Immobilized Stenotrophomonas maltophilia KB2 in Naproxen Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the KB2 Strain Immobilization on the Luffa Sponge
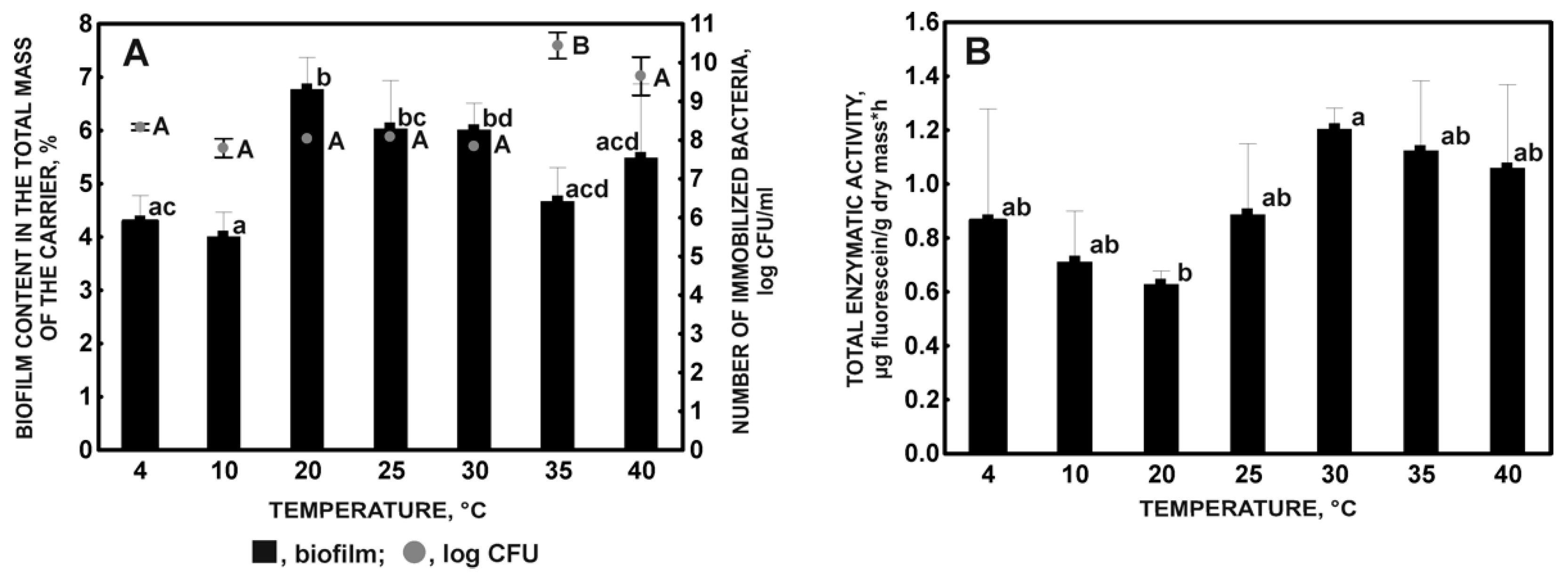
2.1.1. Influence of Temperature
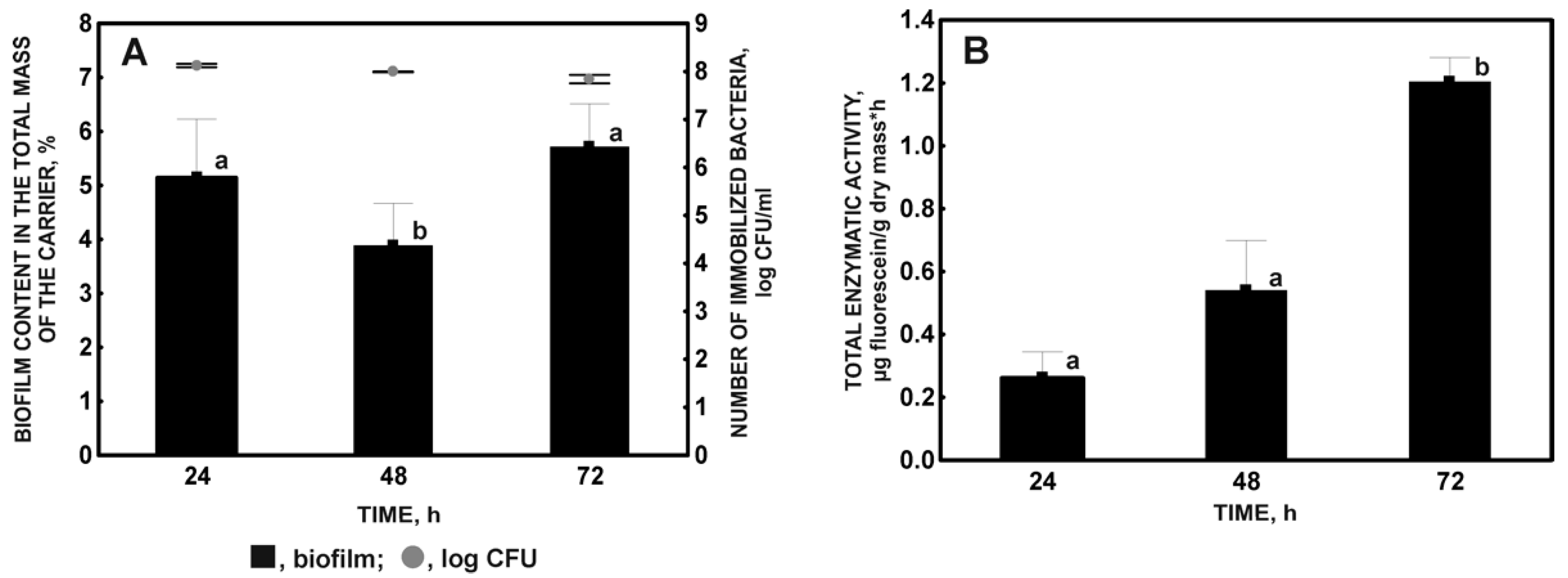
2.1.2. Influence of Incubation Time
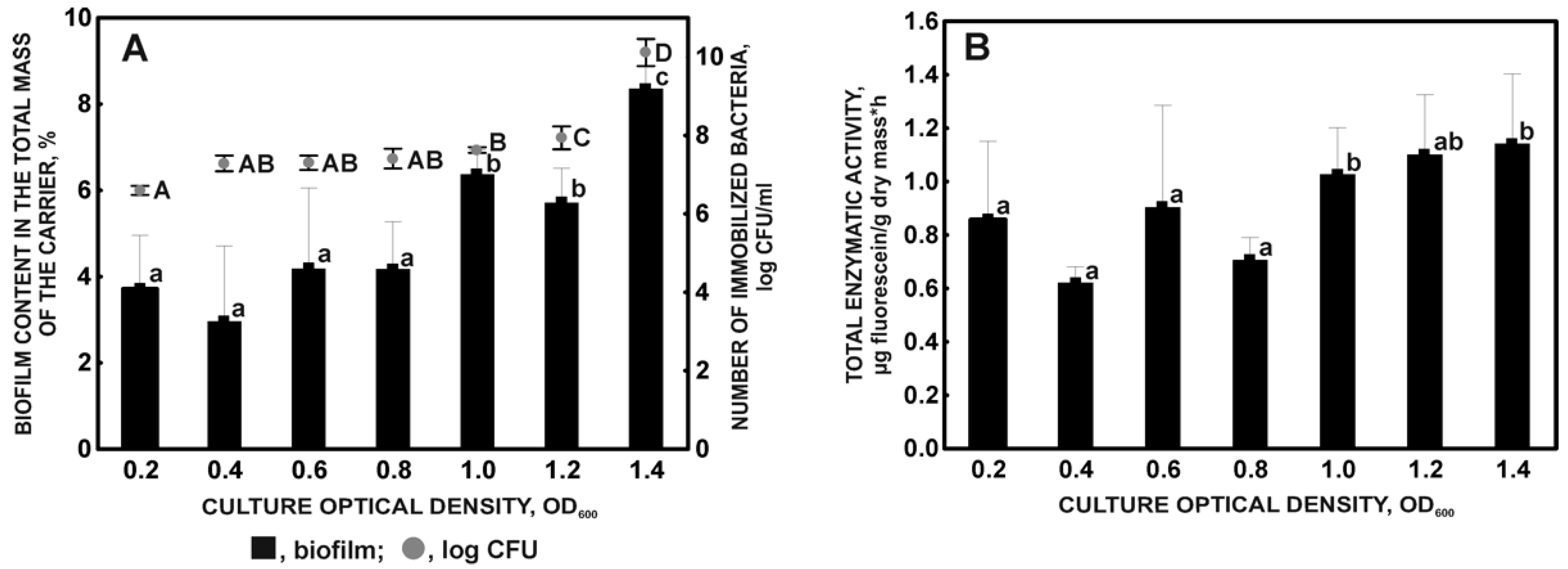
2.1.3. Effect of Initial Culture Density
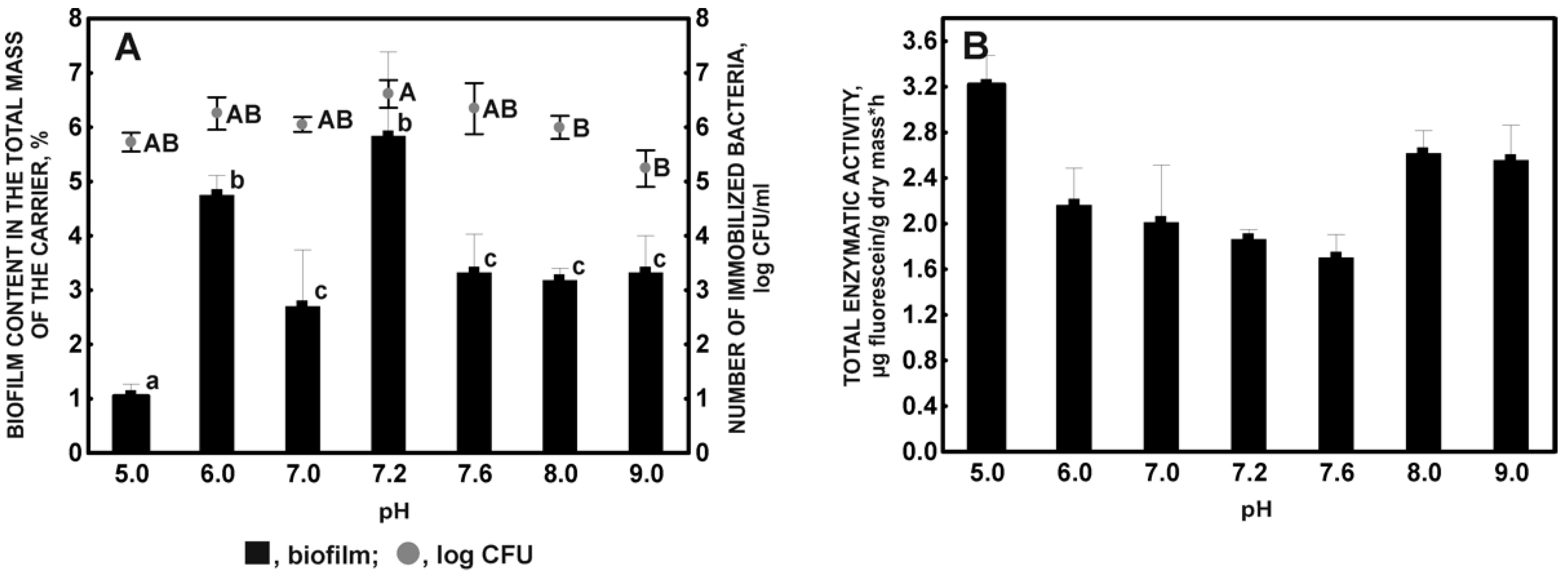
2.1.4. Impact of pH
2.2. Naproxen Degradation by Immobilized KB2 Strain
2.2.1. Naproxen Biodegradation
2.2.2. SEM Visualization of Immobilized Bacteria on a Luffa Sponge during Naproxen Degradation
3. Materials and Methods
3.1. Immobilization of KB2 Strain
3.2. Biochemical Analysis
3.2.1. Assay of Enzymatic Activity of Immobilized Bacteria
3.2.2. Determination of the Dry Mass of Bacteria
3.2.3. Determination of the Number of Colony-Forming Units
3.3. Biofilm Analysis by Scanning Electron Microscopy
3.4. Naproxen Degradation Study
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wojcieszyńska, D.; Guzik, H.; Guzik, U. Non-steroidal anti-inflammatory drugs in the era of the COVID-19 pandemic in the context of the human and the environment. Sci. Total Environ. 2022, 834, 155317. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Hupert-Kocurek, K.; Adamczyk-Habrajska, M.; Guzik, U. Immobilization of Planococcus Sp. S5 strain on the loofah sponge and its application in naproxen removal. Catalysts 2018, 8, 176. [Google Scholar] [CrossRef]
- Bowalgaha, K.; Elliot, D.J.; Mackenzie, P.I.; Knights, K.M.; Swedmark, S.; Miners, J.O.S. Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): Role of UGT2B7 in the elimination of naproxen. Br. J. Clin. Pharmacol. 2005, 60, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Pacheco-Juarez, J.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. A survey of the presence of pharmaceutical residues in wastewaters. Evaluation of their removal using conventional and natural treatment procedures. Molecules 2020, 25, 1639. [Google Scholar] [CrossRef]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. C. R. Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Praveenkumarreddy, Y.; Vimalkumar, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Gopal, C.M.; Vandana, K.E.; Bhat, K.; Udayashankar, H.N.; et al. Assessment of non-steroidal anti-inflammatory drugs from selected wastewater treatment plants of Southwestern India. Emerg. Contam. 2021, 7, 43–51. [Google Scholar] [CrossRef]
- Lahti, M.; Oikari, A. Microbial transformation of pharmaceuticals naproxen, bisoprolol, and diclofenac in aerobic and anaerobic environments. Arch. Environ. Contam. Toxicol. 2011, 61, 202–210. [Google Scholar] [CrossRef]
- Greń, I.; Wojcieszyńska, D.; Guzik, U.; Perkosz, M.; Hupert-Kocurek, K. Enhanced biotransformation of mononitrophenols by Stenotrophomonas maltophilia KB2 in the presence of aromatic compounds of plant origin. World J. Microbiol. Biotechnol. 2010, 2, 289–295. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Hupert-Kocurek, K.; Guzik, U. Factors affecting activity of catechol 2,3-dioxygenase from 2-chlorophenol-degrading Stenotrophomonas maltophilia strain KB2. Biocatal. Biotransform. 2013, 3, 141–147. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Bacterial degradation of naproxen--undisclosed pollutant in the environment. J. Environ. Manag. 2014, 145, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Hebrajska, M.; Guzik, U. Enhanced degradation of naproxen by immobilization of Bacillus thuringiensis B1 (2015b) on loofah sponge. Molecules 2020, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Karczewski, J.; Potocka, I.; Guzik, U. Xanthan gum as a carrier for bacterial cell entrapment: Developing a novel immobilised biocatalyst. Mater. Sci. Eng. C 2021, 118, 111474. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Piński, A.; Michalska, J.; Hupert-Kocurek, K.; Nowak, A.; Wojcieszyńska, D.; Guzik, U. A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. Int. Biodeterior. Biodegrad. 2020, 149, 104919. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; de Toledo, R.A.; Shim, H. Enhanced removal of naproxen and carbamazepine from wastwewater using a novel countercurrent seepage bioreactor immobilized with Phanerochaete chrysosporium unde non-sterile conditions. Biores. Technol. 2015, 197, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2018, 17, 241–257. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M. Loofa (Luffa cylindrica) sponge: Review of development of the biomatrix as a tool for biotechnological applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef]
- He, Q.; Xia, Q.; Wang, Y.; Li, X.; Zhang, Y.; Hu, B.; Wang, F. Biodiesel production: Utilization of loofah sponge to immobilize Rhizopus chinensis CGMCC#3.0232 cells as a whole-cell biocatalyst. J. Microbiol. Biotechnol. 2016, 26, 1278–1284. [Google Scholar] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Dzik, J.; Wojcieszyńska, D.; Guzik, U. Fluorescein diacetate hydrolysis using the whole biofilm as a sensitive tool to evaluate the physiological state of immobilized bacterial cells. Catalysts 2018, 8, 434. [Google Scholar] [CrossRef]
- Pompilio, A.; Piccolomini, R.; Picciani, C.; D’Antonio, D.; Savini, V.; di Bonaventura, G. Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: The role of cell surface hydrophobicity and motility. FEMS Microbiol. Lett. 2008, 287, 41–47. [Google Scholar] [CrossRef]
- Pinski, A.; Zur, J.; Hasterok, R.; Hupert-Kocurek, K. Comparative genomics of Stenotrophomonas maltophilia and Stenotrophomonas rhizophila revealed characteristic features of both species. Int. J. Mol. Sci. 2020, 21, 4922. [Google Scholar] [CrossRef]
- Guzik, U.; Greń, I.; Wojcieszyńska, D.; Łabużek, S. Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Braz. J. Microbiol. 2009, 40, 285–291. [Google Scholar]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Martinez, M.B.; Girón, J.A. Characterization of flagella produced by clinical strains of Stenotrophomonas maltophilia. Emerg. Infect. Dis. 2002, 8, 918–923. [Google Scholar] [CrossRef]
- Jayathilake, P.G.; Jana, S.; Rushton, S.; Swailes, D.; Bridgens, B.; Curtis, T.; Chen, J. Extracellular polymeric substance production and aggregated bacteria colonization influence the competition of microbes in biofilms. Front. Microbiol. 2017, 8, 1865. [Google Scholar] [CrossRef]
- Dawes, E.A.; Ribbons, D.W. Some aspects of the endogenous metabolism of bacteria. Bacteriol. Rev. 1964, 28, 126–149. [Google Scholar] [CrossRef]
- Cox, H.H.J.; Deshusses, M.A. Effect of starvation on the performance and re-acclimation of biotrickling filters for air pollution control. Environ. Sci. Technol. 2002, 36, 3069–3073. [Google Scholar] [CrossRef]
- Roszak, D.B.; Colwell, R.R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef]
- Navarro, L.J.M.; Tormo, A.; Martínez-García, E. Stationary phase in gram-negative bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Kjelleberg, S.; Humphrey, B.A.; Marshall, K.C. Initial phases of starvation and activity of bacteria at surfaces. Appl. Environ. Microbiol. 1983, 46, 978–984. [Google Scholar] [CrossRef]
- Sanin, S.L.; Sanin, F.D.; Bryers, J.D. Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem. 2003, 38, 909–914. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Celligoi, M.A.P.C.; Silva, R.S.F. Development of a statistical model for sorbitol production by free and immobilized Zymomonas mobilis in loofa sponge Luffa cylindrica. Process Biochem. 2006, 41, 240–243. [Google Scholar] [CrossRef]
- Wang, L.; Fan, D.; Chen, W.; Terentjev, E.M. Bacterial growth, detachment and cell size control on polyethylene terephthalate surfaces. Sci. Rep. 2015, 5, 15159. [Google Scholar] [CrossRef]
- Spector, M.P.; Kenyon, W.J. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res. Int. 2012, 45, 455–481. [Google Scholar] [CrossRef]
- van Overbeek, L.S.; Eberl, L.; Givskov, M.; Molin, S.; van Elsas, J.D. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl. Environ. Microbiol. 1995, 61, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.R.; Sauer, K. Small RNAs and their role in biofilm formation. Trends Microbiol. 2013, 21, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Hengge-Aronis, R. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002, 66, 373–395. [Google Scholar] [CrossRef]
- Raiger-Iustman, L.J.; Ruiz, J.A. The alternative sigma factor, σS, affects polyhydroxyalkanoate metabolism in Pseudomonas putida. FEMS Microbiol. Lett. 2008, 284, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opęchowska, M.; Bielecki, S. The role of alternative sigma factor S (σS) and sigma factor B (σB) in bacterial cell stress response and their regulation. Post. Mikrobiol. 2014, 42, 305–317. (In Polish) [Google Scholar]
- Mahdi, O.; Eklund, B.; Fisher, N. Laboratory culture and maintenance of Stenotrophomonas maltophilia. Curr. Protoc. Microbiol. 2014, 32, Unit-6F.1. [Google Scholar] [CrossRef]
- Santander, R.D.; Biosca, E.G. Erwinia amylovora psychrotrophic adaptations: Evidence of pathogenic potential and survival at temperate and low environmental temperatures. PeerJ 2017, 5, e3931. [Google Scholar] [CrossRef] [PubMed]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of microbes for bioremediation of crude oil polluted environments: A mini review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [PubMed]
- Thomas, W.E.; Trintchina, E.; Forero, M.; Vogel, V.; Sokurenko, E.V. Bacterial adhesion to target cells enhanced by shear force. Cell 2002, 109, 913–923. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Pinto, A.R.; Reis, N.M.; Vieira, M.J.; Keevil, C.W. Shear stress, temperature, and inoculation concentration influence the adhesion of water-stressed Helicobacter pylori to stainless steel 304 and polypropylene. Appl. Environ. Microbiol. 2006, 72, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, S.; Rusconi, R.; Shen, Y.; Forsyth, A.; Vlamakis, H.; Kolter, R.; Stone, H.A. Shear stress increases the residence time of adhesion of Pseudomonas aeruginosa. Biophys. J. 2011, 100, 34. [Google Scholar] [CrossRef]
- Gusnaniar, N.; Sjollema, J.; Jong, E.D.; Woudstra, W.; de Vries, J.; Nuryastuti, T.; van der Mei, H.C.; Busscher, H.J. Influence of biofilm lubricity on shear-induced transmission of staphylococcal biofilms from stainless steel to silicone rubber. Microb. Biotechnol. 2017, 10, 1744–1752. [Google Scholar] [CrossRef]
- Pönisch, W.; Weber, C.A.; Juckeland, G.; Biais, N.; Zaburdaev, V. Multiscale modeling of bacterial colonies: How pili mediate the dynamics of single cells and cellular aggregates. New J. Phys. 2017, 19, 015003. [Google Scholar] [CrossRef]
- Jaishankar, J.; Srivastava, P. Molecular basis of stationary phase survival and applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef] [PubMed]
- Možina, S.S.; Klančnik, A.; Raspor, P. Mechanisms of microbial resistance in biofilms. In Biofilms in Bioengineering; Simões, M., Mergulhão, F., Eds.; Nova Science Publishers: New York, NY, USA, 2013; Volume 1, pp. 311–332. [Google Scholar]
- Gao, M.; Zheng, H.; Ren, Y.; Lou, R.; Wu, F.; Yu, W.; Liu, X.; Ma, X. A crucial role for spatial distribution in bacterial quorum sensing. Sci. Rep. 2016, 6, 34695. [Google Scholar] [CrossRef] [Green Version]
- Jucker, B.A.; Harms, H.; Zehnder, A.J.B. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J. Bacteriol. 1996, 178, 5472–5479. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Super-hydrophobic modification of porous natural polymer “luffa sponge” for oil absorption. Polymer 2017, 126, 470–476. [Google Scholar] [CrossRef]
- Diwakar, S.; Rajkumar, K. Preparation of super hydrophobic loofah sponge for fast and efficient separation of oil from seawater. Mater. Today Proc. 2018, 5, 14367–14374. [Google Scholar] [CrossRef]
- Esan, O.S.; Abiola, O.N.; Owoyomi, O.; Aboluwoye, C.O.; Osundiya, M.O. Adsorption of Brilliant Green onto Luffa cylindrica sponge: Equilibrium, kinetics, and thermodynamic studies. ISRN Phys. Chem. 2014, 2014, 743532. [Google Scholar]
- Ams, D.A.; Fein, J.B.; Dong, H.; Maurice, P.A. Experimental measurements of the adsorption of Bacillus subtilis and Pseudomonas mendocina onto Fe-oxyhydroxide-coated and uncoated quartz grains. Geomicrobiol. J. 2004, 21, 511–519. [Google Scholar]
- Yee, N.; Fein, J.B.; Daughney, C.J. Experimental study of the pH, ionic strength, and reversibility behavior of bacteria—mineral adsorption. Geochim. Cosmochim. Acta 2000, 64, 609–617. [Google Scholar] [CrossRef]
- Moorman, M.A.; Thelemann, C.A.; Zhou, S.; Pestka, J.J.; Linz, J.E.; Ryser, E.T. Altered hydrophobicity and membrane composition in stress-adapted Listeria innocua. J. Food Prot. 2008, 71, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, A.; Sauer, U.; Mitter, B.; Preininger, C. Parameters influencing adsorption of Paraburkholderia phytofirmans PsJN onto bentonite, silica and talc for microbial inoculants. Appl. Clay Sci. 2017, 141, 138–145. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A brief recap of microbial adhesion and biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Borkowski, A.; Szala, M.; Cłapa, T. Adsorption studies of the Gram-negative bacteria onto nanostructured silicon carbide. Appl. Biochem. Microbiol. 2015, 175, 1448–1459. [Google Scholar] [CrossRef]
- Booth, I.R. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 1985, 49, 359–378. [Google Scholar] [CrossRef]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. A new pathway for naproxen zutilization by Bacillus thuringiensis B1(2015b) and its decomposition in the presence of organic and inorganic contaminants. J. Environ. Manag. 2019, 239, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Sánchez, B.M.; Pérez-Tapia, S.M.; Vázquez-Leyva, S.; Mejía-Calvo, I.; Macías-Palacios, Z.; Vallejo-Castillo, L.; Flores-Ortiz, C.M.; Guerrero-Barajas, C.; Cruz-Maya, J.A.; Jan-Roblero, J. Utilization of naproxen by Amycolatopsis sp. Poz 14 and detection of the enzymes involved in the degradation metabolic pathway. World J. Microbiol. Biotechnol. 2019, 35, 186. [Google Scholar] [CrossRef] [PubMed]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites. Z. Für Nat. C 2017, 72, 173–179. [Google Scholar] [CrossRef]
- Domaradzka, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Cometabolic degradation of naproxen by Planococcus sp. strain S5. Water Air Soil Pollut. 2015, 226, 297. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1(2015b) is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil Pollut. 2016, 227, 197. [Google Scholar] [CrossRef] [PubMed]
- Surma, R.; Wojcieszyńska, D.; Karcz, J.; Guzik, U. Effect of Pseudomonas moorei KB4 cells’ immobilisation on their degradation potential and tolerance towards paracetamol. Molecules 2021, 26, 820. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saeed, A.; Edyvean, R.G.J.; O’Sullivan, B.; Styring, P. Production of fungal biomass immobilized loofa sponge (FBILS)-discs for the removal of heavy metal ions and chlorinated compounds from aqueous solution. Biotechnol. Lett. 2005, 27, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, J.; Lu, H.; Liu, J.; Yan, C. Optimisation for assay of fluorescein diacetate hydrolytic activity as a sensitive tool to evaluate impacts of pollutants and nutrients on microbial activity in coastal sediments. Mar. Pollut. Bull. 2016, 110, 424–431. [Google Scholar] [CrossRef]
- Nie, M.; Nie, H.; He, M.; Lin, Y.; Wang, L.; Jin, P.; Zhang, S. Immobilization of biofilms of Pseudomonas aeruginosa NY3 and their application in the removal of hydrocarbons from highly concentrated oil-containing wastewater on the laboratory scale. J. Environ. Manag. 2016, 173, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, A.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcieszyńska, D.; Klamka, J.; Marchlewicz, A.; Potocka, I.; Żur-Pińska, J.; Guzik, U. Immobilized Stenotrophomonas maltophilia KB2 in Naproxen Degradation. Molecules 2022, 27, 5795. https://doi.org/10.3390/molecules27185795
Wojcieszyńska D, Klamka J, Marchlewicz A, Potocka I, Żur-Pińska J, Guzik U. Immobilized Stenotrophomonas maltophilia KB2 in Naproxen Degradation. Molecules. 2022; 27(18):5795. https://doi.org/10.3390/molecules27185795
Chicago/Turabian StyleWojcieszyńska, Danuta, Judyta Klamka, Ariel Marchlewicz, Izabela Potocka, Joanna Żur-Pińska, and Urszula Guzik. 2022. "Immobilized Stenotrophomonas maltophilia KB2 in Naproxen Degradation" Molecules 27, no. 18: 5795. https://doi.org/10.3390/molecules27185795





