Bioconversion of Glycosidic Precursors from Sour Guava (Psidium friedrichsthalianum Nied.) Fruit by the Oral Microbiota into Odor-Active Volatile Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Oral Microbiota on Glycosidic Precursors (GP)
2.2. Effect of P. friedrichsthalianum Glycosidic Precursors on the Oral Microbiota
3. Materials and Methods
3.1. Materials
3.2. Plant Material
3.3. Preparation of Human Saliva Samples
3.4. In Vitro Experiments with the Glycosidic Extract (GP) and Human Saliva
3.5. HS-SPME (Headspace Solid-Phase Microextraction) of Volatile Compounds after Incubation of the P. friedrichsthalianum Aroma Precursors with Human Saliva
3.6. GC–MS (Gas Chromatography–Mass Spectrometry) Analysis of Volatile Compounds
3.7. Counting of Microorganisms in Human Saliva
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cuadrado-Silva, C.T.; Pozo-Bayón, M.A.; Osorio, C. Identification of aroma compounds and precursors of sour guava (Psidium friedrichsthalianum Nied.) following a sensomics approach. Eur. Food Res. Technol. 2016, 243, 1–10. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the aroma-active compounds in pink guava (Psidium guajava L.) by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Sefton, M.A.; Francis, I.L. Glycosidic precursors of varietal grape and wine flavor. In Flavor Precursors: Thermal and Enzymatic Conversions; Teranishi, R., Takeoka, G.R., Guntert, M., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 74–86. [Google Scholar]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Williams, P.J. Hydrolytic flavor release in fruit and wines through hydrolysis of nonvolatile precursors. In Flavor Science: Sensible Principles and Techniques; Acree, T.E., Teranishi, R., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 287–308. [Google Scholar]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma precursors in grapes and wine: Flavor release during wine production and consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Onetto, C.; Hixson, J.; Bilogrevic, E.; Schueth, L.; Pisaniello, L.; Borneman, A.; Herderich, M.; de Barros Lopes, M.; Francis, L. Factors contributing to interindividual variation in retronasal odor perception from aroma glycosides: The role of odorant sensory detection threshold, oral microbiota, and hydrolysis in saliva. J. Agric. Food Chem. 2020, 68, 10299–10309. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, K.M.; Alston, M.J.; Chappell, C.G.; Taylor, A.J. Carbohydrate-flavour conjugates in wine. Carbohydr. Polym. 1999, 38, 283–286. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke-derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of a HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2013, 61, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glyconconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Cueva, C.; Pozo-Bayón, M.A.; Moreno-Arribas, M.V. Ability of human oral microbiota to produce wine odorant aglycones from odourless grape glycosidic aroma precursors. Food Chem. 2015, 187, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Black, C.A.; Barker, A.; Pearson, W.; Hayasaya, Y.; Francis, I.L. The contribution of wine-derived monoterpene glycosides to retronasal odour during tasting. Food Chem. 2017, 232, 413–424. [Google Scholar] [CrossRef] [PubMed]
- How, M.S.; Jones, J.R.; Morgenstern, M.P.; Gray-Stuart, E.; Bronlund, J.E.; Saint-Eve, A.; Trelea, I.C.; Souchon, I. Modelling the role of oral processing on in vivo aroma release of white rice: Conceptual model and experimental validation. LWT-Food Sci. Technol. 2021, 141, 110918. [Google Scholar] [CrossRef]
- Tian, Y.; He, X.; Torralba, M.; Yooseph, S.; Nelson, K.E.; Lux, R.; McLean, J.S.; Yu, G.; Shi, W. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol. Oral Microbiol. 2010, 25, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schall, K.P.; Lee, H.J. Actinomycete infections in human-a Review. Gene 1992, 115, 201–211. [Google Scholar] [CrossRef]
- Li, J.; Helmerhorst, E.J.; Leone, C.W.; Troxler, R.F.; Yaskell, T.; Haffajee, A.D.; Socransky, S.S.; Oppenheim, F.G. Identification of early microbial colonizers in human dental biofilm. J. Appl. Microbiol. 2004, 97, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
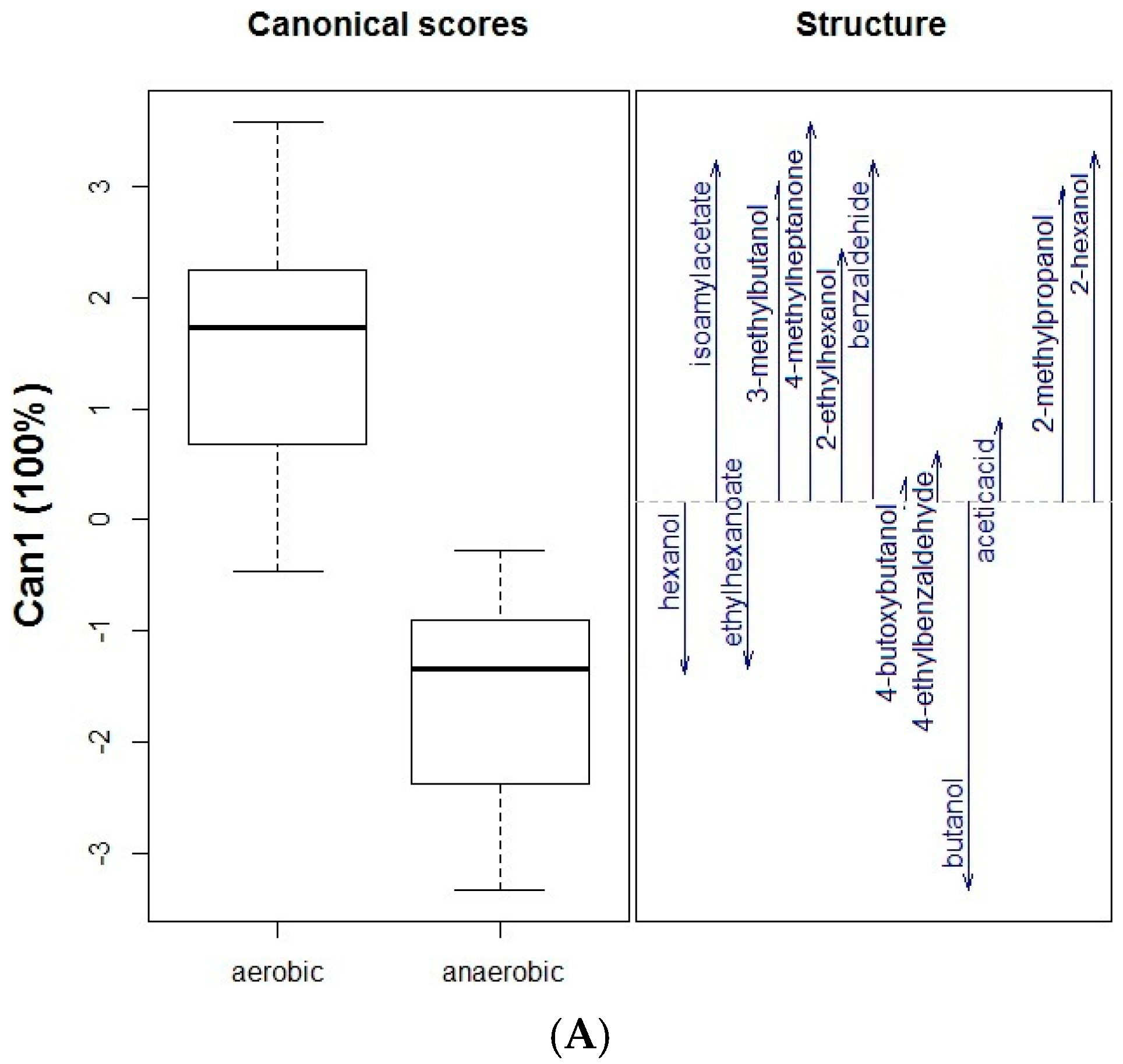
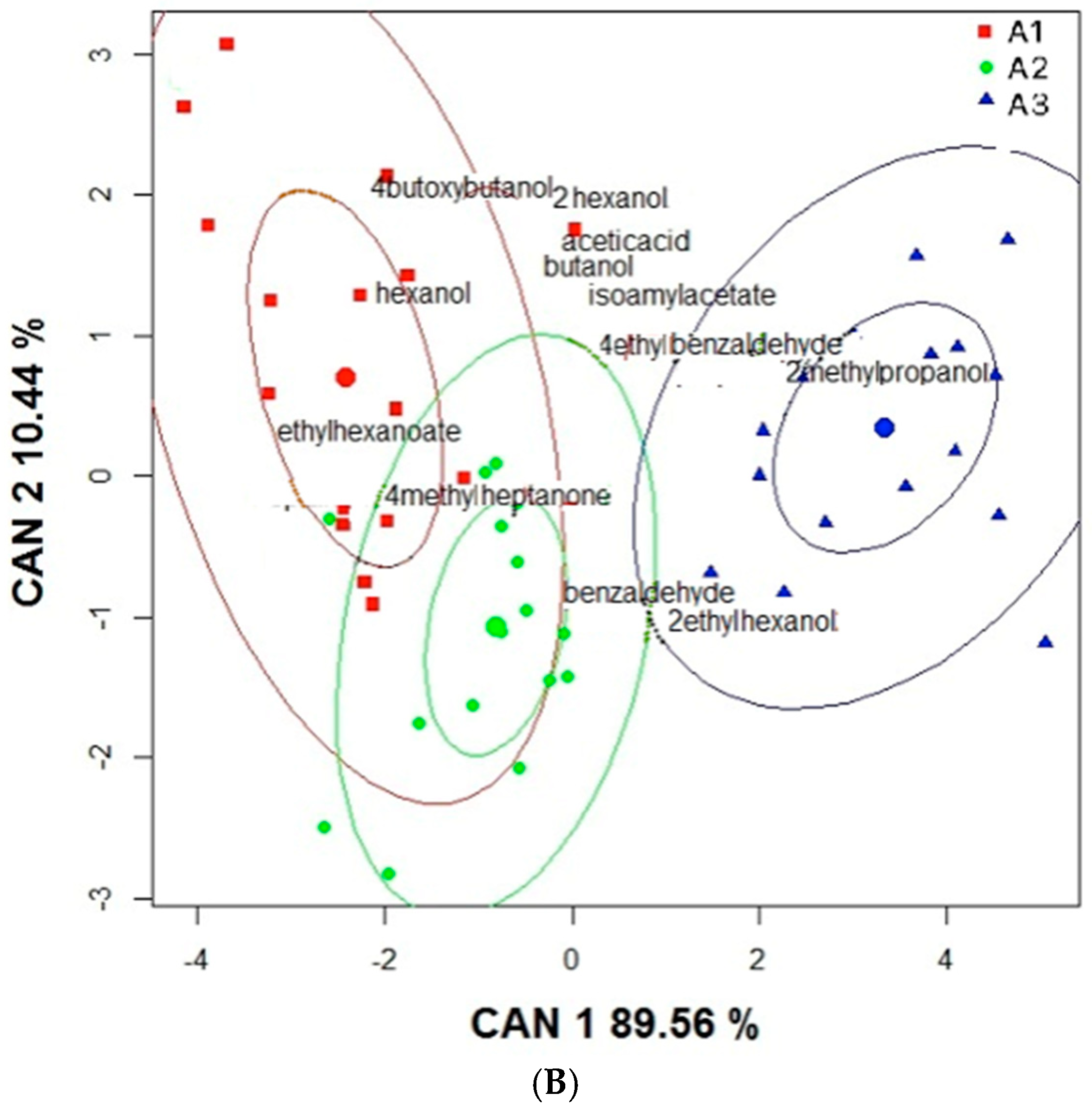
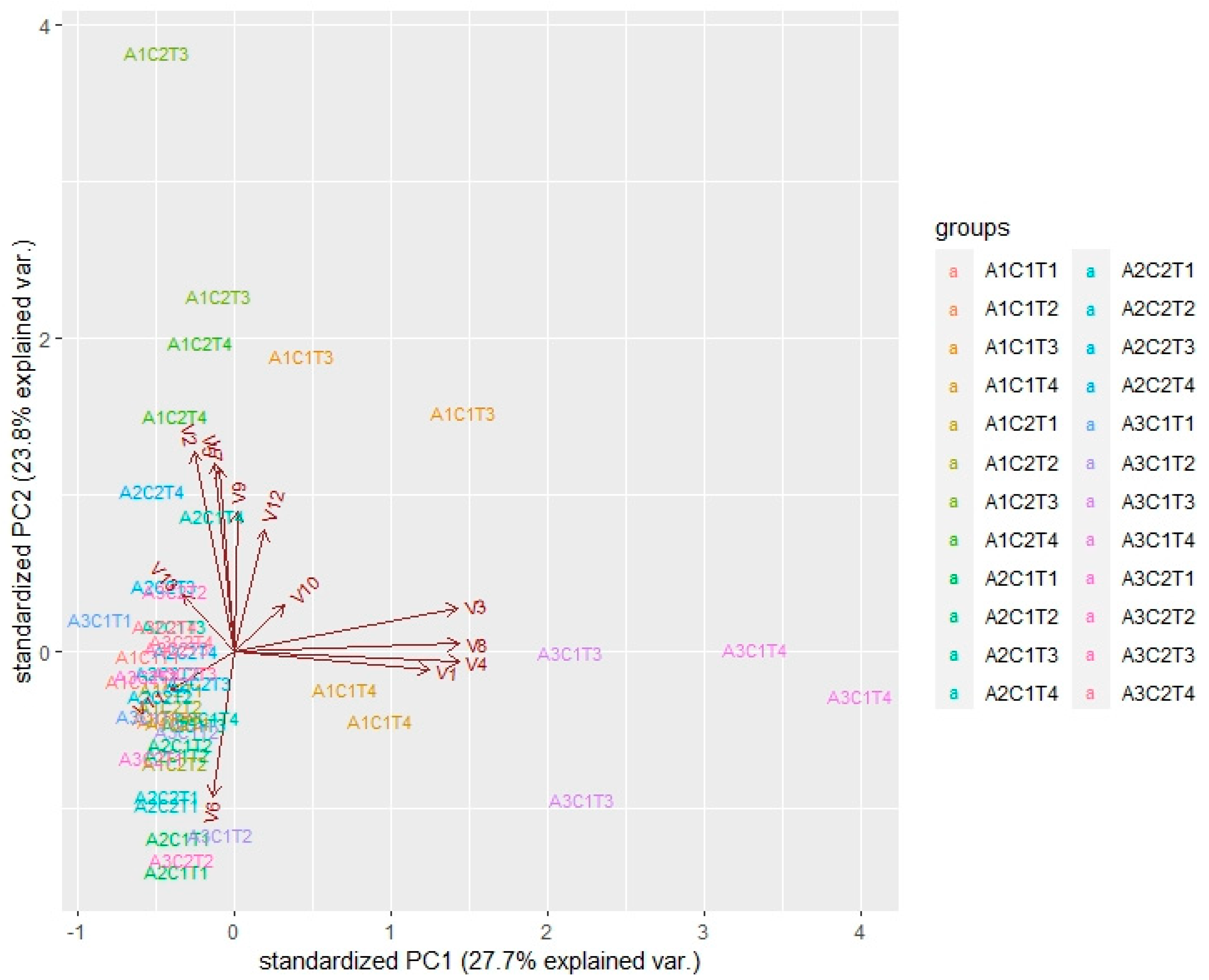
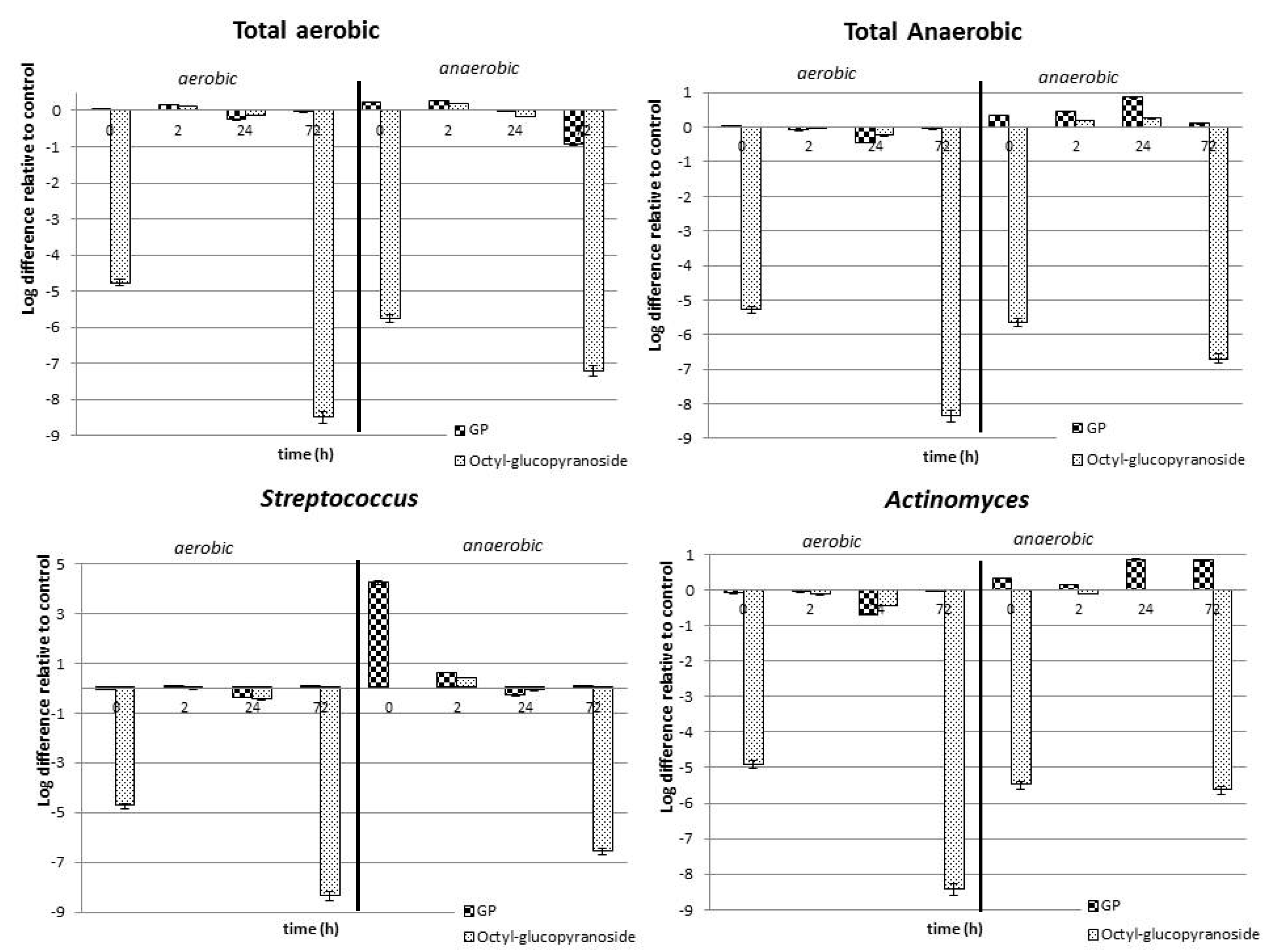
| No. | Compound a | RIDB-wax | Aerobic Conditions (C1, Amount in ppm) e | Anaerobic Conditions (C2, Amount in ppm) e | ||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A1 | A2 | A3 | |||
| V1 | 2-Methyl-1-propanol b | 1085 | 0.00315 | 0.00168 | 0.00297 | 0.00178 | 0.00064 | - |
| V2 | 1-Butanol b | 1176 | 0.02510 | 0.01480 | 0.03870 | 0.02450 | 0.01400 | 0.03430 |
| V3 | 2-Hexanol b,d | 1197 | 0.05350 | - | 0.05800 | 0.01510 | - | - |
| V4 | 3-Methyl-1-butanol | 1213 | 0.03060 | 0.00476 | 0.05430 | 0.01620 | 0.00406 | 0.02960 |
| V5 | Hexanol c,d | 1368 | 0.02200 | 0.03800 | - | 0.19100 | - | - |
| V6 | 2-Ethyl-1-hexanol b | 1494 | 0.14400 | 0.20400 | 0.15800 | 0.13100 | 0.14500 | 0.13700 |
| V7 | 4-Butoxy-1-butanol b | 1691 | 0.01680 | 0.00707 | 0.01270 | 0.01360 | 0.00874 | 0.00944 |
| V8 | Isoamyl acetate b,d | 1159 | 0.02500 | - | 0.03220 | - | - | - |
| V9 | Ethyl hexanoate c | 1238 | 0.00592 | 0.00304 | - | 0.00877 | 0.00207 | - |
| V10 | 4-Methyl-2-heptanone b | 1250 | 0.00898 | 0.00805 | - | 0.00550 | 0.00269 | - |
| V11 | Benzaldehyde | 1528 | 0.42000 | 0.14800 | 0.54000 | 0.23900 | 0.16500 | 0.25000 |
| V12 | 4-Ethyl benzaldehyde b | 1786 | 0.02020 | 0.01430 | 0.02860 | 0.01960 | 0.01450 | 0.02200 |
| V13 | Acetic acid | 1452 | 0.08790 | 0.01740 | 0.10900 | 0.01150 | 0.01920 | 0.05060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadrado-Silva, C.T.; Muñoz-González, C.; Giraldo, R.; Del Pozo-Bayón, M.Á.; Osorio, C. Bioconversion of Glycosidic Precursors from Sour Guava (Psidium friedrichsthalianum Nied.) Fruit by the Oral Microbiota into Odor-Active Volatile Compounds. Molecules 2022, 27, 1269. https://doi.org/10.3390/molecules27041269
Cuadrado-Silva CT, Muñoz-González C, Giraldo R, Del Pozo-Bayón MÁ, Osorio C. Bioconversion of Glycosidic Precursors from Sour Guava (Psidium friedrichsthalianum Nied.) Fruit by the Oral Microbiota into Odor-Active Volatile Compounds. Molecules. 2022; 27(4):1269. https://doi.org/10.3390/molecules27041269
Chicago/Turabian StyleCuadrado-Silva, Carmen Tatiana, Carolina Muñoz-González, Ramón Giraldo, María Ángeles Del Pozo-Bayón, and Coralia Osorio. 2022. "Bioconversion of Glycosidic Precursors from Sour Guava (Psidium friedrichsthalianum Nied.) Fruit by the Oral Microbiota into Odor-Active Volatile Compounds" Molecules 27, no. 4: 1269. https://doi.org/10.3390/molecules27041269








