Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita
Abstract
:1. Introduction
2. Results
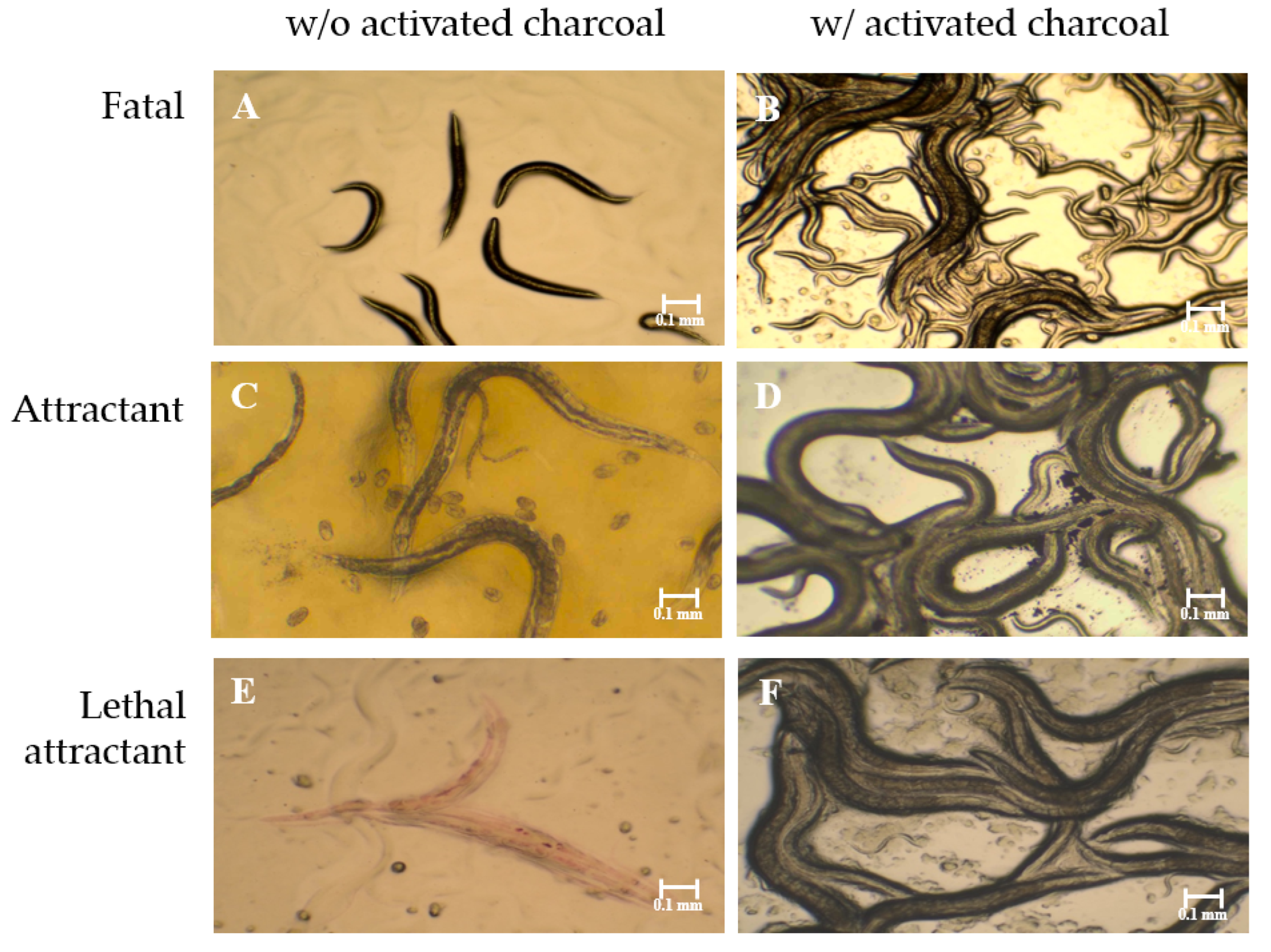
2.1. Fumigant Activity of Bacterial Strains toward C. elegans
2.2. Identification of the VOCs
2.3. Fumigant Activity of Pure VOCs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Mediums
4.2. Bacterial Strains
4.3. C. elegans and M. incognita
4.4. Fumigant Activity of Bacterial Strains against C. elegans
4.5. Extraction of Bacterial VOCs by SPME
4.6. Identification of VOCs by GC-MS
4.7. Fumigant Activity of VOCs against C. elegans and M. incognita
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, S.; Zhang, W.; Xu, C.; Li, B.; Zhang, D.; Mu, W.; Liu, F. Nematicidal activity of trans-2-hexenal against southern root-knot nematode (Meloidogyne incognita) on tomato plants. J. Agric. Food Chem. 2017, 65, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Lu, H.; Wang, X.; Zhang, K.Q.; Li, G.H. Effect of volatile organic compounds from bacteria on nematodes. Chem. Biodivers 2015, 12, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef]
- Kihika, R.; Murungi, L.K.; Coyne, D.; Ng’ang’a, M.; Hassanali, A.; Teal, P.E.A.; Torto, B. Parasitic nematode Meloidogyne incognita interactions with different Capsicum annum cultivars reveal the chemical constituents modulating root herbivory. Sci. Rep. 2017, 7, 2903. [Google Scholar] [CrossRef]
- Riga, E. The effects of Brassica green manures on plant parasitic and free living nematodes used in combination with reduced rates of synthetic nematicides. J. Nematol. 2011, 43, 119–121. [Google Scholar]
- Abdel-Rahman, F.H.; Alaniz, N.M.; Saleh, M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health B 2013, 48, 16–22. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos Trans. R Soc. Lond B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Whorton, M.D.; Foliart, D.E. Mutagenicity, carcinogenicity and reproductive effects of dibromochloropropane (DBCP). Mutat. Res. 1983, 123, 13–30. [Google Scholar] [CrossRef]
- Hasan, M.M.; Aikins, M.J.; Schilling, M.W.; Phillips, T.W. Comparison of methyl bromide and phosphine for fumigation of Necrobia rufipes (Coleoptera: Cleridae) and Tyrophagus putrescentiae (Sarcoptiformes: Acaridae), pests of high-value stored products. J. Econ. Entomol. 2020, 113, 1008–1014. [Google Scholar] [CrossRef]
- Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Rajaofera, M.J.N.; Wang, Y.; Dahar, G.Y.; Jin, P.; Fan, L.; Xu, L.; Liu, W.; Miao, W. Volatile organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pestic. Biochem. Physiol. 2019, 156, 170–176. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.T.; Chung, F.Y.; Moyle, P.M.; Eltanahy, E.G.; Schenk, P.M. Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth. Sci. Total Environ. 2019, 692, 267–280. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 2018, 44, 88–97. [Google Scholar] [CrossRef]
- Campos, V.; Pinho, R.; Freire, E. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciência E Agrotecnologia 2010, 34, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Liu, R.; Zhao, J.L.; Khan, R.A.A.; Li, Y.; Ling, J.; Liu, W.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Volatile organic compounds of Bacillus cereus strain Bc-cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Dis. 2021, 105, 904–911. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lee, C.C.; Leu, W.M.; Wu, J.J.; Huang, Y.C.; Meng, M. Fungicidal activity of volatile organic compounds emitted by Burkholderia gladioli strain BBB-01. Molecules 2021, 26, 745. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterisation of volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Guan, X.L.; Wu, P.F.; Wang, S.; Zhang, J.J.; Shen, Z.C.; Luo, H.; Chen, H.; Long, L.H.; Chen, J.G.; Wang, F. Dimethyl sulfide protects against oxidative stress and extends lifespan via a methionine sulfoxide reductase A-dependent catalytic mechanism. Aging Cell 2017, 16, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.P.; Campos, V.P.; Barros, A.F.; Pedroso, M.P.; Terra, W.C.; Lopez, L.E.; de Souza, J.T. Plant volatiles reduce the viability of the root-knot nematode Meloidogyne incognita either directly or when retained in water. Plant Dis. 2018, 102, 2170–2179. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.; Risi, G. Caenorhabditis elegans: Nature and nurture gift to nematode parasitologists. Parasitology 2018, 145, 979–987. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Kim, M.; Kim, E.; Choi, H.; Kim, Y.; Lee, J. Indole-associated predator-prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 2017, 19, 1776–1790. [Google Scholar] [CrossRef]
- Ferchichi, M.; Hemme, D.; Nardi, M.; Pamboukdjian, N. Production of methanethiol from methionine by Brevibacterium linens CNRZ 918. J. Gen. Microbiol. 1985, 131, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.S.; Zhou, H.; Li, H.M.; Yuan, Z.P.; Chen, T.; Tang, Y.J. Metabolism of L-methionine linked to the biosynthesis of volatile organic sulfur-containing compounds during the submerged fermentation of Tuber melanosporum. Appl. Microbiol. Biotechnol. 2013, 97, 9981–9992. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wang, J.; Huang, D.; Cheng, W.; Shao, Z.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Volatile organic compounds from Bacillus aryabhattai MCCC 1K02966 with multiple modes against Meloidogyne incognita. Molecules 2021, 27, 103. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.-Q.; Mo, M.-H.; Zhou, J.-P.; Zou, C.-S.; Zhang, K.-Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest. Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
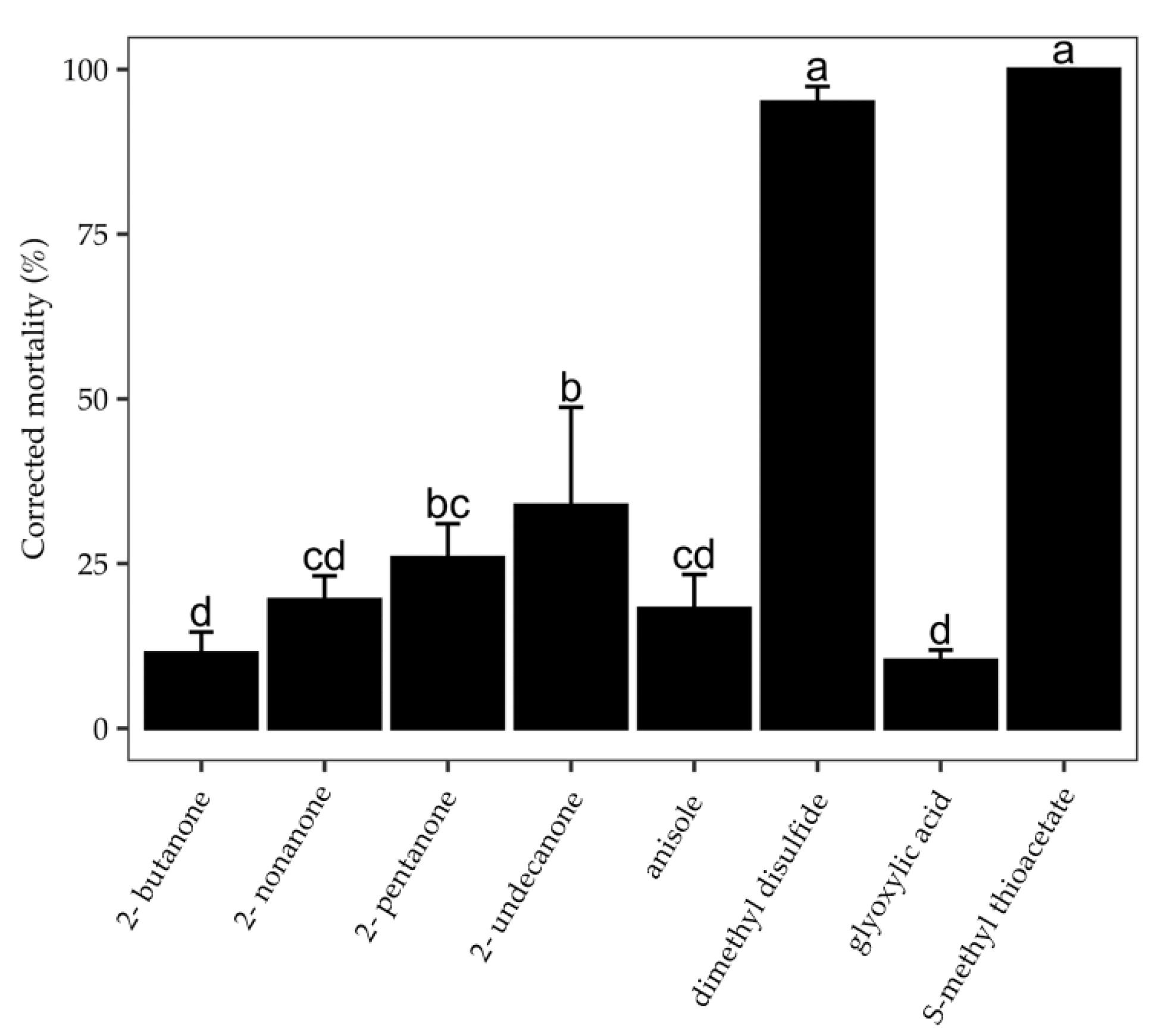

| Compound | n | LC50 (95% CI) (µg/cm3 Air) | LC95 (95% CI) (µg/cm3 Air) | Slope ± SD | Chi-Square |
|---|---|---|---|---|---|
| DMDS | 6000 | 8.57 (7.26–9.86) | 42.25 (32.95–60.18) | 2.37 ± 0.23 | 1.46 |
| MTA | 9600 | 1.43 (1.10–1.81) | 4.58 (3.27–8.39) | 3.26 ± 0.44 | 5.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diyapoglu, A.; Chang, T.-H.; Chang, P.-F.L.; Yen, J.-H.; Chiang, H.-I.; Meng, M. Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules 2022, 27, 4714. https://doi.org/10.3390/molecules27154714
Diyapoglu A, Chang T-H, Chang P-FL, Yen J-H, Chiang H-I, Meng M. Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules. 2022; 27(15):4714. https://doi.org/10.3390/molecules27154714
Chicago/Turabian StyleDiyapoglu, Ali, Tao-Ho Chang, Pi-Fang Linda Chang, Jyh-Herng Yen, Hsin-I Chiang, and Menghsiao Meng. 2022. "Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita" Molecules 27, no. 15: 4714. https://doi.org/10.3390/molecules27154714








