Anti-α-Glucosidase, SAR Analysis, and Mechanism Investigation of Indolo[1,2-b]isoquinoline Derivatives
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. α-Glucosidase Activity Evaluation
2.3. SAR Analysis
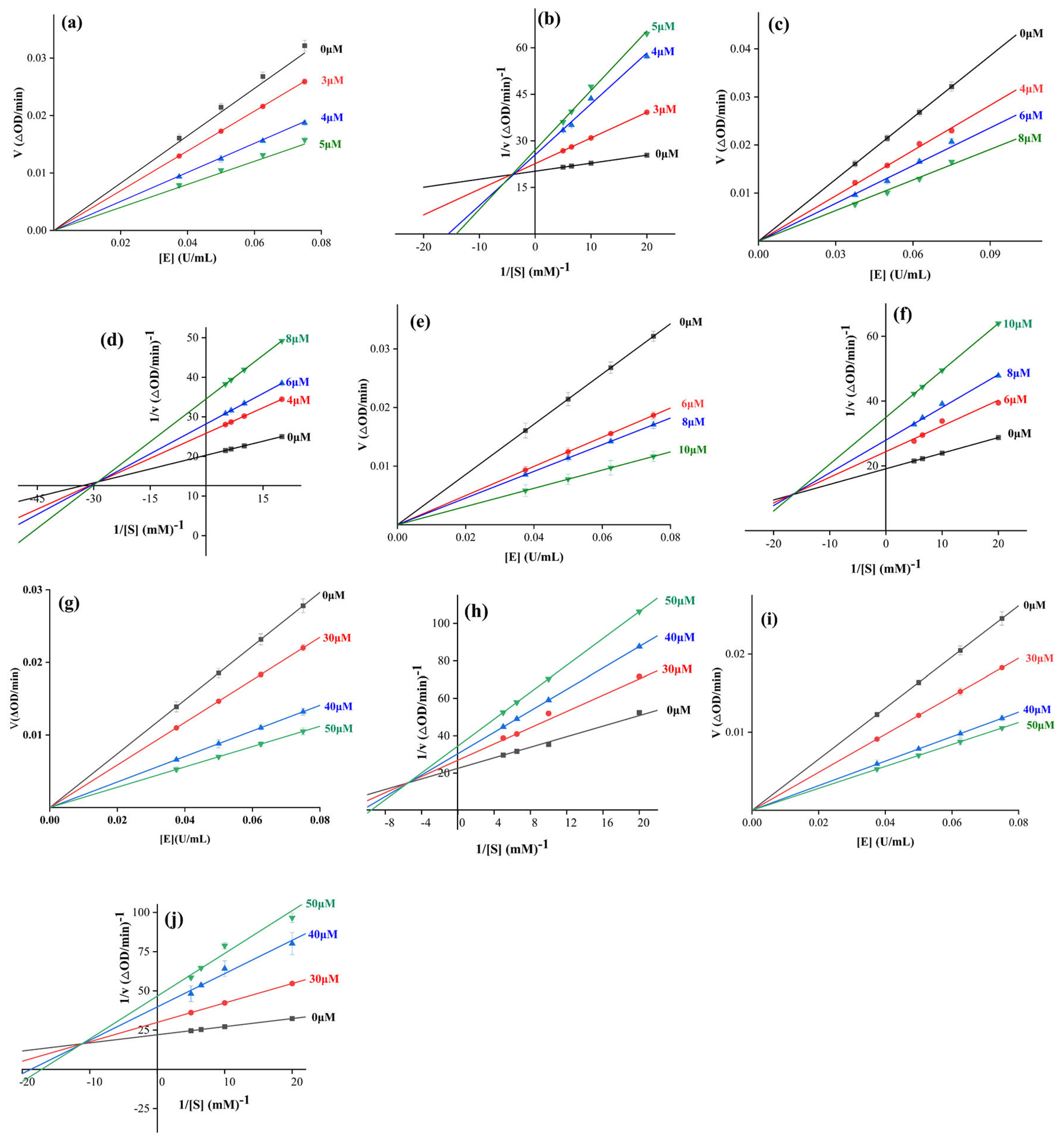
2.4. Inhibition Kinetics Study
2.5. 3D Fluorescence Spectra Assay
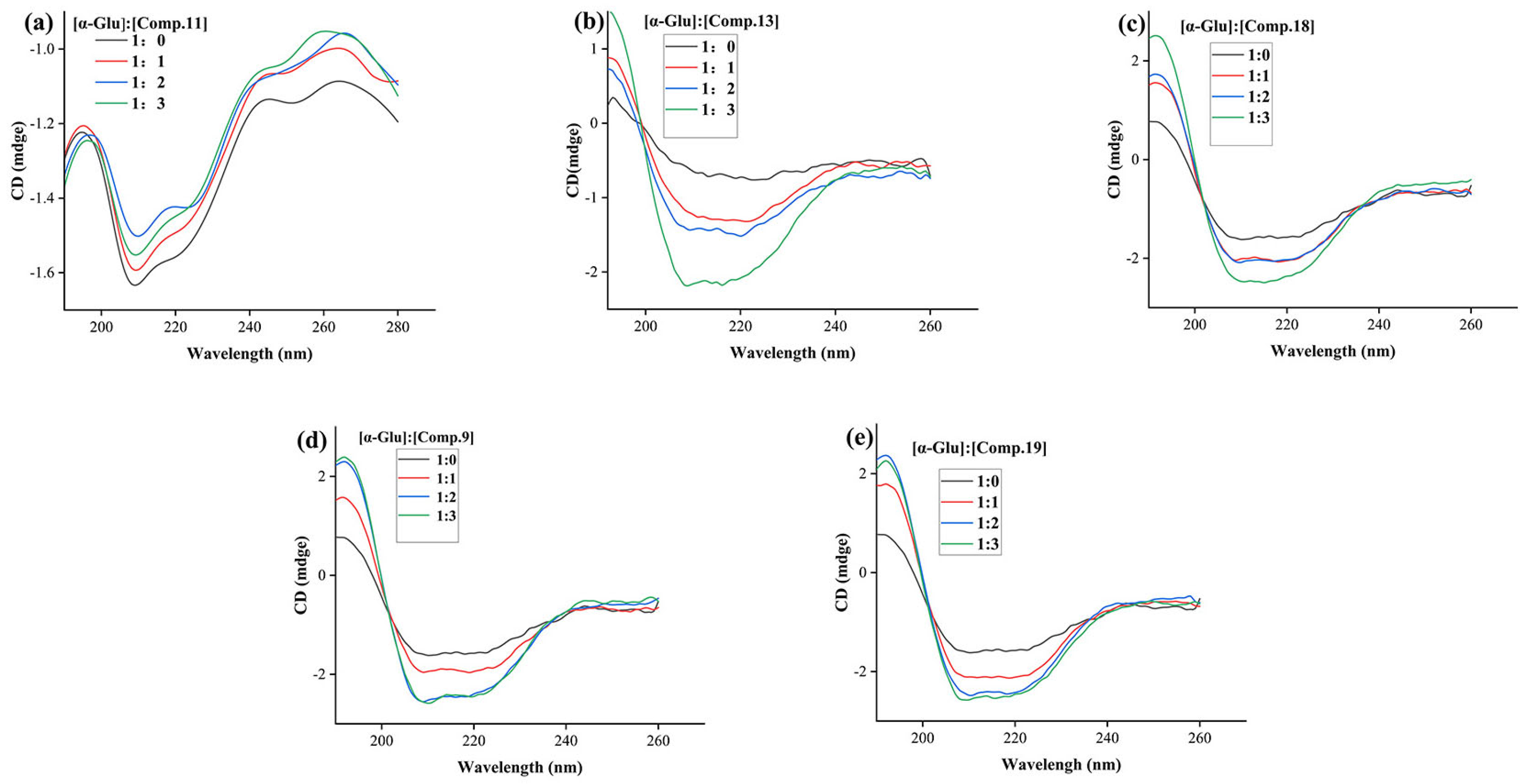
2.6. CD Spectra Assay
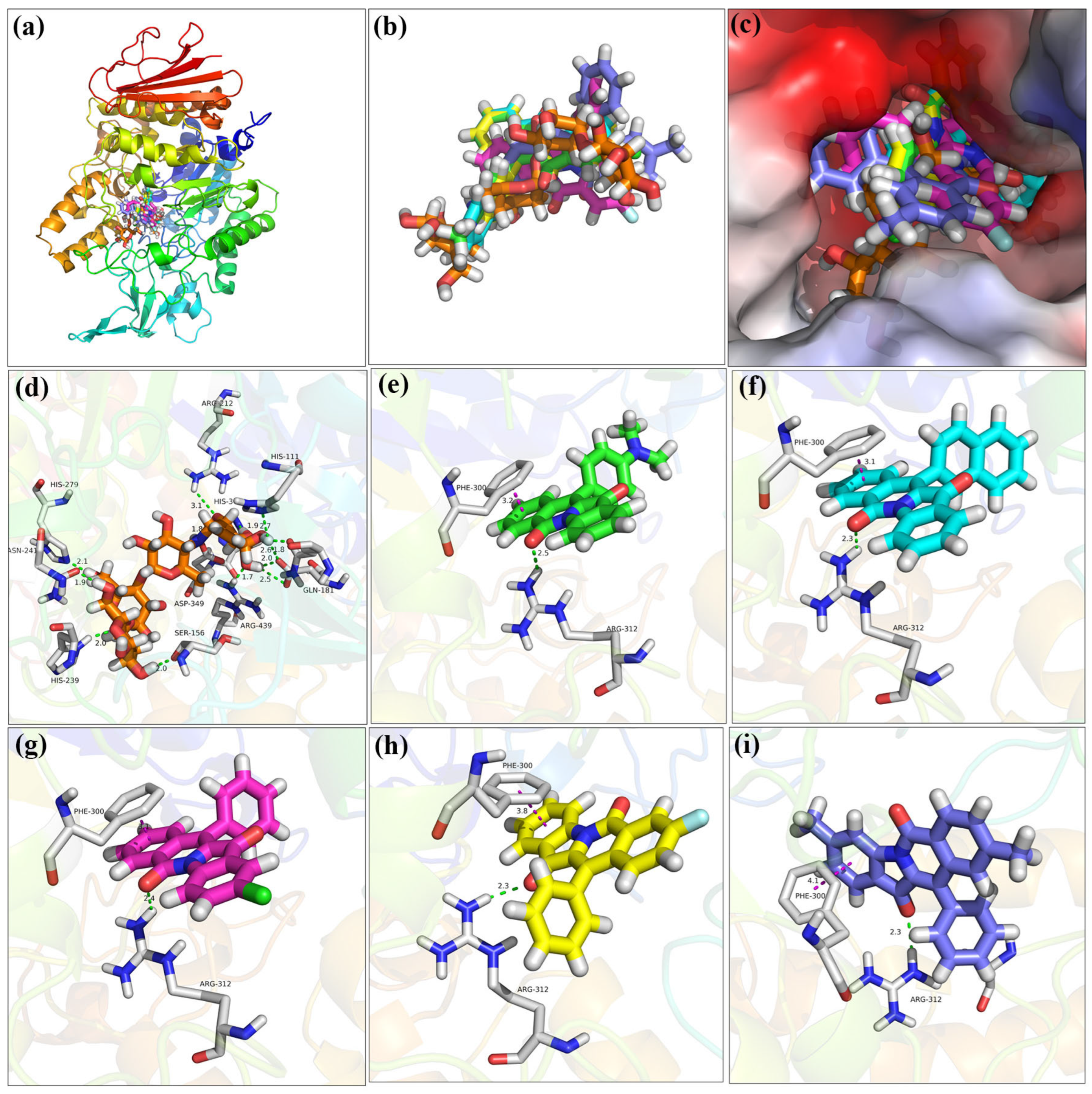
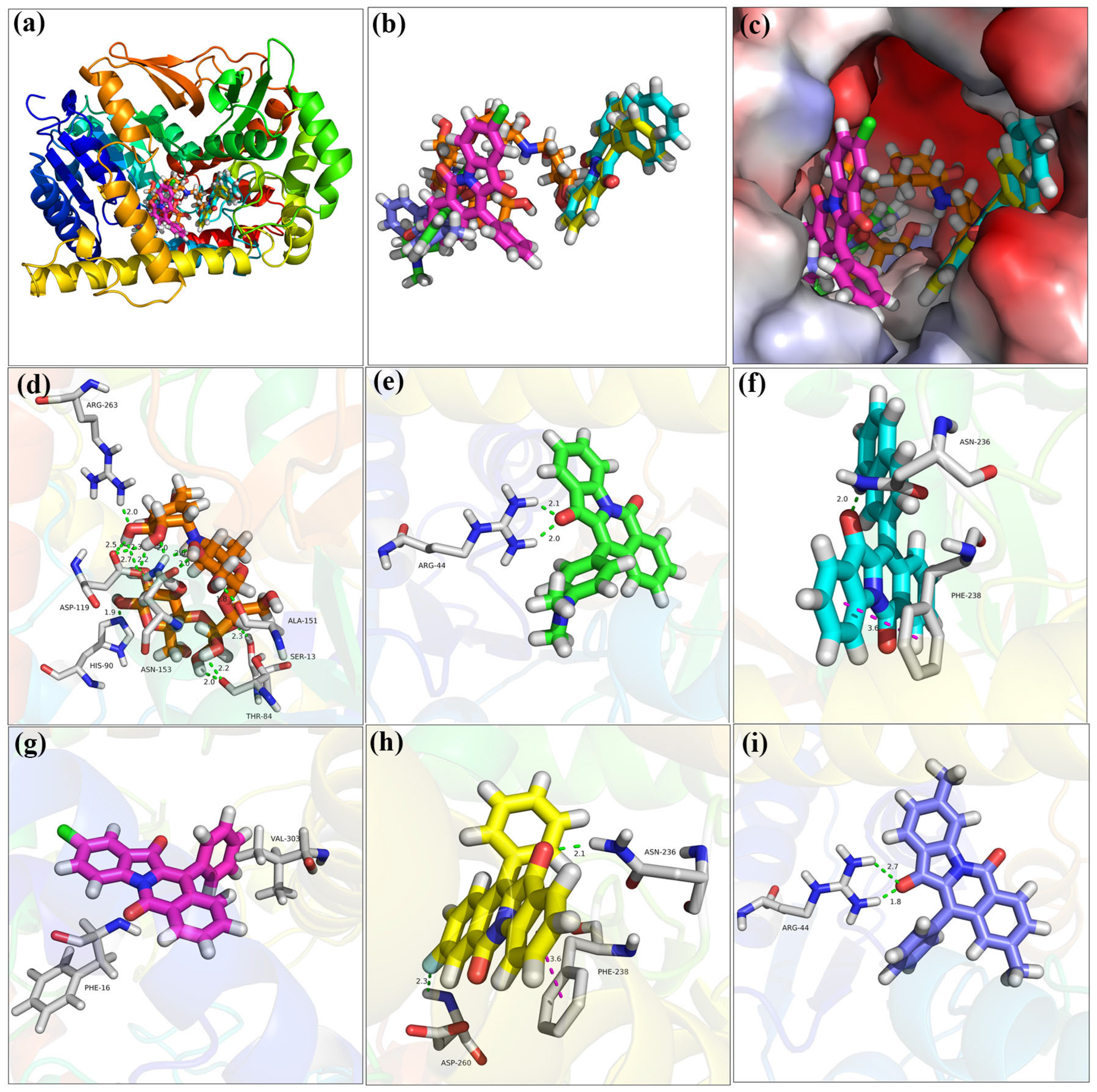
2.7. Molecular Docking
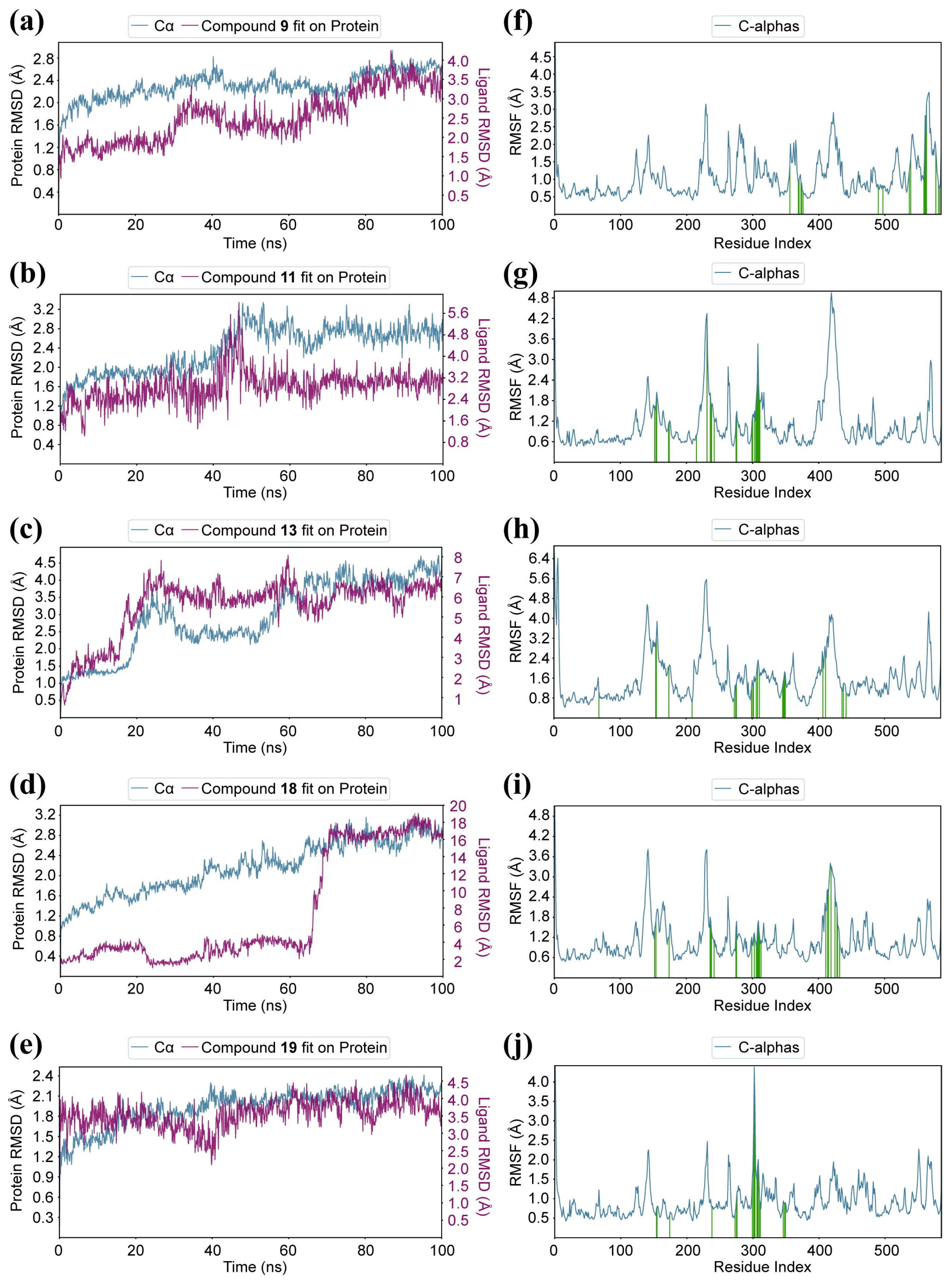
2.8. Molecular Dynamics Simulation
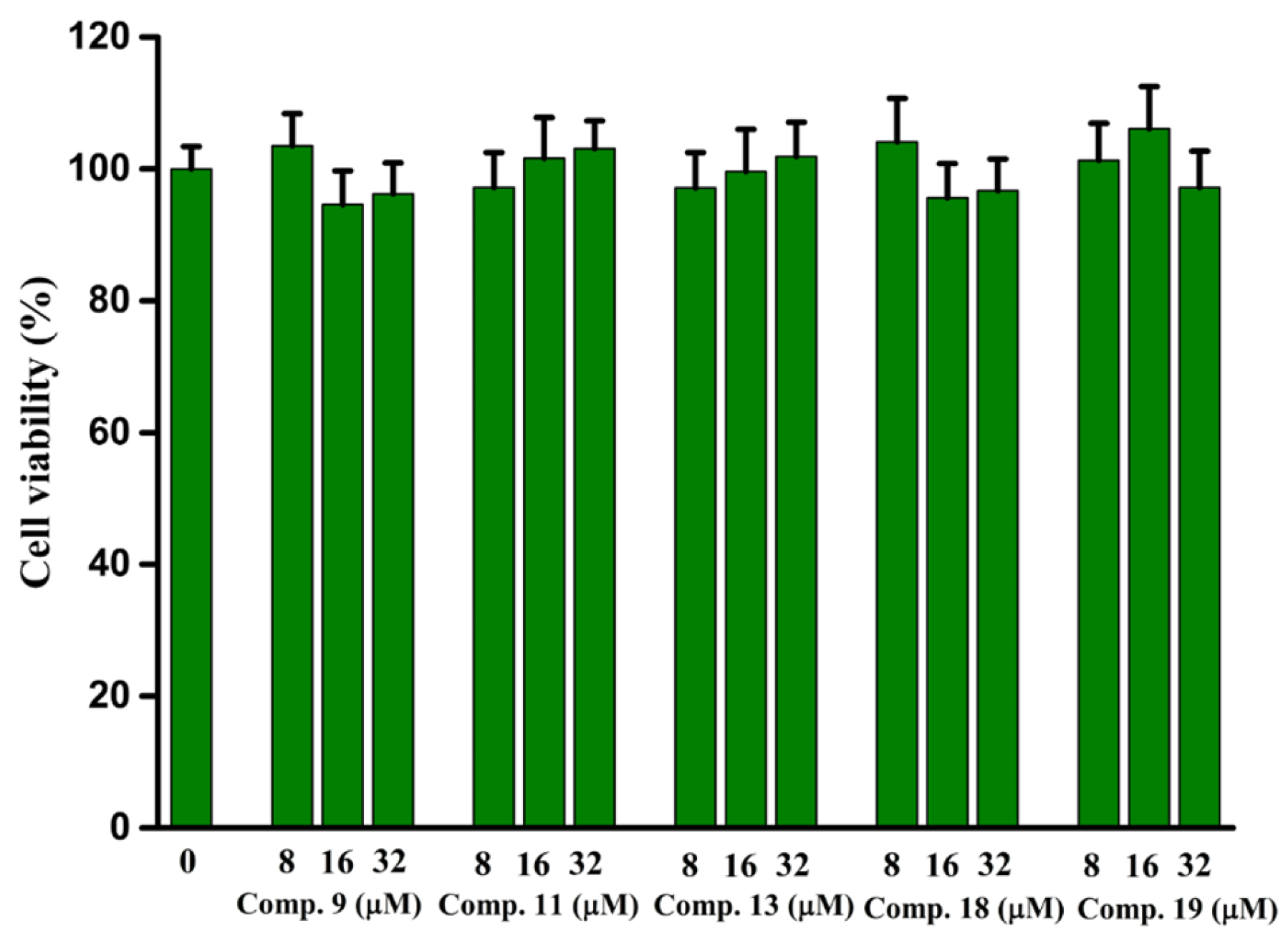
2.9. In Vitro Cytotoxicity
2.10. Drug-Like Properties
3. Experimental
3.1. Synthesis of Indolo[1,2-b]isoquinoline Derivatives (1–20)
3.2. Materials and Methods
3.3. α-Glucosidase Inhibition and Kinetics Assay
3.4. D Fluorescence Spectra
3.5. CD Spectra
3.6. Molecular Docking
3.7. Molecular Dynamics Simulation
3.8. MTT Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mushtaq, A.; Azam, U.; Mehreen, S.; Naseer, M.M. Synthetic alpha-glucosidase inhibitors as promising anti-diabetic agents: Recent developments and future challenges. Eur. J. Med. Chem. 2023, 249, 115119. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Bennett, P.; Chair, S.C.; Gregg, E.; Narayan, K.V.; Schmidt, M.I.; Sobngwi, E.; Tajima, N.; Tandon, N.; Unwin, N.; et al. Reprint of: Classification of diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 108972. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.W.; LeRoith, D. The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 93–96. [Google Scholar] [CrossRef]
- He, M.; Li, Y.J.; Shao, J.; Li, Y.S.; Cui, Z.N. Synthesis and biological evaluation of 2,5-disubstituted furan derivatives containing 1,3-thiazole moiety as potential alpha-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2023, 83, 129173. [Google Scholar] [CrossRef]
- Moghadam, E.S.; Al-Sadi, A.M.; Al-Harthy, T.; Faramarzi, M.A.; Shongwe, M.; Amini, M.; Abdel-Jalil, R. Synthesis, bioactivity, and molecular docking of benzimidazole-2-carbamate derivatives as potent α-glucosidase inhibitors. J. Mol. Struct. 2023, 1278, 134931. [Google Scholar] [CrossRef]
- Tshiluka, N.R.; Bvumbi, M.V.; Mnyakeni-Moleele, S.S. Synthesis, cytotoxicity and in vitro α-glucosidase inhibition of new N-substituted glitazone and rhodanine derivatives. Russ. J. Bioorg. Chem. 2023, 49, 384–389. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mulpuru, V.; Mishra, N. Discovery of novel coumarin analogs against the alpha-glucosidase protein target of diabetes mellitus: Pharmacophore-based QSAR, docking, and molecular dynamics simulation studies. ACS Omega 2020, 5, 32234–32249. [Google Scholar] [CrossRef]
- Tan, K.; Tesar, C.; Wilton, R.; Jedrzejczak, R.P.; Joachimiak, A. Interaction of antidiabetic alpha-glucosidase inhibitors and gut bacteria alpha-glucosidase. Protein Sci. 2018, 27, 1498–1508. [Google Scholar] [CrossRef] [Green Version]
- Serra-Barcellona, C.; Habib, N.C.; Honore, S.M.; Sánchez, S.S.; Genta, S.B. Enhydrin regulates postprandial hyperglycemia in diabetic tats by inhibition of alpha-glucosidase activity. Plant Foods Hum. Nutr. 2017, 72, 156–160. [Google Scholar] [CrossRef]
- Acar Çevik, U.; Celik, I.; Paşayeva, L.; Fatullayev, H.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. New benzimidazole-oxadiazole derivatives: Synthesis, alpha-glucosidase, alpha-amylase activity, and molecular modeling studies as potential antidiabetic agents. Arch. Pharm. 2023, 356, e2200663. [Google Scholar] [CrossRef]
- Deng, X.; Ke, J.; Zheng, Y.; Li, D.; Zhang, K.; Zheng, X.; Wu, J.; Xiong, Z.; Wu, P.; Xu, X.T. Synthesis and bioactivities evaluation of oleanolic acid oxime ester derivatives as α-glucosidase and α-amylase inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 451–461. [Google Scholar] [CrossRef]
- Moghadam Farid, S.; Noori, M.; Nazari Montazer, M.; Khalili Ghomi, M.; Mollazadeh, M.; Dastyafteh, N.; Irajie, C.; Zomorodian, K.; Mirfazli, S.M.; Mojtabavi, S.; et al. Synthesis and structure-activity relationship studies of benzimidazole-thioquinoline derivatives as alpha-glucosidase inhibitors. Sci. Rep. 2023, 13, 4392. [Google Scholar] [CrossRef]
- Pogaku, V.; Gangarapu, K.; Basavoju, S.; Tatapudi, K.K.; Katragadda, S.B. Design, synthesis, molecular modelling, ADME prediction and anti-hyperglycemic evaluation of new pyrazole-triazolopyrimidine hybrids as potent alpha-glucosidase inhibitors. Bioorg. Chem. 2019, 93, 103307. [Google Scholar] [CrossRef]
- Fan, M.; Yang, W.; Liu, L.; Peng, Z.; He, Y.; Wang, G. Design, synthesis, biological evaluation, and docking study of chromone-based phenylhydrazone and benzoylhydrazone derivatives as antidiabetic agents targeting alpha-glucosidase. Bioorg. Chem. 2023, 132, 106384. [Google Scholar] [CrossRef]
- Talaviya, P.A.; Saboo, B.D.; Dodiya, H.G.; Rao, S.K.; Joshi, S.R.; Modh, V.B.; Ghadiya, S.V. Retrospective comparison of voglibose or acarbose as an add-on therapy to sulfonylureas in Western Indian patients with uncontrolled overweight/obese type 2 diabetes. Diabetes Metab. Syndr. 2016, 10, 88–91. [Google Scholar] [CrossRef]
- Fattaheian-Dehkordi, S.; Hojjatifard, R.; Saeedi, M.; Khanavi, M. A Review on Antidiabetic Activity of Centaurea spp.: A New Approach for Developing Herbal Remedies. Evid.-Based Complement. Altern. Med. 2021, 2021, 5587938. [Google Scholar] [CrossRef]
- Khan, I.; Khan, A.; Halim, S.A.; Khan, M.; Zaib, S.; Al-Yahyaei, B.E.M.; Al-Harrasi, A.; Ibrar, A. Utilization of the common functional groups in bioactive molecules: Exploring dual inhibitory potential and computational analysis of keto esters against alpha-glucosidase and carbonic anhydrase-II enzymes. Int. J. Biol. Macromol. 2021, 167, 233–244. [Google Scholar] [CrossRef]
- Wongon, M.; Limpeanchob, N. Inhibitory effect of Artocarpus lakoocha Roxb and oxyresveratrol on alpha-glucosidase and sugar digestion in Caco-2 cells. Heliyon 2020, 6, e03458. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, O.M. Recent progress in biological activities of indole and indole alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S.M. Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats. J. Funct. Foods 2012, 4, 79–86. [Google Scholar] [CrossRef]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Mohammadi-Khanaposhtani, M.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Moghadamnia, A.A.; Larijani, B.; Mahdavi, M. Synthesis and biological evaluation of new dihydroindolizino[8,7-b]indole derivatives as novel α-glucosidase inhibitors. J. Mol. Struct. 2021, 1224, 129290. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, T.; Huo, Y.; Du, W. Exploration of isoquinoline alkaloids as potential inhibitors against human islet amyloid polypeptide. ACS Chem. Neurosci. 2022, 13, 2164–2175. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Qi, Z.; Yang, A.H.; Ma, A.J.; Peng, J.B. Palladium-catalyzed carbonylative sonogashira/annulation reaction: Synthesis of indolo[1,2-b]isoquinolines. Org. Lett. 2022, 24, 1201–1206. [Google Scholar] [CrossRef]
- Qin, B.; Huang, S.; Chen, J.-Q.; Xiao, W.; Wu, J. Metal-free synthesis of sulfonylated indolo[2,1-a]isoquinolines from sulfur dioxide. Org. Chem. Front. 2022, 9, 3521–3526. [Google Scholar] [CrossRef]
- Ramana, C.; Swami, A. Target cum Flexibility: Synthesis of indolo[1,2-b]isoquinoline derivatives via cobalt-catalyzed [2+2+2] cyclotrimerization. Synlett 2015, 26, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Sanz, R.; Ignacio, J.M.; Castroviejo, M.P.; Fañanás, F.J. Synthesis of new indolo[1,2-b]isoquinoline derivatives from N-(2-bromobenzyl)indole. Arkivoc 2007, 4, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Zheng, Y.Y.; Lin, A.T.; Zhang, X.; Wu, X.Z.; Lin, J.; Xu, X.T.; Xiong, Z. Design, synthesis and evaluation of indole-based bisacylhydrazone derivatives as α-glucosidase inhibitors. J. Mol. Struct. 2023, 1271, 134124. [Google Scholar] [CrossRef]
- Fan, M.; Zhong, X.; Huang, Y.; Peng, Z.; Wang, G. Synthesis, biological evaluation and molecular docking studies of chromone derivatives as potential α-glucosidase inhibitors. J. Mol. Struct. 2023, 1274, 134575. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.Y.; Hu, C.M.; Wu, X.Z.; Lin, J.; Xiong, Z.; Zhang, K.; Xu, X.T. Synthesis and biological evaluation of coumarin derivatives containing oxime ester as α-glucosidase inhibitors. Arab. J. Chem. 2022, 15, 104072. [Google Scholar] [CrossRef]
- Xu, X.T.; Deng, X.Y.; Chen, J.; Liang, Q.M.; Zhang, K.; Li, D.L.; Wu, P.P.; Zheng, X.; Zhou, R.P.; Jiang, Z.Y.; et al. Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2020, 189, 112013. [Google Scholar] [CrossRef]
- Shamim, S.; Khan, K.M.; Ullah, N.; Mahdavi, M.; Faramarzi, M.A.; Larijani, B.; Salar, U.; Rafique, R.; Taha, M.; Perveen, S. Synthesis, in vitro, and in silico evaluation of Indazole Schiff bases as potential α-glucosidase inhibitors. J. Mol. Struct. 2021, 1242, 130826. [Google Scholar] [CrossRef]
- Hameed, S.; Seraj, F.; Rafique, R.; Chigurupati, S.; Wadood, A.; Rehman, A.U.; Venugopal, V.; Salar, U.; Taha, M.; Khan, K.M. Synthesis of benzotriazoles derivatives and their dual potential as alpha-amylase and alpha-glucosidase inhibitors in vitro: Structure-activity relationship, molecular docking, and kinetic studies. Eur. J. Med. Chem. 2019, 183, 111677. [Google Scholar] [CrossRef]
- Zheng, P.F.; Xiong, Z.; Liao, C.Y.; Zhang, X.; Feng, M.; Wu, X.Z.; Lin, J.; Lei, L.S.; Zhang, Y.C.; Wang, S.H.; et al. In vitro and in silico studies of bis (indol-3-yl) methane derivatives as potential alpha-glucosidase and alpha-amylase inhibitors. J. Enzyme Inhib. Med. Chem. 2021, 36, 1938–1951. [Google Scholar] [CrossRef]
- Wang, G.; Chen, M.; Qiu, J.; Xie, Z.; Cao, A. Synthesis, in vitro α-glucosidase inhibitory activity and docking studies of novel chromone-isatin derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 113–116. [Google Scholar] [CrossRef]
- Hussain, F.; Khan, Z.; Jan, M.S.; Ahmad, S.; Ahmad, A.; Rashid, U.; Ullah, F.; Ayaz, M.; Sadiq, A. Synthesis, in-vitro α-glucosidase inhibition, antioxidant, in-vivo antidiabetic and molecular docking studies of pyrrolidine-2,5-dione and thiazolidine-2,4-dione derivatives. Bioorg. Chem. 2019, 2019, 103128. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Wang, J.; Li, X.; Li, J. Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur. J. Med. Chem. 2017, 125, 423–429. [Google Scholar] [CrossRef]
- Özil, M.; Parlak, C.; Baltaş, N. A simple and efficient synthesis of benzimidazoles containing piperazine or morpholine skeleton at C-6 position as glucosidase inhibitors with antioxidant activity. Bio. Chem. 2017, 76, 68–77. [Google Scholar] [CrossRef]
- Hu, C.M.; Wang, W.J.; Ye, Y.N.; Kang, Y.; Lin, J.; Wu, P.P.; Li, D.L.; Bai, L.P.; Xu, X.T.; Li, B.Q.; et al. Novel cinnamic acid magnolol derivatives as potent α-glucosidase and α-amylase inhibitors: Synthesis, in vitro and in silico studies. Bioorg. Chem. 2021, 116, 105291. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xiao, D.; Lu, L.; Liang, B.; Xiong, Z.; Xu, X. New β-carboline derivatives as potential α-glucosidase inhibitor: Synthesis and biological activity evaluation. J. Mol. Struct. 2023, 1283, 135279. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Zhu, W.-J.; Lu, L.; Hu, C.-M.; Zheng, Y.-Y.; Zhang, X.; Lin, J.; Wu, J.-Y.; Xiong, Z.; Zhang, K.; et al. Synthesis and anti-α-glucosidase activity evaluation of betulinic acid derivatives. Arab. J. Chem. 2023, 16, 104659. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, X.; Kang, Y.; Xiong, Z.; Zhang, K.; Xu, X.; Bai, L.; Li, H. Novel coumarin derivatives as potential tyrosinase inhibitors: Synthesis, binding analysis and biological evaluation. Arab. J. Chem. 2023, 16, 104724. [Google Scholar] [CrossRef]
- Fan, M.; Yang, W.; Peng, Z.; He, Y.; Wang, G. Chromone-based benzohydrazide derivatives as potential α-glucosidase inhibitor: Synthesis, biological evaluation and molecular docking study. Bioorg. Chem. 2023, 131, 106276. [Google Scholar] [CrossRef]

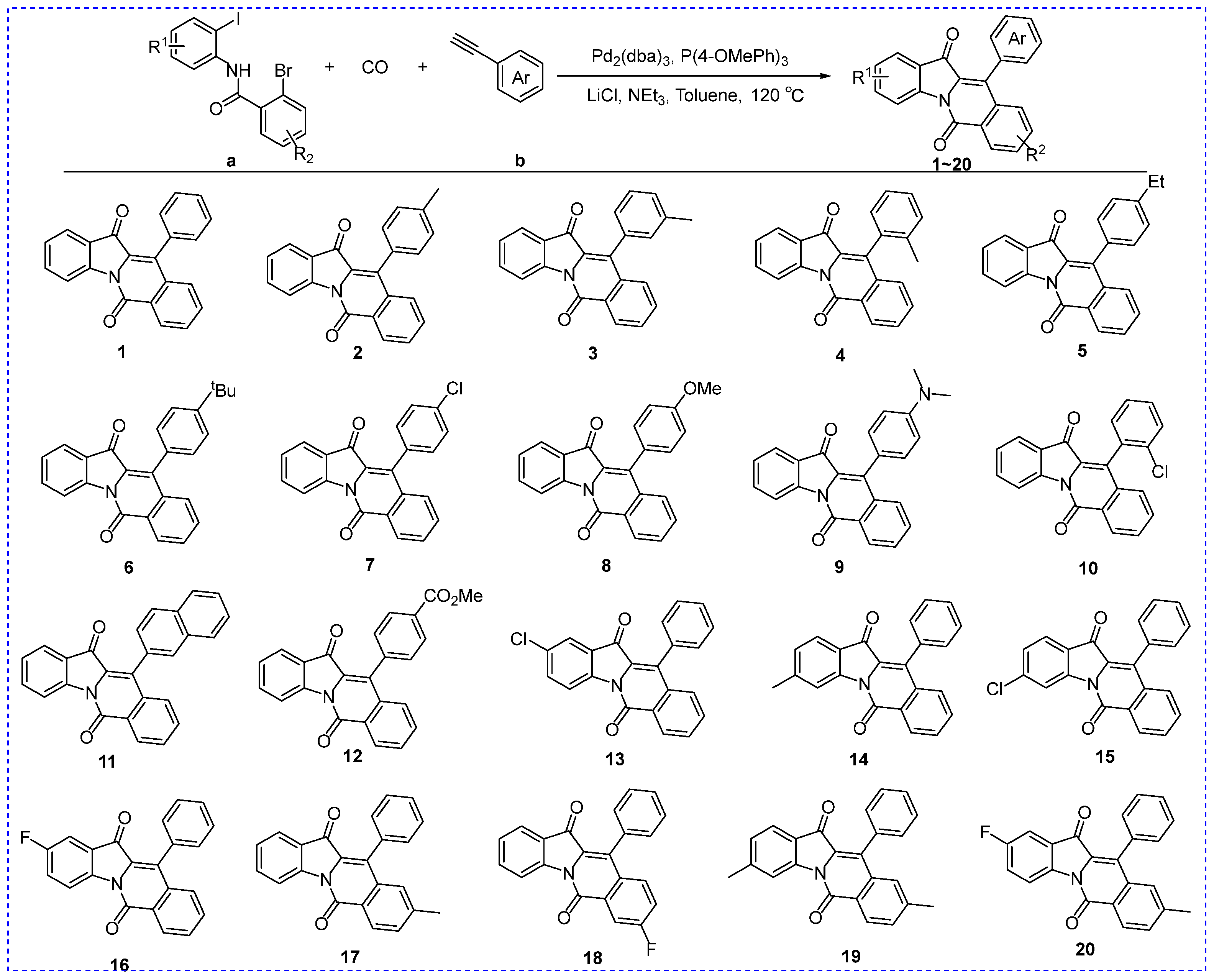
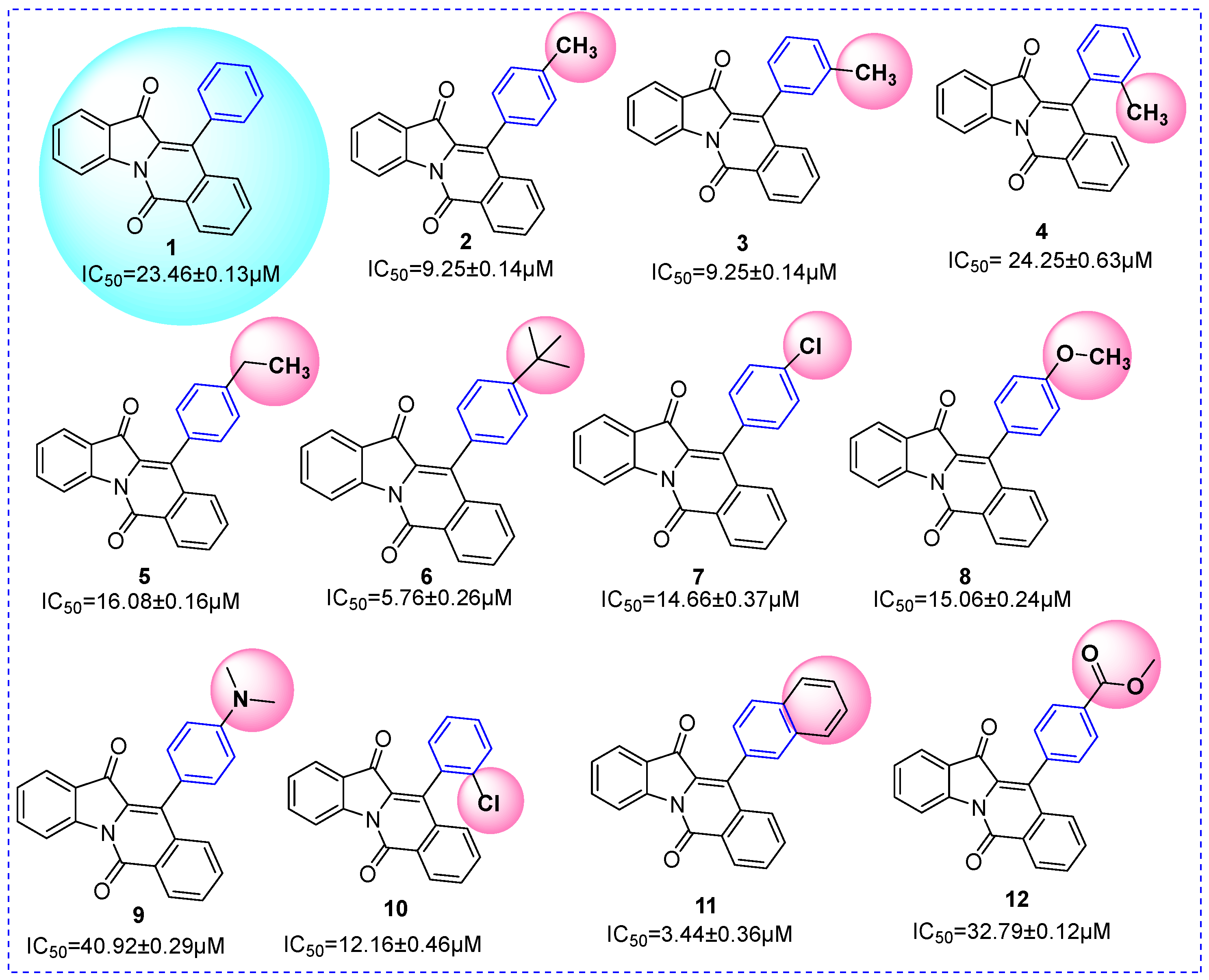
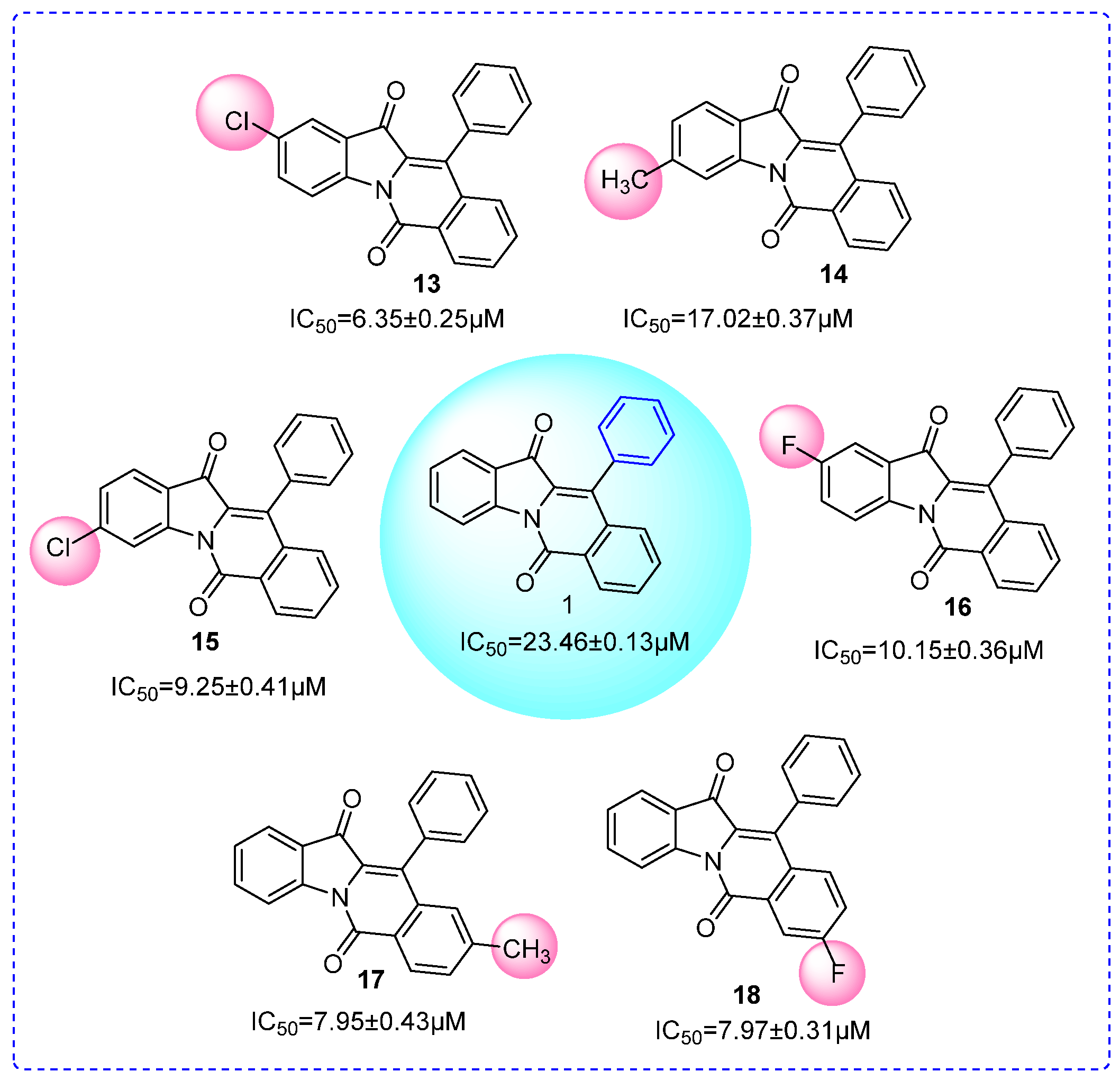


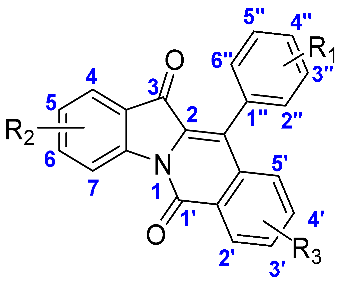 | ||||
| Compound | R1 | R2 | R3 | IC50 (μM) |
| 1 | H | H | H | 23.46 ± 0.23 |
| 2 | 4-CH3 | H | H | 17.37 ± 0.25 |
| 3 | 3-CH3 | H | H | 9.25 ± 0.14 |
| 4 | 2-CH3 | H | H | 22.25 ± 0.63 |
| 5 | 4-CH2CH3 | H | H | 16.08 ± 0.46 |
| 6 | 4-C(CH3)3 | H | H | 5.76 ± 0.26 |
| 7 | 4-Cl | H | H | 14.66 ± 0.37 |
| 8 | 4-OCH3 | H | H | 15.06 ± 0.44 |
| 9 | 4-N(CH3)2 | H | H | 40.92 ± 0.69 |
| 10 | 2-Cl | H | H | 12.16 ± 0.46 |
| 11 | naphthalene | H | H | 3.44 ± 0.36 |
| 12 | 4-COOCH3 | H | H | 32.79 ± 0.72 |
| 13 | H | 5-Cl | H | 6.35 ± 0.25 |
| 14 | H | 6-CH3 | H | 17.02 ± 0.37 |
| 15 | H | 6-Cl | H | 9.25 ± 0.41 |
| 16 | H | 5-F | H | 10.15 ± 0.36 |
| 17 | H | H | 4-CH3 | 7.95 ± 0.43 |
| 18 | H | H | 3-F | 7.97 ± 0.31 |
| 19 | H | 6-CH3 | 4-CH3 | 41.24 ± 0.76 |
| 20 | H | 5-F | 4-CH3 | 11.87 ± 0.36 |
| Acarbose | 640.57 ± 5.13 | |||
| Compound | Ki (μM) | Kis (μM) | Km (μM) | Vmax (μM·min−1·mg−1) |
|---|---|---|---|---|
| 9 | 30.46 | 96.19 | 1.67 | 0.12 |
| 11 | 0.46 | 14.57 | 0.12 | 0.06 |
| 13 | 3.62 | 11.65 | 0.60 | 0.08 |
| 18 | 4.62 | 12.34 | 0.49 | 0.08 |
| 19 | 8.38 | 42.30 | 0.01 | 0.05 |
| Comp. | Molar Ratio [α-Glu]:[Comp.] | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Rndm Coil (%) |
|---|---|---|---|---|---|
| 9 | 1:0 | 9.40 | 38.40 | 19.50 | 35.40 |
| 1:1 | 10.80 | 35.60 | 19.30 | 35.10 | |
| 1:2 | 13.00 | 31.90 | 19.30 | 34.60 | |
| 1:3 | 13.10 | 31.70 | 19.10 | 33.40 | |
| 11 | 1:0 | 8.50 | 34.10 | 19.80 | 35.50 |
| 1:1 | 8.40 | 34.30 | 20.00 | 35.60 | |
| 1:2 | 8.20 | 34.70 | 20.00 | 35.60 | |
| 1:3 | 7.80 | 35.20 | 20.10 | 35.80 | |
| 13 | 1:0 | 9.40 | 38.40 | 19.50 | 35.40 |
| 1:1 | 8.80 | 40.60 | 19.30 | 35.60 | |
| 1:2 | 9.10 | 39.60 | 19.40 | 35.40 | |
| 1:3 | 11.20 | 34.60 | 19.60 | 35.10 | |
| 18 | 1:0 | 9.40 | 38.40 | 19.50 | 35.40 |
| 1:1 | 11.10 | 35.30 | 19.40 | 35.00 | |
| 1:2 | 11.20 | 35.00 | 19.30 | 35.00 | |
| 1:3 | 12.80 | 35.00 | 19.30 | 34.70 | |
| 19 | 1:0 | 9.40 | 38.40 | 19.50 | 35.40 |
| 1:1 | 12.90 | 31.80 | 19.40 | 34.60 | |
| 1:2 | 11.50 | 34.20 | 19.30 | 34.70 | |
| 1:3 | 12.80 | 34.40 | 19.30 | 34.90 |
| Compd. | ΔGbind (kcal/mol) | ΔEVDW (kcal/mol) | ΔEele (kcal/mol) | ΔEGB (kcal/mol) | ΔEGA (kcal/mol) |
|---|---|---|---|---|---|
| 9 | −47.23 ± 5.09 | −42.34 ± 2.31 | −6.89 ± 1.71 | 26.57 ± 5.10 | −14.76 ± 2.04 |
| 11 | −66.94 ± 4.24 | −46.50 ± 2.22 | −8.60 ± 2.89 | 21.62 ± 2.72 | −26.42 ± 1.71 |
| 13 | −60.27 ± 3.20 | −45.21 ± 1.78 | −0.78 ± 1.55 | 18.45 ± 1.84 | −24.08 ± 1.18 |
| 18 | −52.87 ± 5.81 | −42.34 ± 2.31 | −12.64 ± 3.99 | 25.44 ± 1.47 | −14.76 ± 2.03 |
| 19 | −51.49 ± 4.20 | −35.25 ± 2.46 | −3.73 ± 3.40 | 12.92 ± 1.86 | −20.70 ± 1.29 |
| Comp. | MW | RB | HBA | HBD | PPSA | Log Po/w | WS |
|---|---|---|---|---|---|---|---|
| 9 | 373.4 | 1 | 2 | 0 | 39.07 | 4.83 | Poorly |
| 11 | 373.4 | 1 | 2 | 0 | 39.07 | 4.83 | Poorly |
| 13 | 357.79 | 1 | 2 | 0 | 39.07 | 4.46 | Poorly |
| 18 | 341.33 | 1 | 3 | 0 | 39.07 | 4.23 | Poorly |
| 19 | 351.40 | 1 | 2 | 0 | 39.07 | 4.61 | Poorly |
| MF | MW | RB | HBA | HBD | MolVol | BBB | MolLPSA |
|---|---|---|---|---|---|---|---|
| 9 | 373.4 | 1 | 2 | 0 | 262.08 A3 | 4.10 | 27.92 A2 |
| 11 | 373.11 | 1 | 2 | 0 | 262.08 A2 | 4.10 | 27.92 A2 |
| 13 | 357.79 | 1 | 2 | 0 | 357.74 A3 | 4.69 | 38.19 A2 |
| 18 | 341.33 | 1 | 2 | 0 | 342.46 A3 | 4.69 | 28.19 A2 |
| 19 | 1 | 2 | 0 | 378.50 A3 | 4.66 | 28.19 A2 | 1 |
| MF | MW | RB | Acceptors | Donors | VDss | Log P | WS |
|---|---|---|---|---|---|---|---|
| 9 | 373.4 | 1 | 3 | 0 | −0.61 | 5.355 | −8.256 |
| 11 | 373.4 | 1 | 3 | 0 | −0.61 | 5.355 | −8.256 |
| 13 | 357.79 | 1 | 3 | 0 | −0.511 | 4.8555 | −4.52 |
| 18 | 341.33 | 1 | 3 | 0 | −0.604 | 4.3412 | −4.522 |
| 19 | 351.40 | 1 | 3 | 0 | −0.363 | 4.81894 | −5.622 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, L.; Lu, L.; Xu, X.; Hu, J.; Peng, J.-B. Anti-α-Glucosidase, SAR Analysis, and Mechanism Investigation of Indolo[1,2-b]isoquinoline Derivatives. Molecules 2023, 28, 5282. https://doi.org/10.3390/molecules28135282
Li M, Li L, Lu L, Xu X, Hu J, Peng J-B. Anti-α-Glucosidase, SAR Analysis, and Mechanism Investigation of Indolo[1,2-b]isoquinoline Derivatives. Molecules. 2023; 28(13):5282. https://doi.org/10.3390/molecules28135282
Chicago/Turabian StyleLi, Mengyue, Lin Li, Li Lu, Xuetao Xu, Jinhui Hu, and Jin-Bao Peng. 2023. "Anti-α-Glucosidase, SAR Analysis, and Mechanism Investigation of Indolo[1,2-b]isoquinoline Derivatives" Molecules 28, no. 13: 5282. https://doi.org/10.3390/molecules28135282





