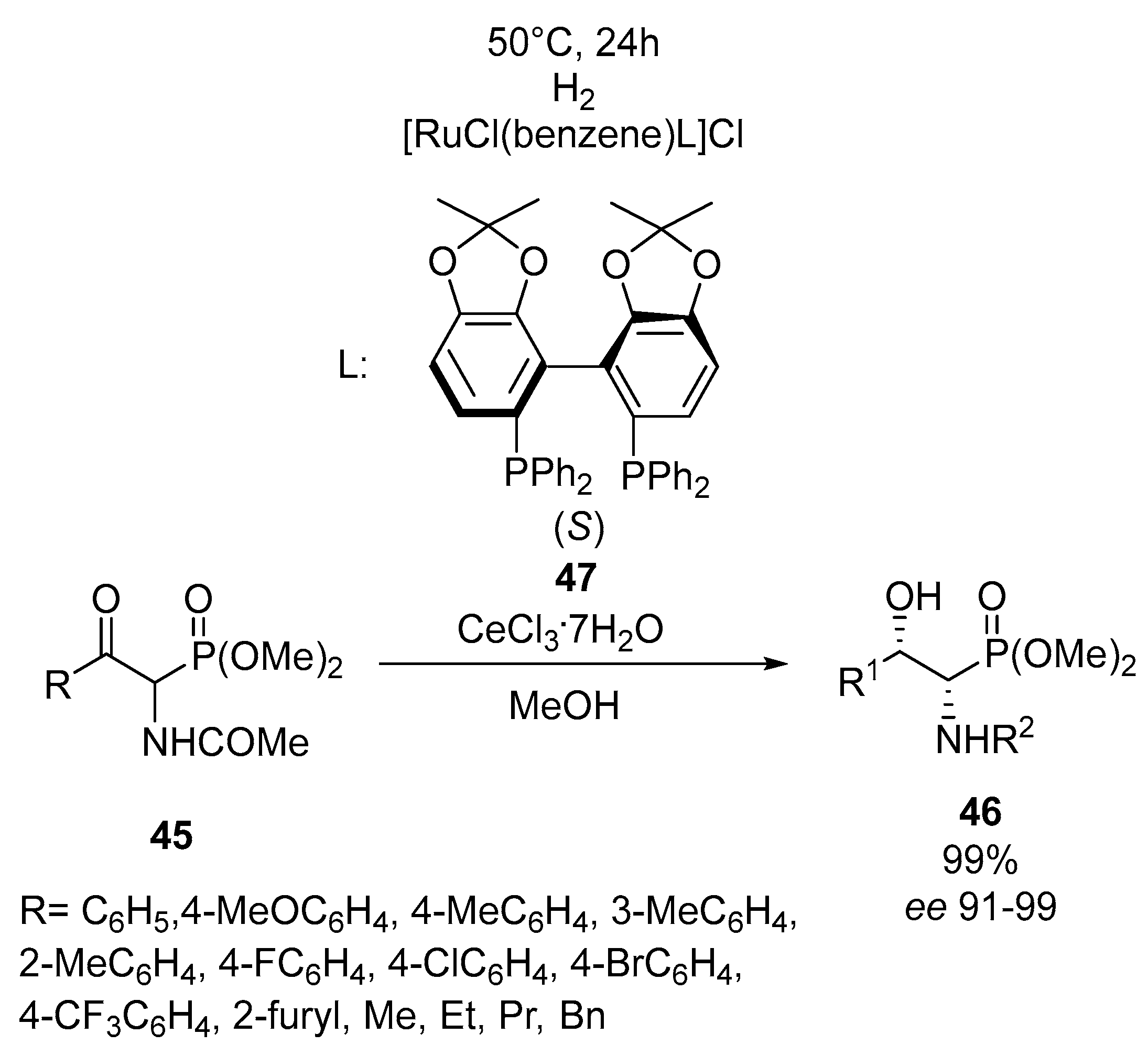
2.3. Synthesis of Enantiopure α-Aminophosphonates by the Asymmetric Hydrogenation of Iminophosphonates
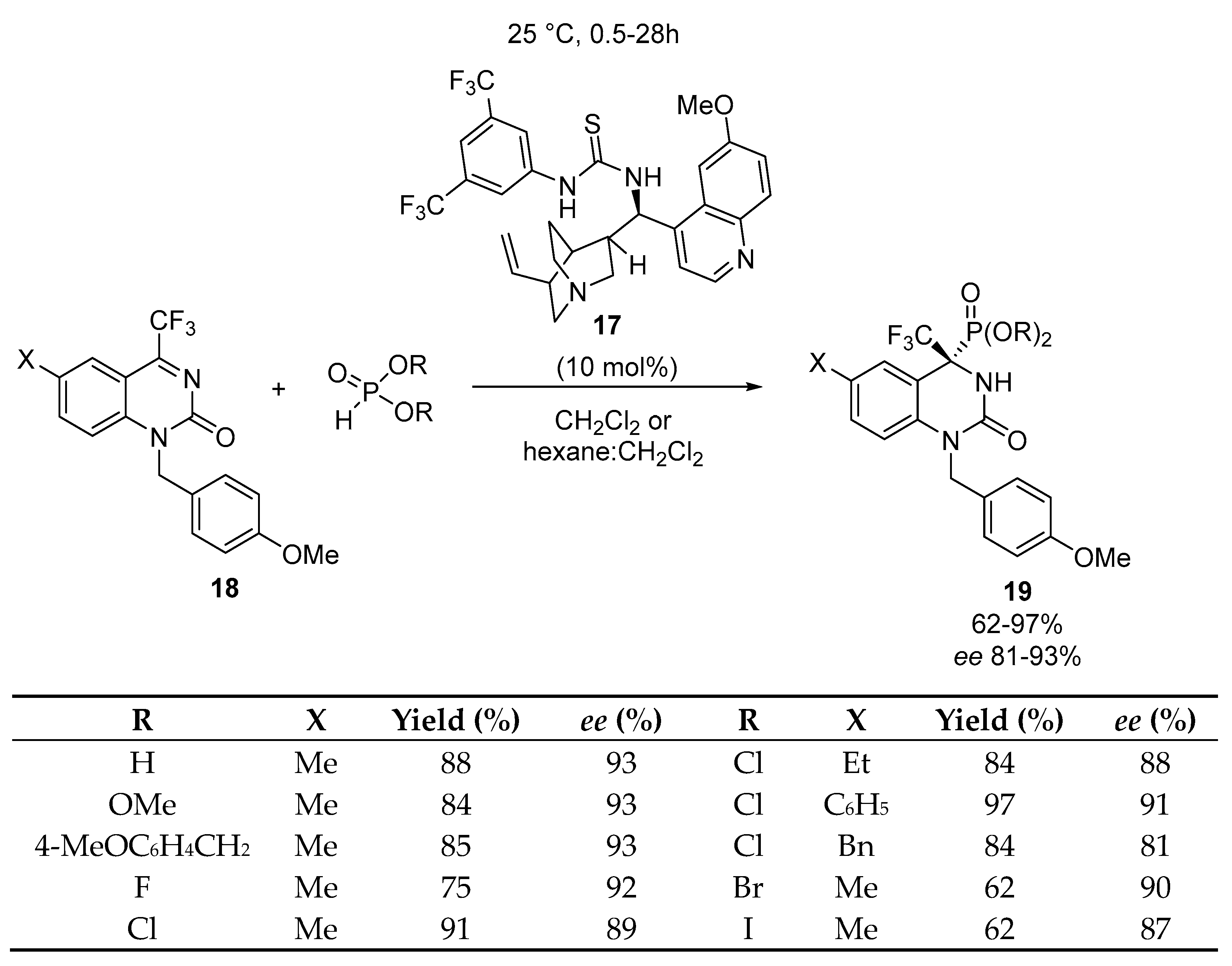
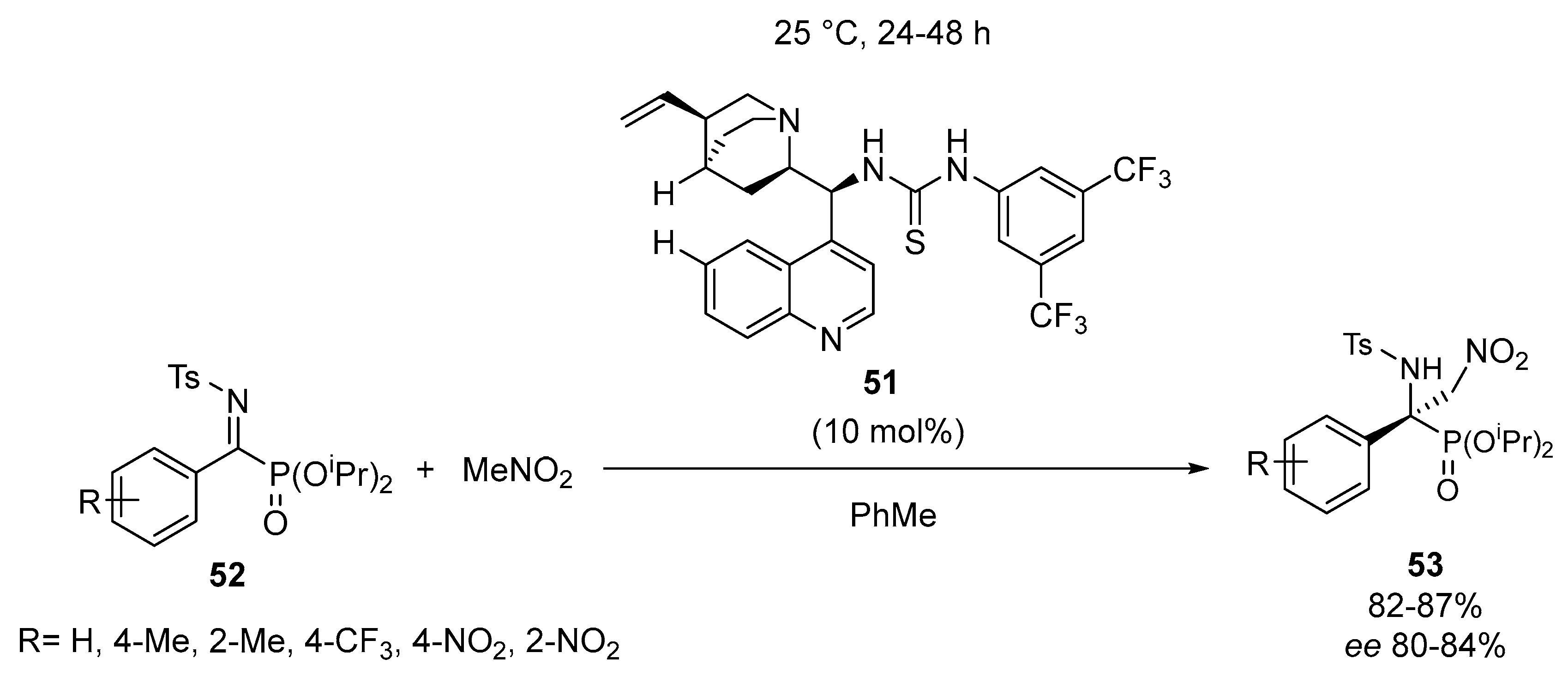
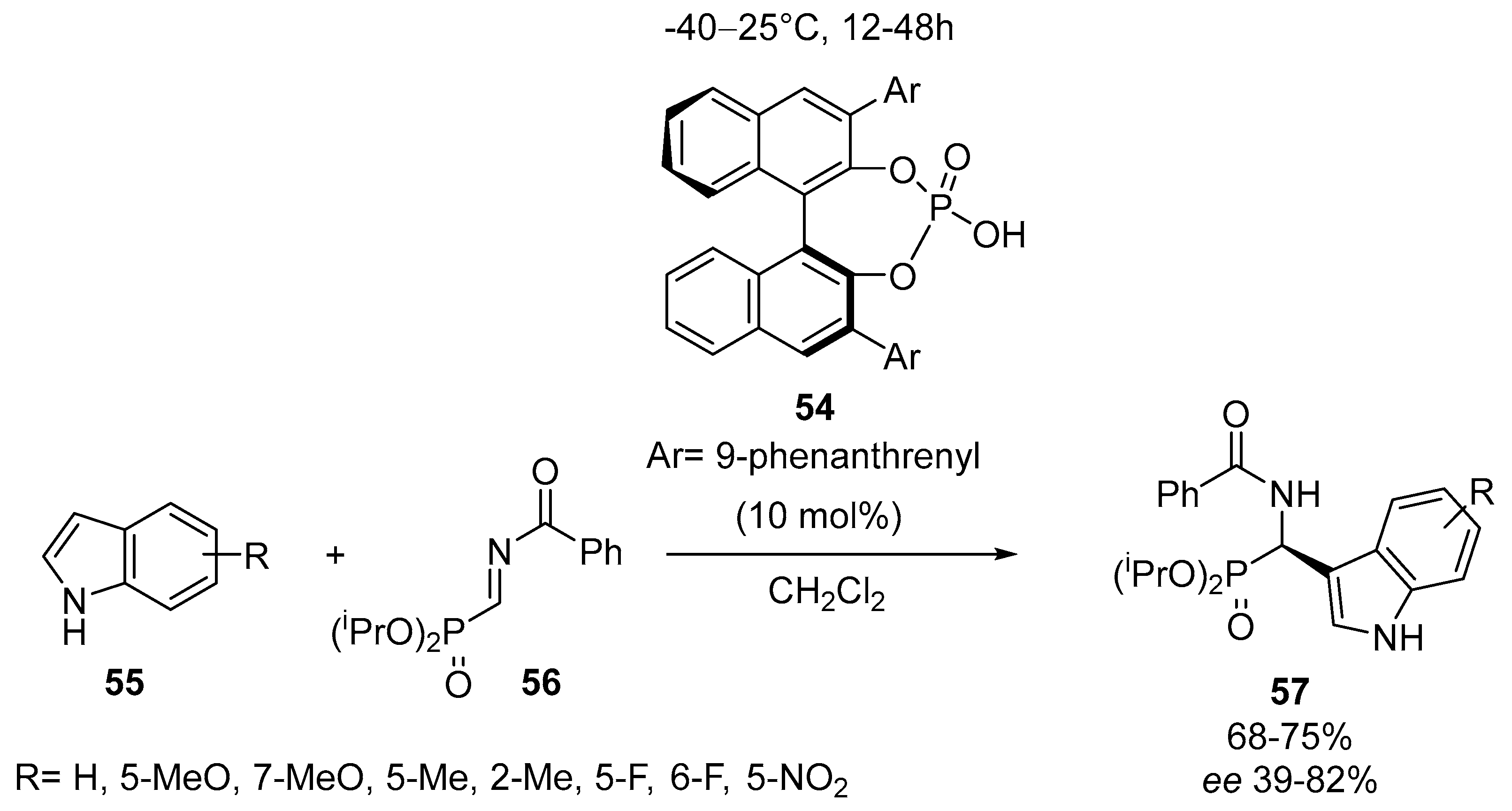
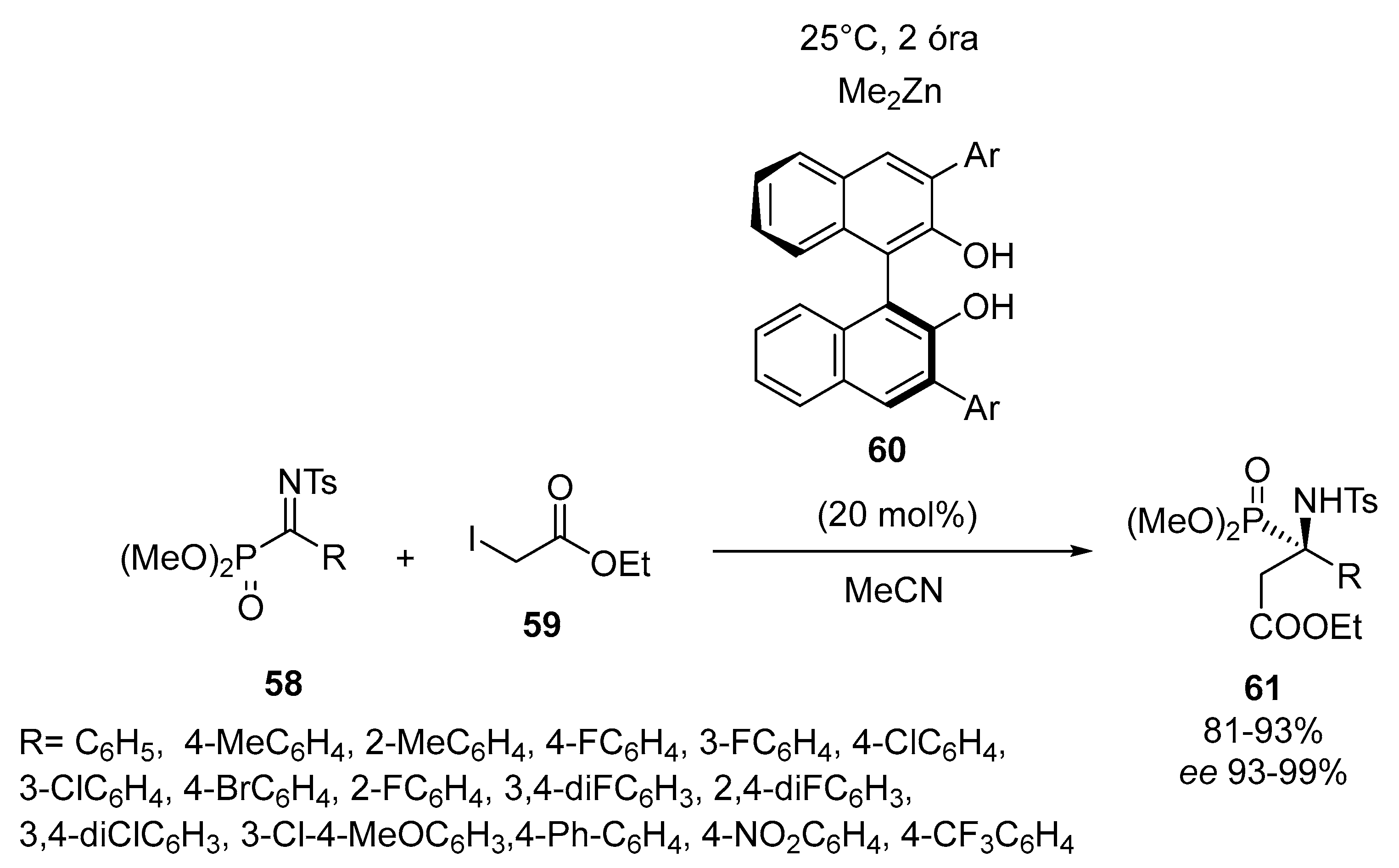
2.4. Optically Active α-Aminophosphonates by Miscellenious Methods
2.5. Synthesis of Enantioenriched α-Aminophosphonate Derivatives from Optically Active Starting Materials
3. Conclusions
Funding
Conflicts of Interest
Sample Availability
References
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Sravya, G.; Balakrishna, A.; Zyryanov, G.V.; Mohan, G.; Reddy, C.S.; Bakthavatchala Reddy, N. Synthesis of α-aminophosphonates by the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2020, 196, 353–381. [Google Scholar] [CrossRef]
- Ordóñez, M.; Viveros-Ceballos, J.L.; Cativiela, C.; Sayago, F.J. An update on the stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2015, 71, 1745–1784. [Google Scholar] [CrossRef]
- Maestro, A.; del Corte, X.; López-Francés, A.; Martínez de Marigorta, E.; Palacios, F.; Vicario, J. Asymmetric synthesis of tetrasubstituted α-aminophosphonic acid derivatives. Molecules 2021, 26, 3202. [Google Scholar] [CrossRef]
- Albrecht, L.; Albrecht, A.; Krawczyk, H.; Jorgensen, K. Organocatalytic asymmetric synthesis of organophosphorus compounds. Chem. Eur. J. 2010, 16, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Namboothiri, I.N.N. Asymmetric synthesis of quaternary α-amino acids and their phosphonate analogues. Asian J. Org. Chem. 2014, 3, 1234–1260. [Google Scholar] [CrossRef]
- Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. α-Iminophosphonates. Useful intermediates for the enantioselective synthesis of α-aminophosphonates. Asian J. Org. Chem. 2020, 9, 538–548. [Google Scholar] [CrossRef]
- Ordóñez, M.; Sayago, F.J.; Cativiela, C. Synthesis of quaternary α-aminophosphonic acids. Tetrahedron 2012, 68, 6369–6412. [Google Scholar] [CrossRef]
- Turcheniuk, K.V.; Kukhar, V.P.; Röschenthaler, G.-V.; Aceña, J.L.; Soloshonok, V.A.; Sorochinsky, A.E. Recent advances in the synthesis of fluorinated aminophosphonates and aminophosphonic acids. RSC Adv. 2013, 3, 6693. [Google Scholar] [CrossRef]
- Dzięgielewski, M.; Pięta, J.; Kamińska, E.; Albrecht, Ł. Organocatalytic synthesis of optically active organophosphorus compounds. Eur. J. Org Chem. 2015, 4, 677–702. [Google Scholar] [CrossRef]
- Mucha, A. Synthesis and modifications of phosphinic dipeptide analogues. Molecules 2012, 17, 13530–13568. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Nakamura, S.; Shibataa, N. Direct enantioselective three-component Kabachnik–Fields reaction catalyzed by chiral bis(imidazoline)-Zinc(II) Catalysts. Adv. Synth. Catal. 2011, 353, 3285–3289. [Google Scholar] [CrossRef]
- Wang, L.; Cui, S.; Meng, W.; Zhang, G.; Nie, J.; Ma, J. Asymmetric synthesis of α-aminophosphonates by means of direct organocatalytic three-component hydrophosphonylation. Chin. Sci. Bull. 2010, 55, 1729–1731. [Google Scholar] [CrossRef]
- Thorat, P.B.; Goswami, S.V.; Magar, R.L.; Patil, B.R.; Bhusare, S.R. An efficient organocatalysis: A one-pot highly enantioselective synthesis of α-aminophosphonates. Eur. J. Org. Chem. 2013, 2013, 5509–5516. [Google Scholar] [CrossRef]
- Zou, L.; Huang, J.; Liao, N.; Liu, Y.; Guo, Q.; Peng, Y. Catalytic asymmetric three-component reaction of 2-alkynylbenzaldehydes, amines, and dimethylphosphonate. Org. Lett. 2020, 22, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Xu, D. Asymmetric synthesis of α-amino phosphonates by using cinchona alkaloid-based chiral phase transfer catalyst. Eur. J. Org. Chem. 2018, 39, 5422–5426. [Google Scholar] [CrossRef]
- Zhang, G.; Hao, G.; Pan, J.; Zhang, J.; Hu, D.; Song, B. Asymmetric synthesis and bioselective activities of α-amino-phosphonates based on the dufulin motif. J. Agric. Food Chem. 2016, 64, 4207–4213. [Google Scholar] [CrossRef]
- Xie, H.; Song, A.; Zhang, X.; Chen, X.; Li, H.; Sheng, C.; Wang, W. Quinine-thiourea catalyzed enantioselective hydrophosphonylation of trifluoromethyl 2(1H)-quinazolinones. Chem. Commun. 2013, 49, 928–930. [Google Scholar] [CrossRef]
- Iwanejko, J.; Brol, A.; Szyja, B.; Daszkiewicz, M.; Wojaczyńska, E.; Olszewski, T.K. Hydrophosphonylation of chiral hexahydroquinoxalin-2(1H)-one derivatives as an effective route to new bicyclic compounds: Aminophosphonates, enamines and imines. Tetrahedron 2019, 75, 1431–1439. [Google Scholar] [CrossRef]
- George, J.; Sridhar, B.; Reddy, B.V.S. First example of quininesquaramide catalyzed enantioselective addition of diphenyl phosphite to ketimines derived from isatins. Org. Biomol. Chem. 2014, 12, 1595–1602. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, Y.; Kim, D.Y. Enantioselective addition of diphenyl phosphonate to ketimines derived from isatins catalyzed by binaphthyl-modified organocatalysts. Belstein J. Org. Chem. 2016, 12, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Mo, F.; Li, L.; Lin, X. Highly enantioselective hydrophosphonylation of imines catalyzed by SPINOL-phosphoric acid. RSC Adv. 2013, 3, 11895. [Google Scholar] [CrossRef]
- Yin, L.; Bao, Y.; Kumagai, N.; Shibasaki, M. Catalytic asymmetric hydrophosphonylation of ketimines. J. Am. Chem. Soc. 2013, 135, 10338–10341. [Google Scholar] [CrossRef]
- Xie, H.-P.; Yan, Z.; Wu, B.; Hu, S.-B.; Zhou, Y.-G. Synthesis of electron-deficient (Sa,R,R)-(CF3)2-C3-TunePhos and its applications in asymmetric hydrogenation of α-iminophosphonates. Tetrahedron Lett. 2018, 59, 2960–2964. [Google Scholar] [CrossRef]
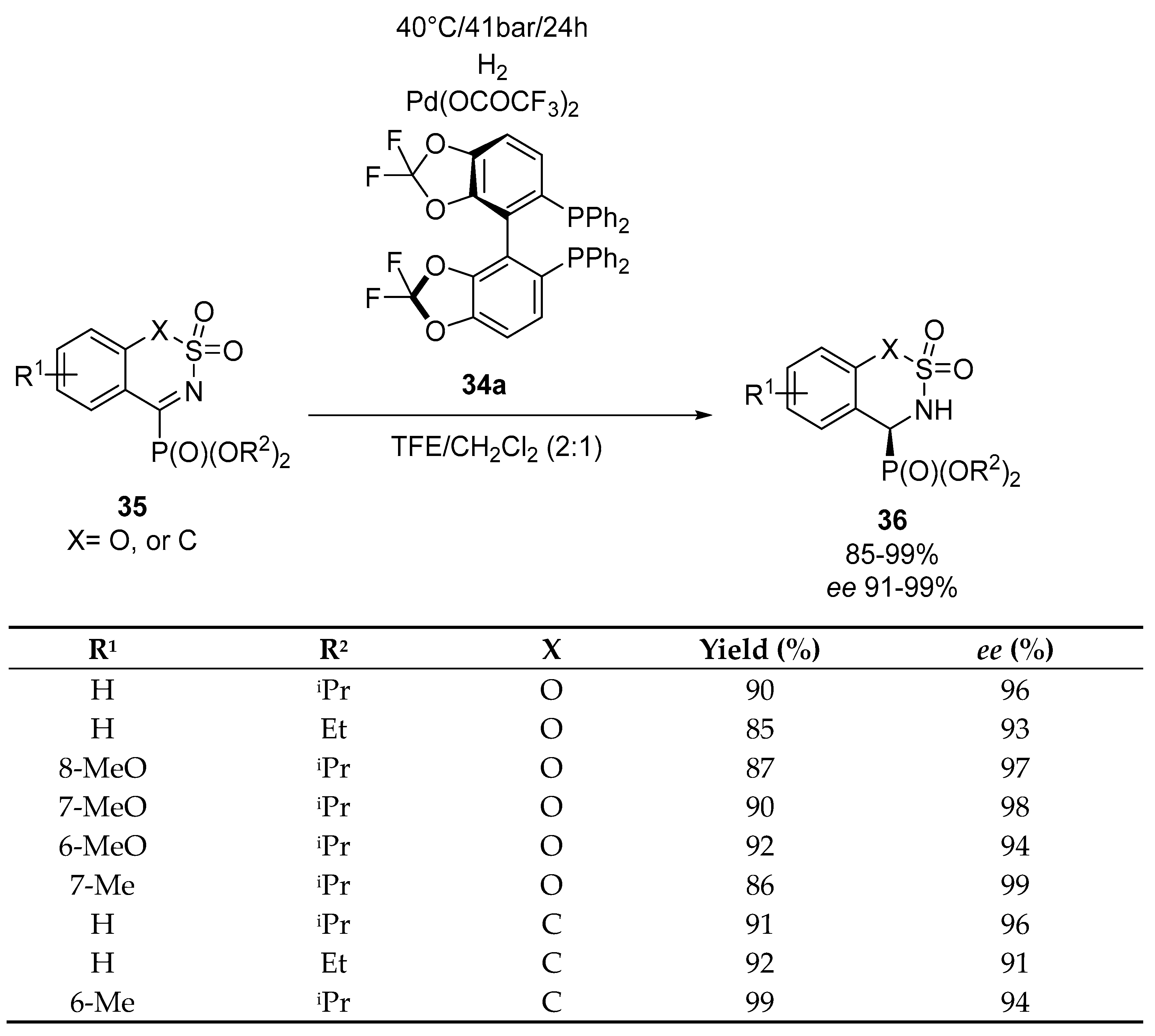
- Yan, Z.; Wu, B.; Gao, X.; Chen, M.-W.; Zhou, Y.-G. Enantioselective synthesis of α-amino phosphonates via Pd-catalyzed asymmetric hydrogenation. Org. Lett. 2016, 18, 692–695. [Google Scholar] [CrossRef]
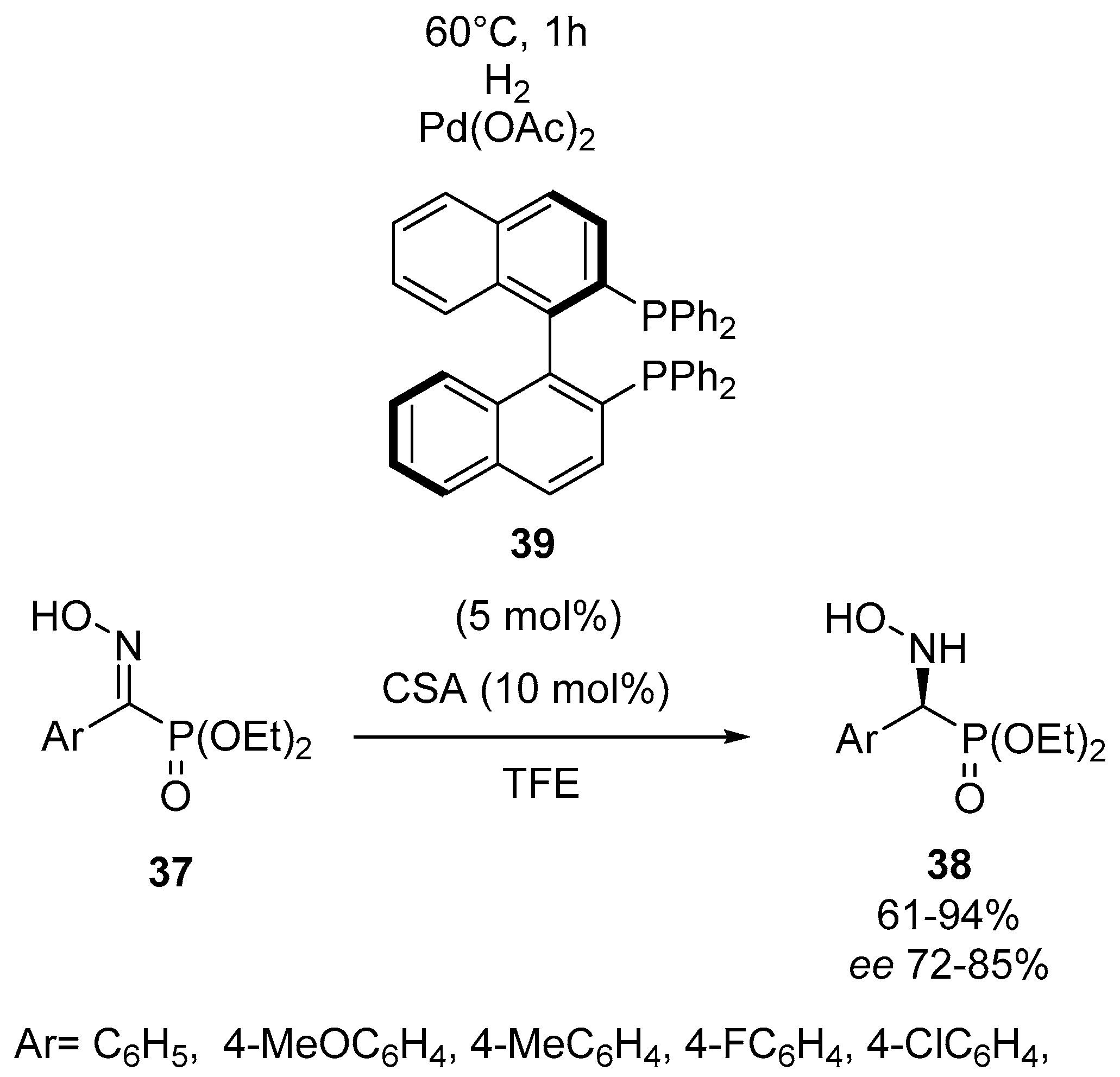
- Goulioukina, N.S.; Shergold, I.A.; Bondarenko, G.N.; Ilyin, M.M.; Davankov, V.A.; Beletskaya, I.P. Palladium-catalyzed asymmetric hydrogenation of N-hydroxy-α-imino phosphonates using Brønsted acid as activator: The first catalytic enantioselective approach to chiral N-hydroxy-α-amino phosphonates. Adv. Synth. Catal. 2012, 354, 2727–2733. [Google Scholar] [CrossRef]
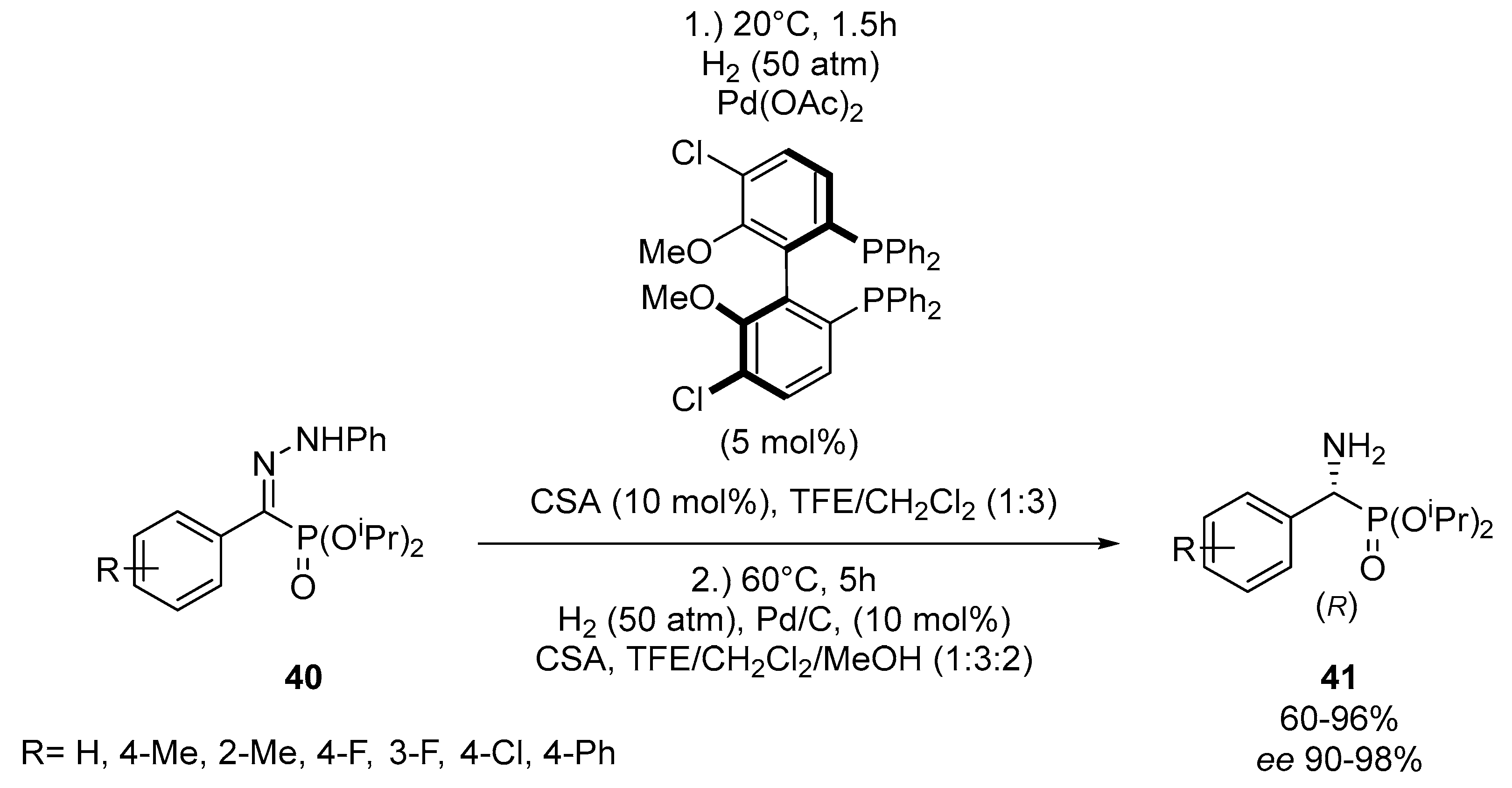
- Goulioukina, N.S.; Shergold, I.A.; Rybakov, V.B.; Beletskaya, I.P. One-pot two-step synthesis of optically active α-amino phosphonates by palladium-catalyzed hydrogenation/hydrogenolysis of α-hydrazono phosphonates. Adv. Synth. Catal. 2016, 359, 153–162. [Google Scholar] [CrossRef]
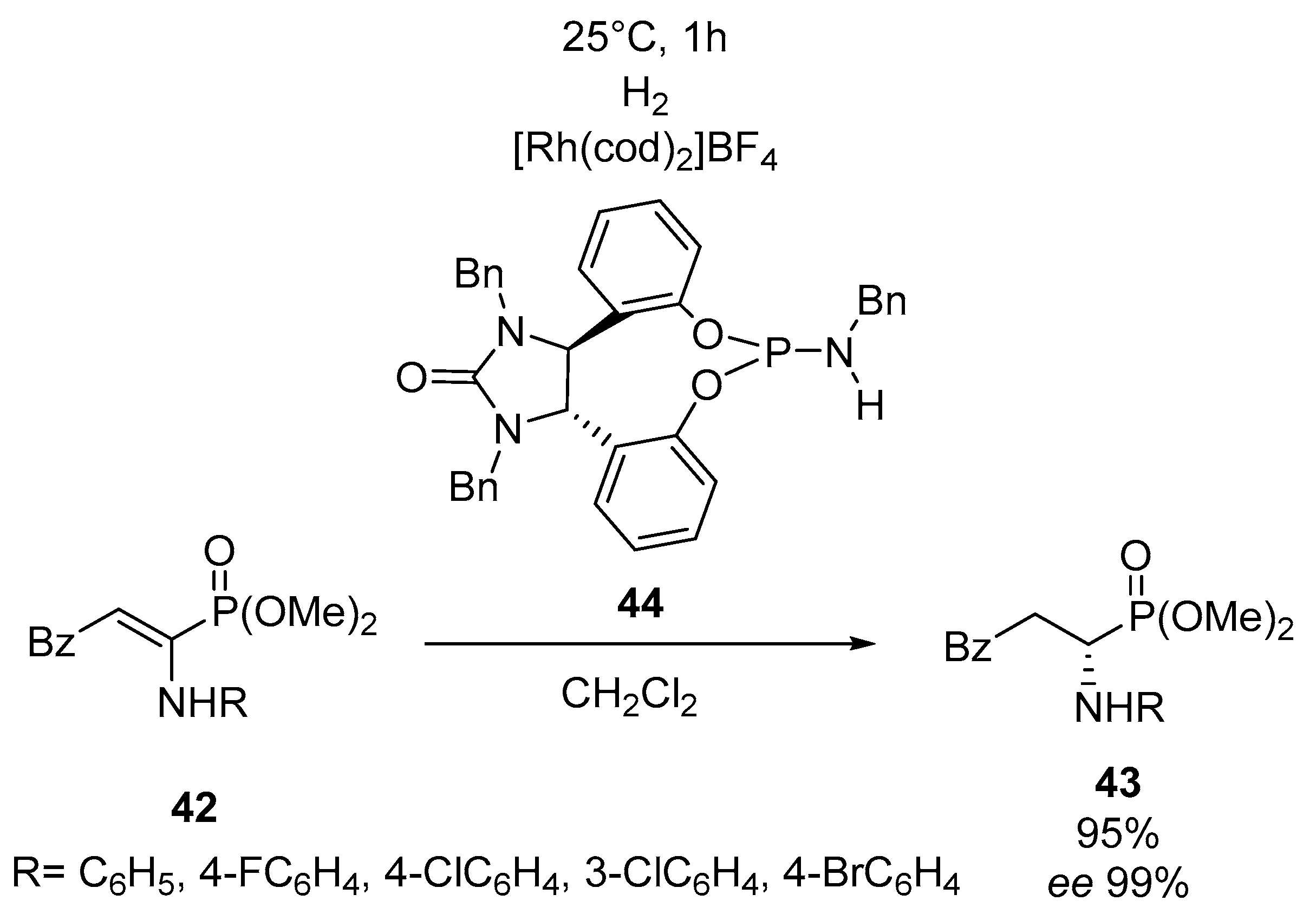
- Zhang, J.; Li, Y.; Wang, Z.; Ding, K. Asymmetric hydrogenation of α- and β-enamido phosphonates: Rhodium(I)/monodentate phosphoramidite catalyst. Angew. Chem. 2011, 123, 11947–11951. [Google Scholar] [CrossRef]
- Tao, X.; Li, W.; Li, X.; Xie, X.; Zhang, Z. Diastereo- and enantioselective asymmetric hydrogenation of α-amido-β-keto phosphonates via dynamic kinetic resolution. Org. Lett. 2012, 15, 72–75. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Albrecht, Ł. An organocatalytic biomimetic approach to α-aminophosphonates. Chem. Commun. 2015, 51, 3981–3984. [Google Scholar] [CrossRef]
- Vicario, J.; Ortiz, P.; Ezpeleta, J.M.; Palacios, F. Asymmetric synthesis of functionalized tetrasubstituted α-aminophosphonates through enantioselective aza-Henry reaction of phosphorylated ketimines. J. Org. Chem. 2014, 80, 156–164. [Google Scholar] [CrossRef]
- Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective α-aminophosphonate functionalization of indole ring through an organocatalyzed Friedel-Crafts reaction. J. Org. Chem. 2018, 84, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective aza-Reformatsky reaction with ketimines. Org. Lett. 2019, 21, 9473–9477. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Nam, L.C.; Dutton, M.J.; Yoshimoto, S.; Kobayashi, S. Catalytic asymmetric endo-selective [3+2] cycloaddition reactions of Schiff bases of α-aminophosphonates with olefins using chiral metal amides. Chem. Commun. 2015, 51, 17064–17067. [Google Scholar] [CrossRef] [PubMed]
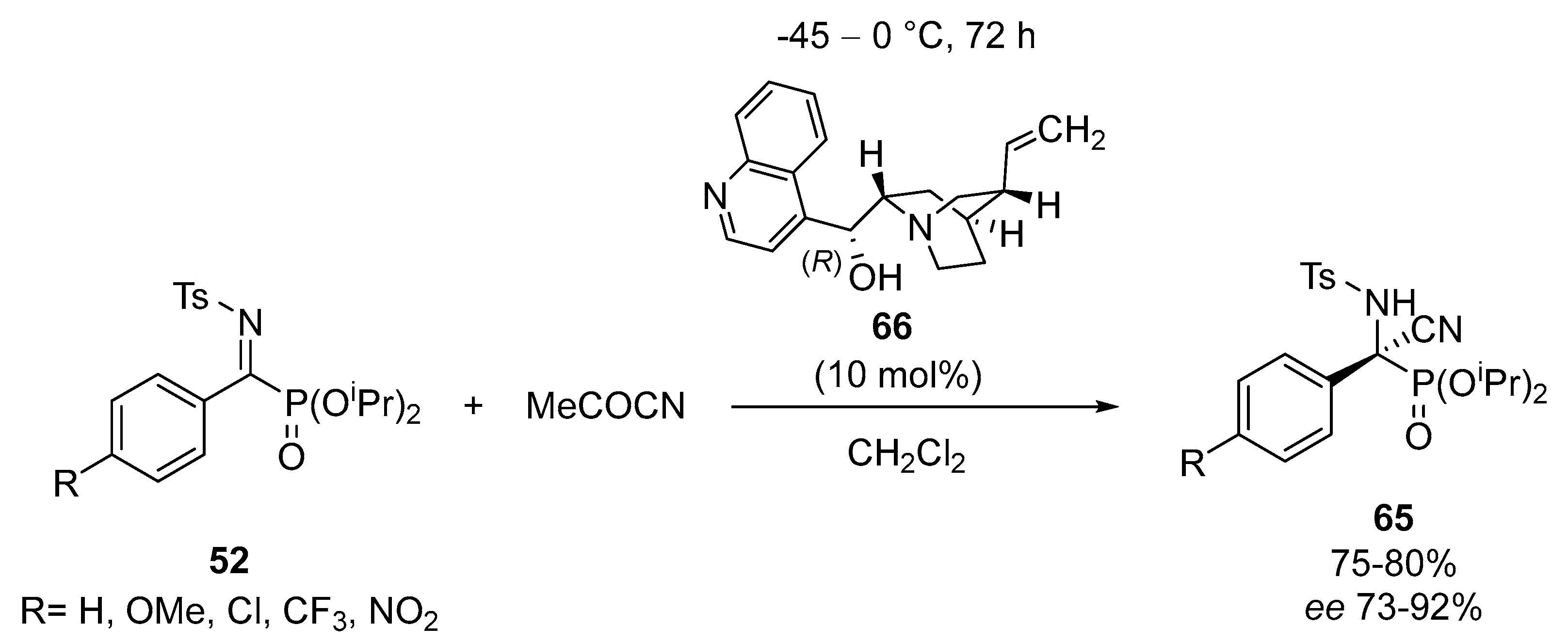
- Vicario, J.; Ezpeleta, J.M.; Palacios, F. Asymmetric cyanation of α-ketiminophosphonates catalyzed by cinchona alkaloids: Enantioselective synthesis of tetrasubstituted α-aminophosphonic acid derivatives from trisubstituted α-aminophosphonates. Adv. Synth. Catal. 2012, 354, 2641–2647. [Google Scholar] [CrossRef]
- Yan, Z.; Gao, X.; Zhou, Y.-G. Enantioselective synthesis of quaternary α-aminophosphonates by organocatalytic Friedel–Crafts reactions of indoles with cyclic α-ketiminophosphonates. Chin. J. Catal. 2017, 38, 784–791. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, B.; Gao, X.; Zhou, Y.-G. Enantioselective synthesis of quaternary α-aminophosphonates by Pd-catalyzed arylation of cyclic α-ketiminophosphonates with arylboronic acids. Chem. Commun. 2016, 52, 10882–10885. [Google Scholar] [CrossRef]
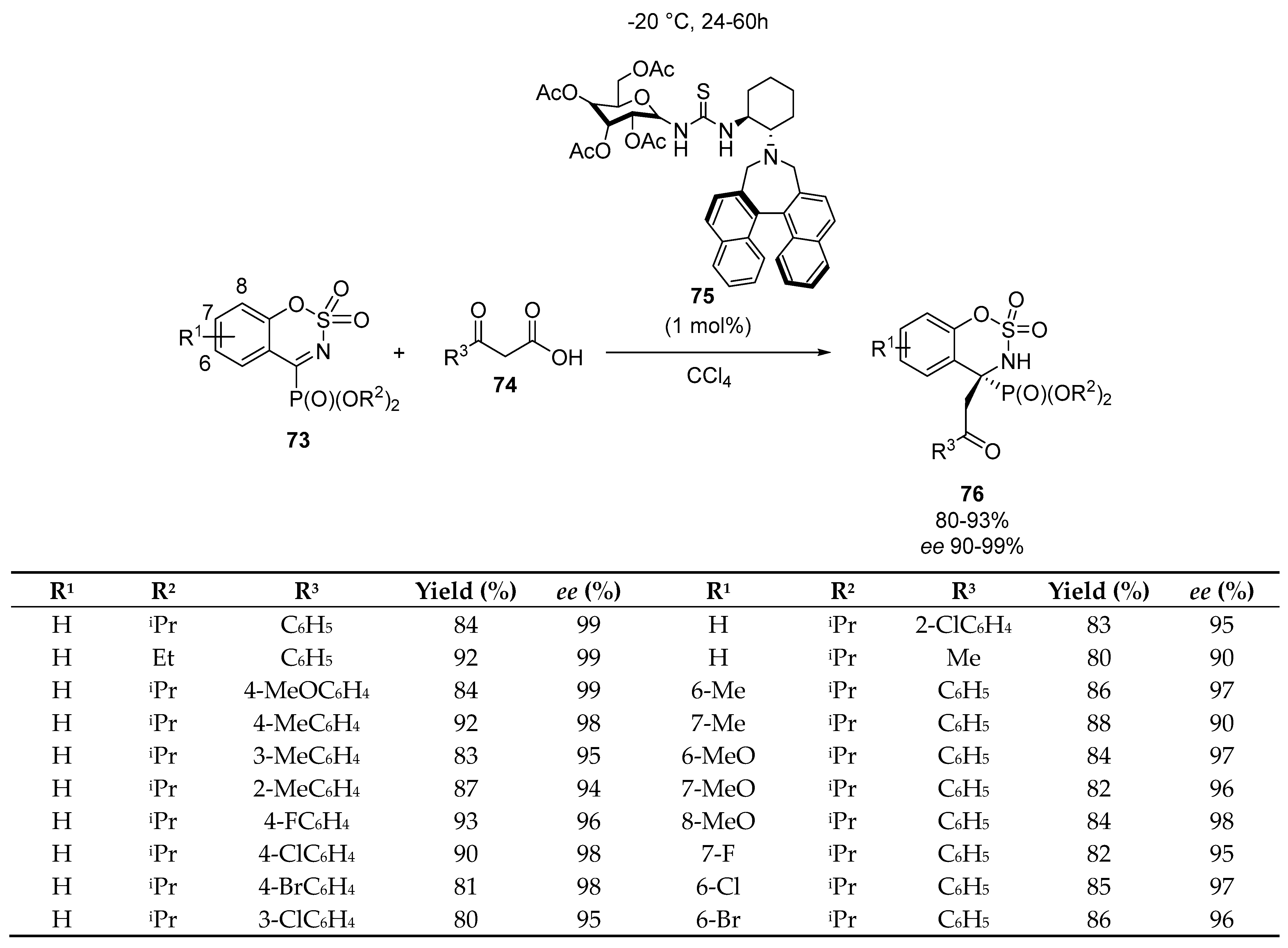
- Li, X.-A.; Li, J.-Y.; Nie, J.; Jun-An, M. Organocatalytic asymmetric decarboxylative Mannich reaction of β-keto acids with cyclic α-ketiminophosphonates: Access to quaternary α-aminophosphonates. Org. Lett. 2018, 20, 3643–3646. [Google Scholar] [CrossRef]
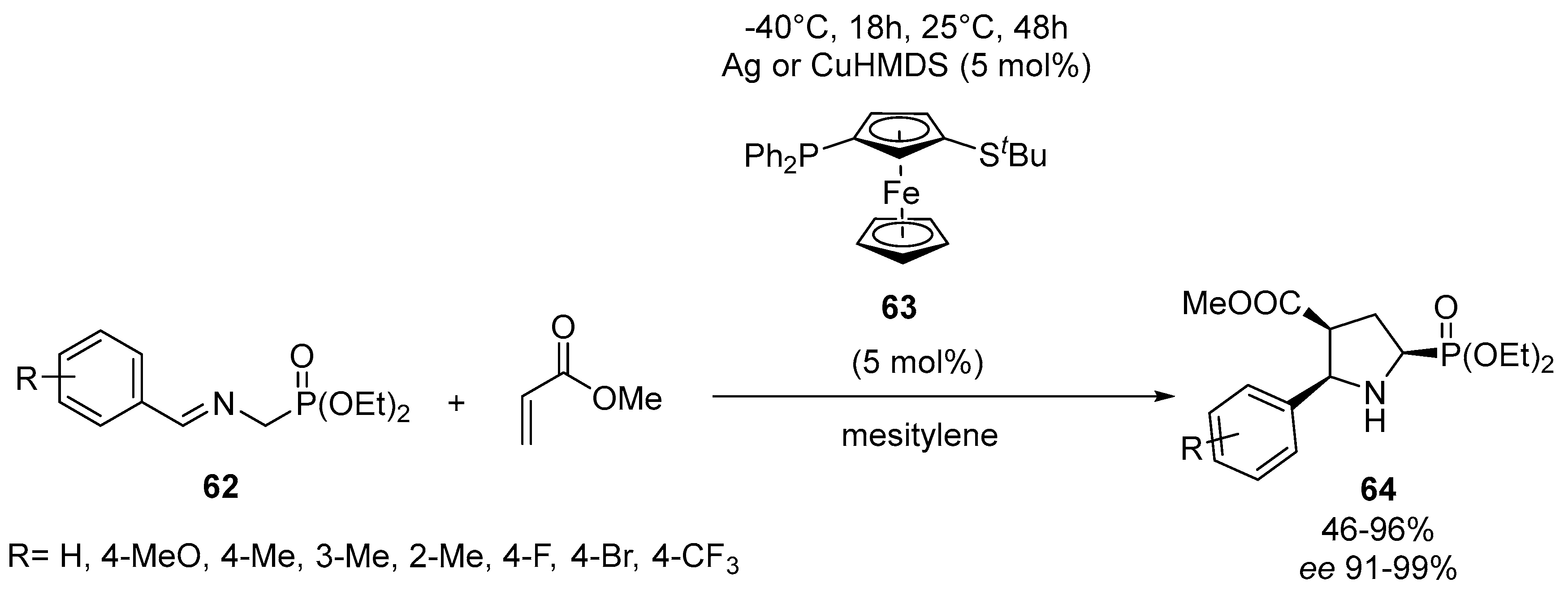
- Sun, J.; Mou, C.; Liu, C.; Huang, R.; Zhang, S.; Zheng, P.; Chi, Y.R. Enantioselective access to multi-cyclic α-amino phosphonates via carbene-catalyzed cycloaddition reactions between enals and six- introduction 60 membered cyclic imines. Org. Chem. Front. 2018, 5, 2992–2996. [Google Scholar] [CrossRef]
- Ordóñez, M.; Arizpe, A.; Sayago, F.; Jiménez, A.; Cativiela, C. Practical and efficient synthesis of α-aminophosphonic acids containing 1,2,3,4-tetrahydroquinoline or 1,2,3,4-tetrahydroisoquinoline heterocycles. Molecules 2016, 21, 1140. [Google Scholar] [CrossRef]
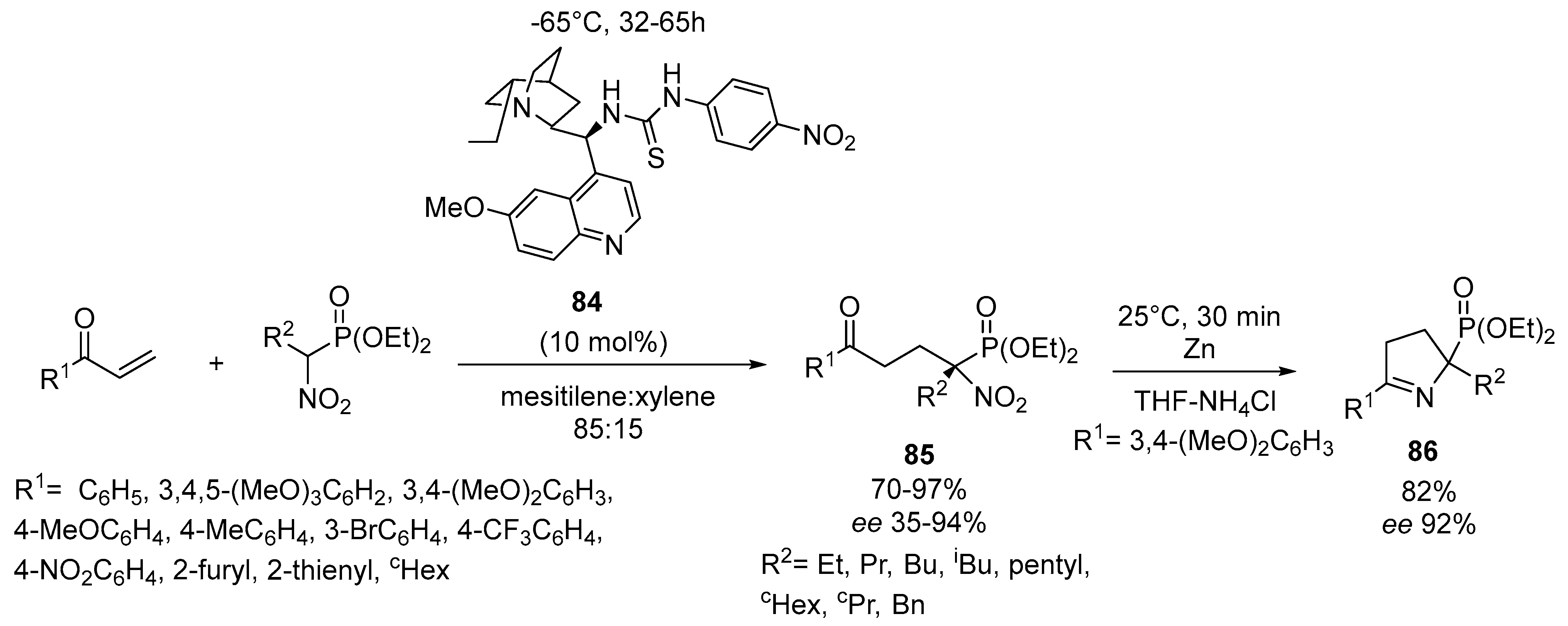
- Bera, K.; Namboothiri, I.N.N. Enantioselective synthesis of quaternary α-aminophosphonates via conjugate addition of α-nitrophosphonates to enones. Org. Lett. 2012, 14, 980–983. [Google Scholar] [CrossRef] [PubMed]
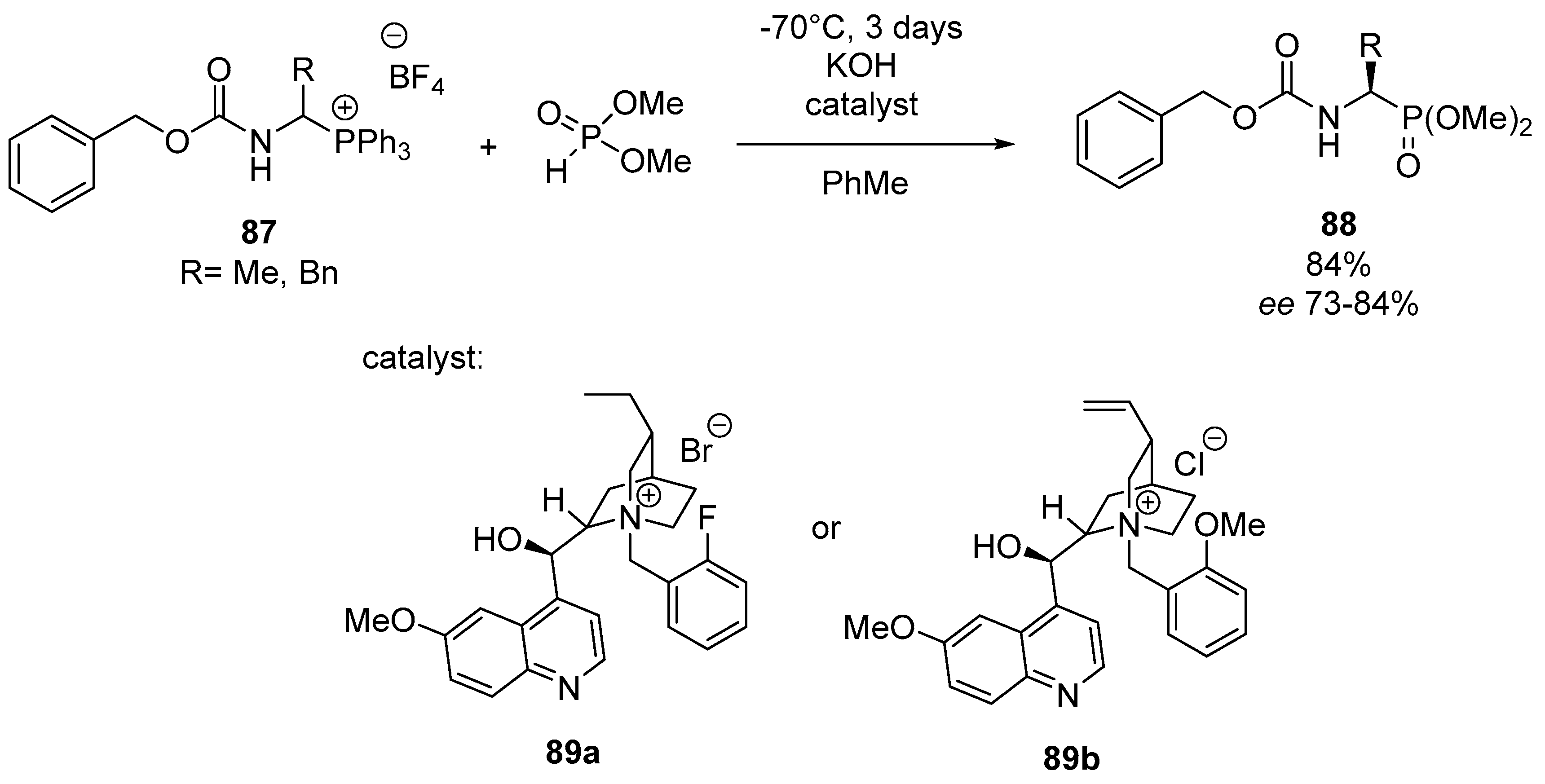
- Walęcka-Kurczyk, A.; Walczak, K.; Kuźnik, A.; Stecko, S.; Październiok-Holewa, A. The synthesis of α-aminophosphonates via enantioselective organocatalytic reaction of 1-(N-acylamino)alkylphosphonium salts with dimethyl phosphite. Molecules 2020, 25, 405. [Google Scholar] [CrossRef] [PubMed]
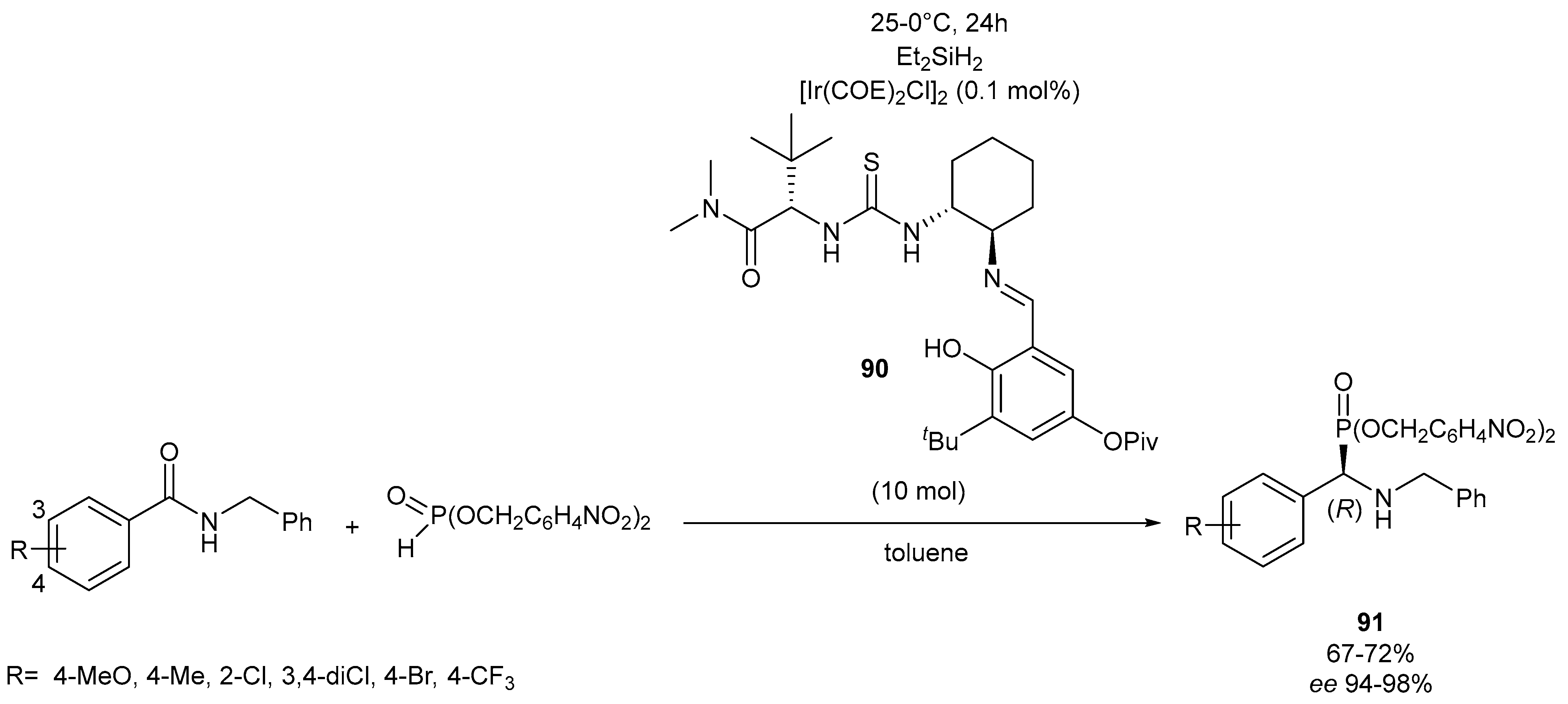
- Chen, D.; Sun, W.; Zhu, C.; Lu, G.; Wu, D.; Wang, A.; Huang, P. Enantioselective reductive cyanation and phosphonylation of secondary amides by iridium and chiral thiourea sequential catalysis. Angew. Chem. Int. Edit. 2021, 60, 8827–8831. [Google Scholar] [CrossRef] [PubMed]
- Bálint, E.; Tajti, Á.; Kalocsai, D.; Mátravölgyi, B.; Karaghiosoff, K.; Czugler, M.; Keglevich, G. Synthesis and utilization of optically active α-aminophosphonate derivatives by Kabachnik-Fields reaction. Tetrahedron 2017, 73, 5659–5667. [Google Scholar] [CrossRef]
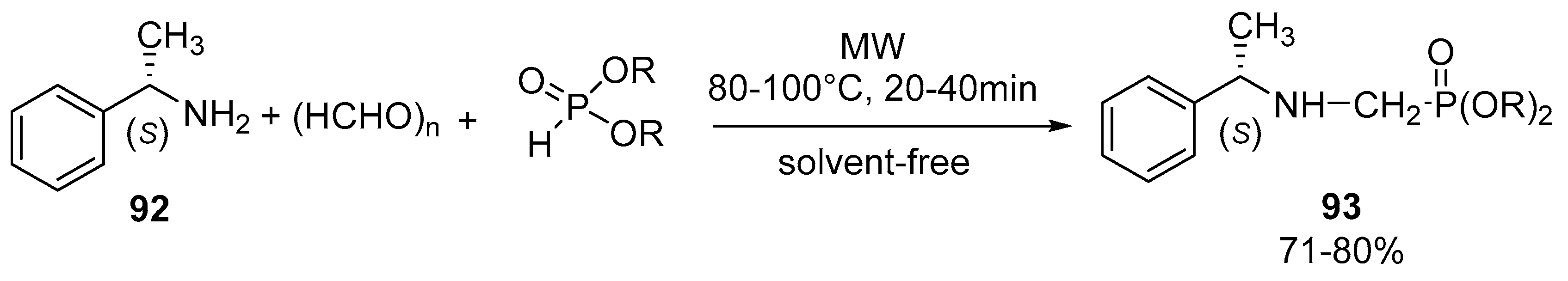
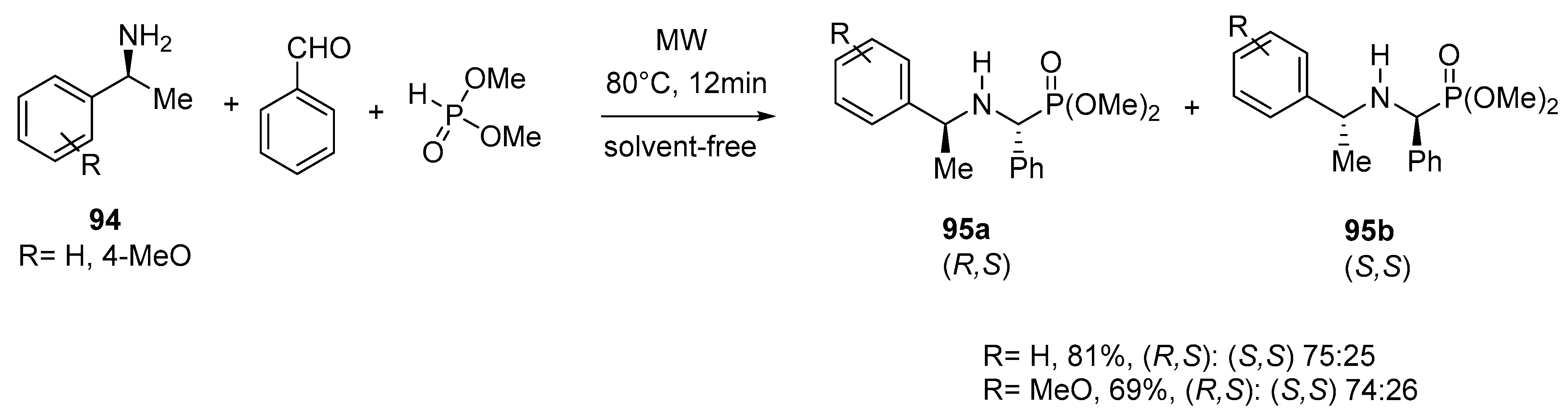
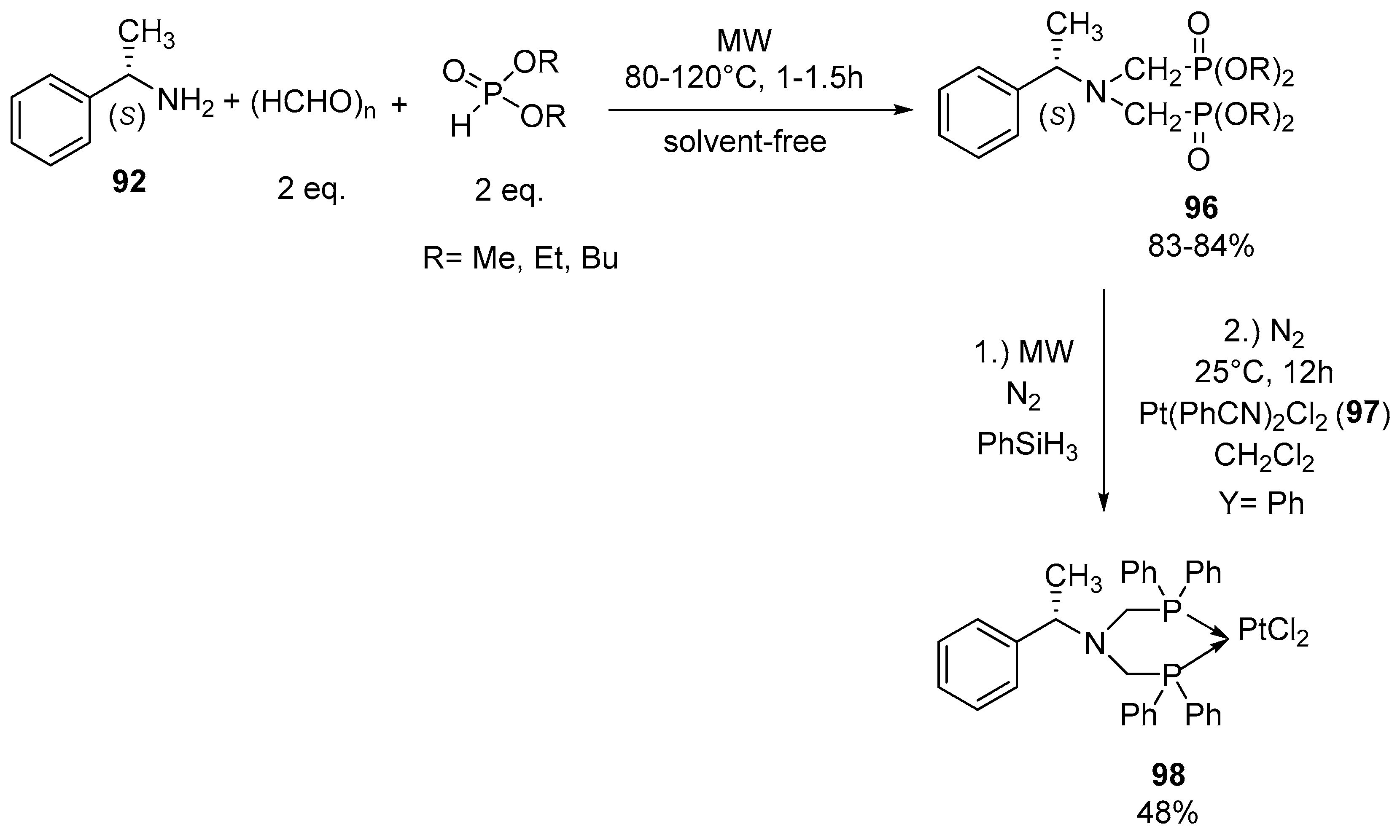
- Tajti, Á.; Bálint, E.; Keglevich, G. Microwave-assisted synthesis of α-aminophosphonates and related derivatives by the Kabachnik-Fields reaction. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 379–381. [Google Scholar] [CrossRef]
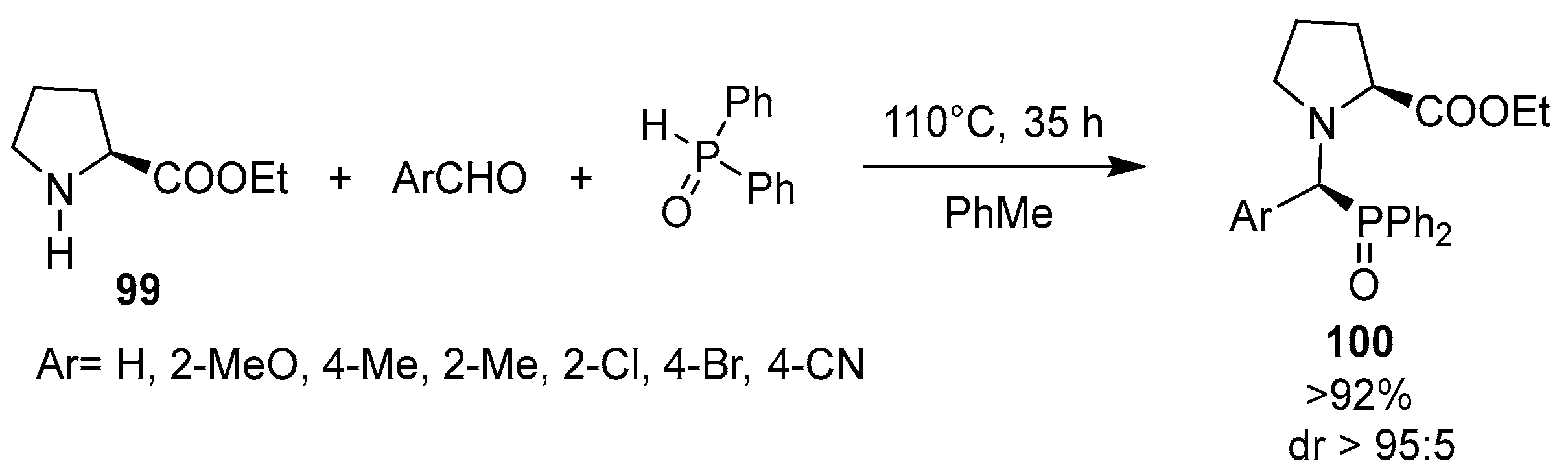
- Tibhe, G.D.; Reyes-González, M.A.; Cativiela, C.; Ordóñez, M. Microwave-assisted high diastereoselective synthesis of α-aminophosphonates under solvent and catalyst free-conditions. J. Mex. Chem. Soc. 2012, 56, 183–187. [Google Scholar] [CrossRef]
- Li, X.-A.; Li, J.-Y.; Yang, B.; Yang, S.-D. Catalyst-free three-component reaction to synthesize chiral α-amino phosphine oxides. RSC Adv. 2014, 4, 39920–39923. [Google Scholar] [CrossRef]
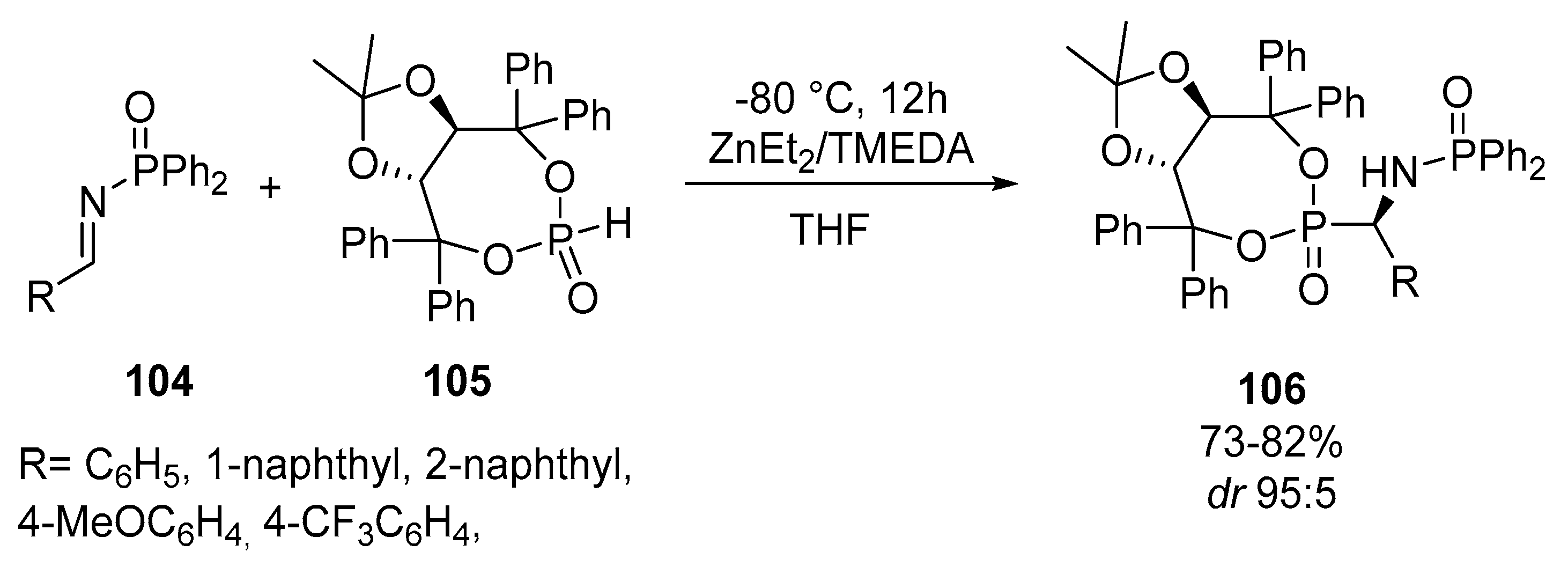
- Gbubele, J.D.; Olszewski, T.K. Asymmetric synthesis of organophosphorus compounds using H–P reagents derived from chiral alcohols. Org. Biomol. Chem. 2021, 19, 2823–2846. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I.; Grishkun, E.V.; Sheiko, S.; Demchuk, O.; Thoennessen, H.; Jones, P.G.; Schmutzler, R. Chiral symmetric phosphoric acid esters as sources of optically active organophosphorus compounds. Tetrahedron Asymmetry 1998, 9, 1645–1649. [Google Scholar] [CrossRef]
- Palacios, F.; Olszewski, T.K.; Vicario, J. Diastereoselective hydrophosphonylation of imines using (R,R)-TADDOL phosphite. Asymmetric synthesis of α-aminophosphonic acid derivatives. Org. Biomol. Chem. 2010, 8, 4255–4258. [Google Scholar] [CrossRef]
- Gbubele, J.D.; Misiaszek, T.; Siczek, M.; Olszewski, T.K. α-Amido sulphones as useful intermediates in the preparation of C-chiral α-aminophosphonates and α-aminophosphonic acids. Org. Biomol. Chem. 2023, 21, 6180–6191. [Google Scholar] [CrossRef] [PubMed]
- Łyżwa, P. Double asymmetric induction in the synthesis of enantiomeric α-aminophosphonic acids mediated by sulfinimines. Heteroatom Chem. 2013, 25, 15–19. [Google Scholar] [CrossRef]
- Łyżwa, P.; Mikołajczyk, M. Chiral sulfinimines in asymmetric synthesis of enantiomeric aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 1174–1192. [Google Scholar] [CrossRef]
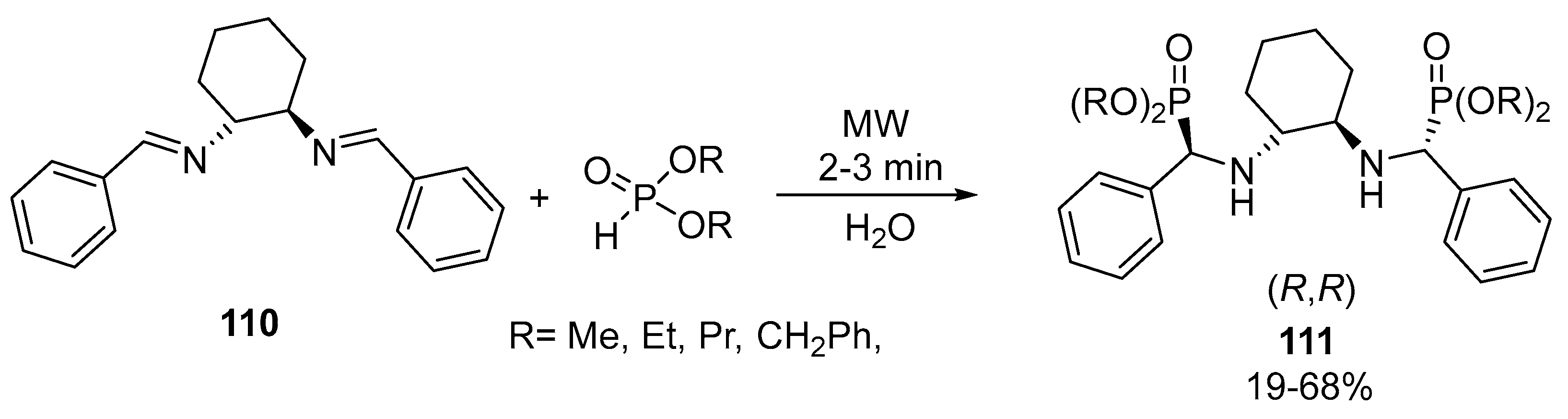
- Lewkowski, J.; Tokarz, P.; Lis, T.; Ślepokura, K. Synthesis and resolution of diastereomers of (R,R)-1,2-cyclohexylenediamino-di-phenylmethylphosphonates. Tetrahedron Asymmetry 2012, 23, 482–488. [Google Scholar] [CrossRef]



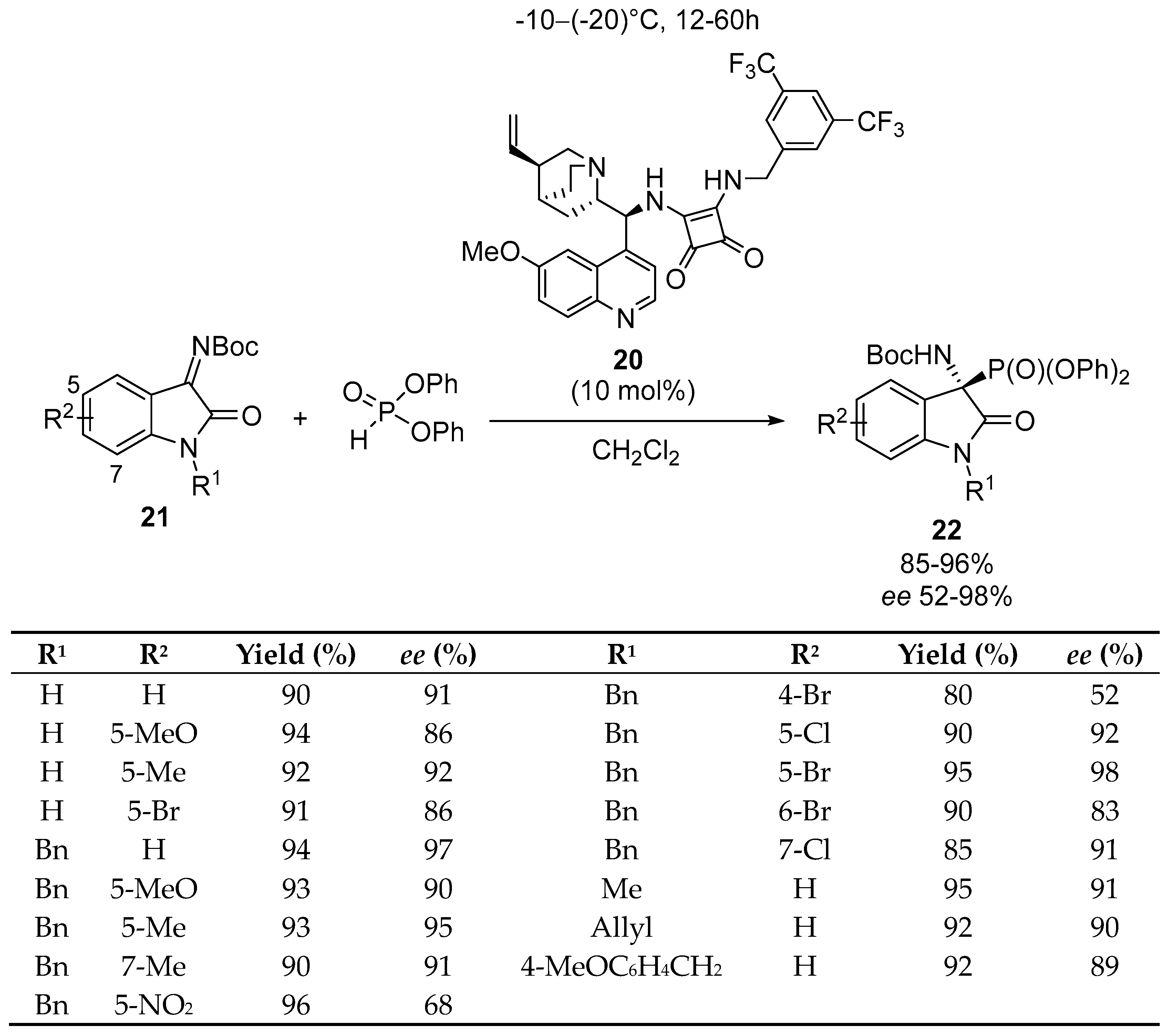
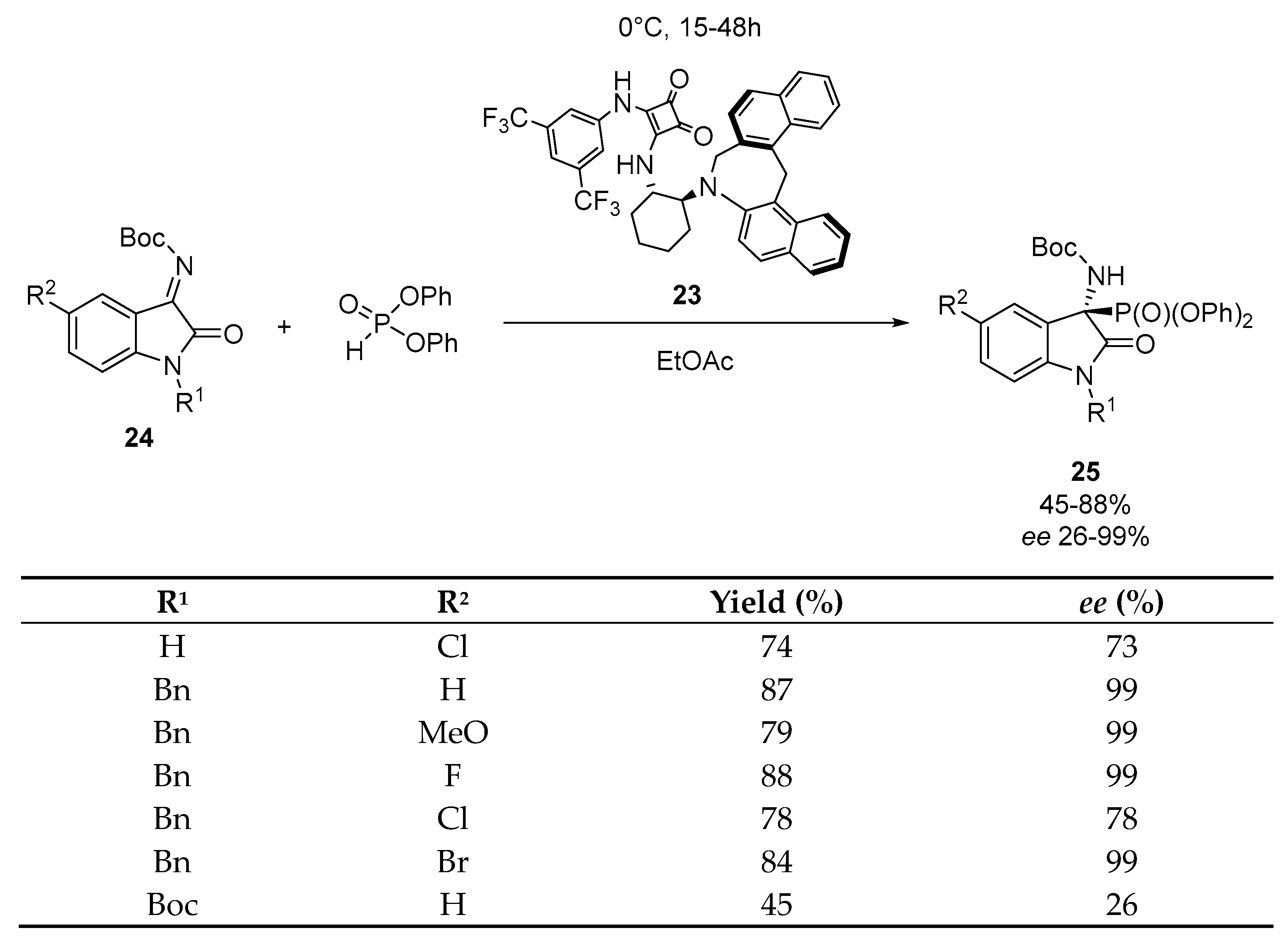

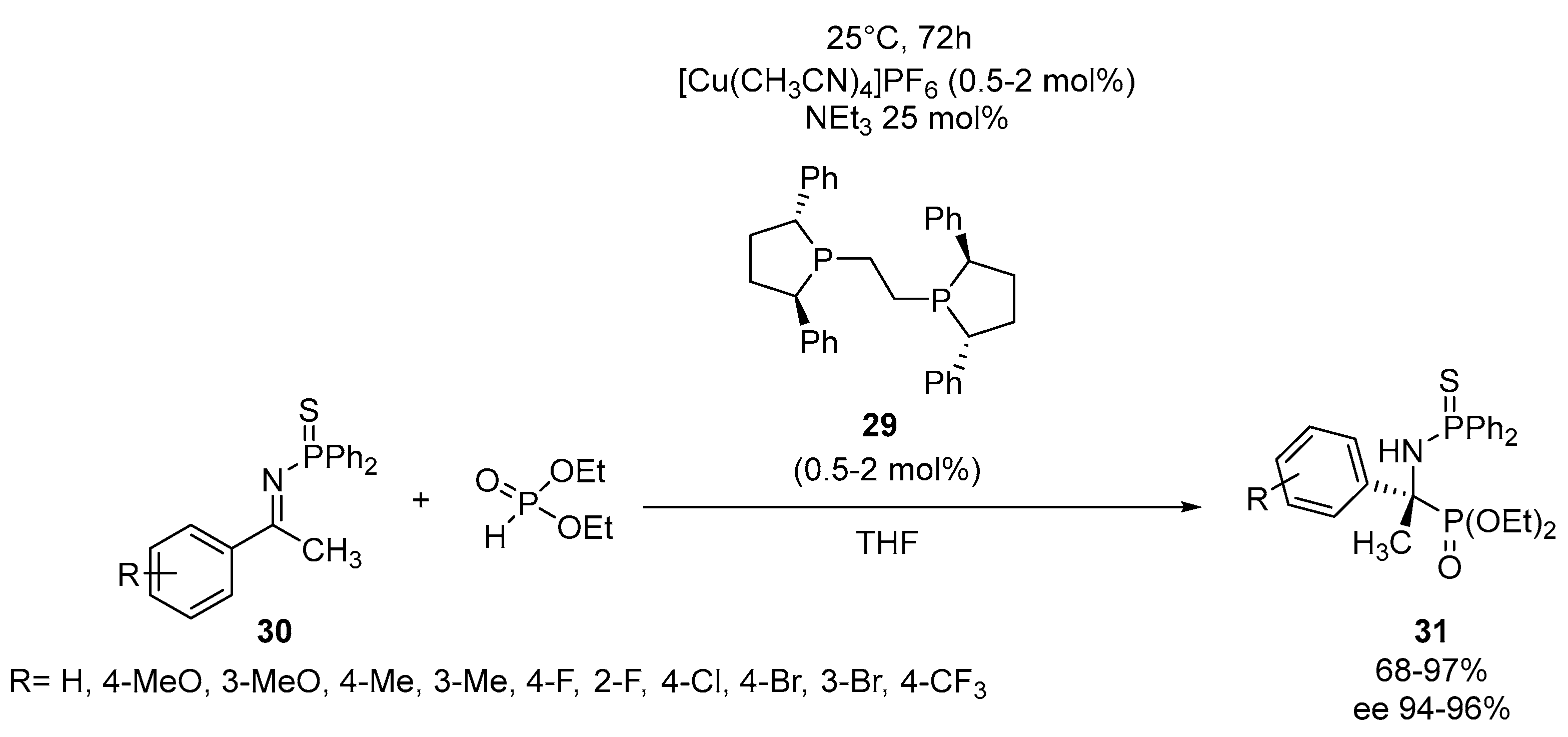
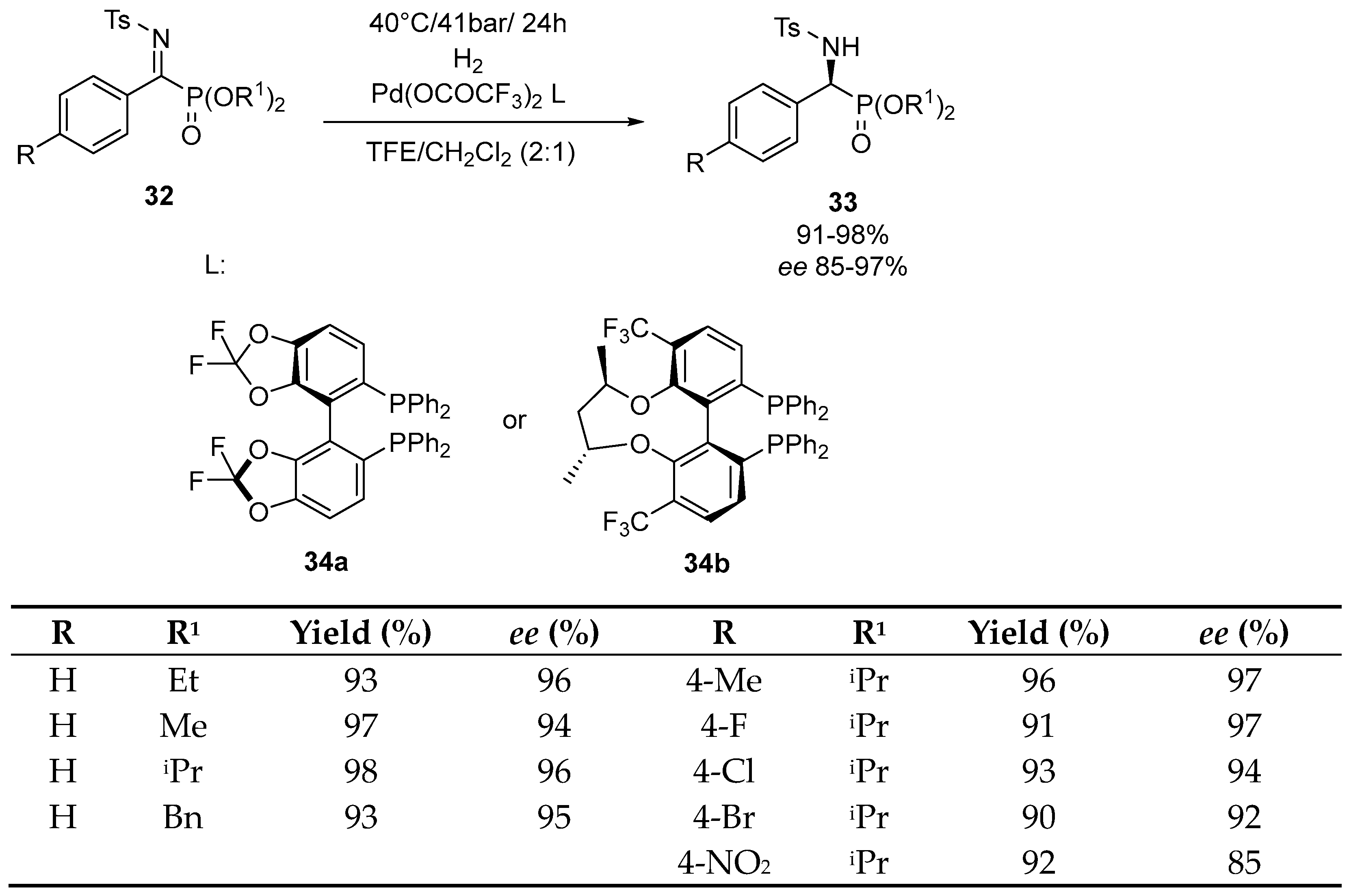






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, P.R.; Keglevich, G. The Last Decade of Optically Active α-Aminophosphonates. Molecules 2023, 28, 6150. https://doi.org/10.3390/molecules28166150
Varga PR, Keglevich G. The Last Decade of Optically Active α-Aminophosphonates. Molecules. 2023; 28(16):6150. https://doi.org/10.3390/molecules28166150
Chicago/Turabian StyleVarga, Petra R., and György Keglevich. 2023. "The Last Decade of Optically Active α-Aminophosphonates" Molecules 28, no. 16: 6150. https://doi.org/10.3390/molecules28166150






