Weathering of Antibacterial Melt-Spun Polyfilaments Modified by Pine Rosin
Abstract
:1. Introduction
2. Background
3. Materials and Methods
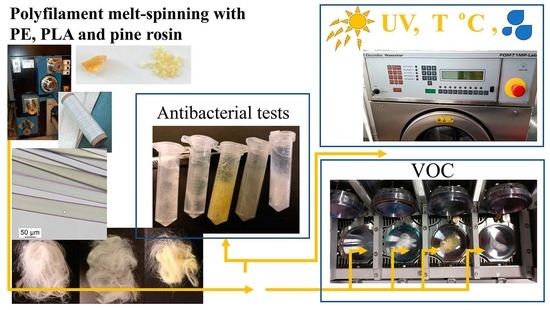
3.1. Polymer Raw Materials and Fibre Melt-Spinning
3.2. Antibacterial Activity
3.3. Volatile Organic Compounds Analysis
3.4. Conditioning by UV Radiation
3.5. Conditioning by Thermal Cycling
3.6. Conditioning by Standard Washing
3.7. Mechanical Testing of Fibres from Polyfilaments
4. Results and Analysis
4.1. Performance of As-Spun Polyfilaments in the Room Conditions
4.2. Stability of Polyfilaments in Terms of Volatile Organic Compounds at Elevated Temperatures
4.3. Long-Term Performance of Polyfilament Fibres
5. Discussion
6. Conclusions
- A high 20 w-% of rosin content was be applied for melt-spun PE and PLA polyfilaments by using a copolymer-type surfactant (F-127, Pluronic) but the ultimate mechanical properties at ambient conditions were not improved;
- The total VOC emissions from the melt-spun PE fibres were lower for those fibres that were modified with 10 w-% of rosin and when analysed over a temperature range of 25…60 °C;
- PE fibres with a 10 w-% of rosin were mechanically durable against UV-radiation, thermal ageing at 60 °C and standard washing cycles at 70 °C as well as in terms of a strong antibacterial response against S. aureus.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sandin, G.; Peters, G. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Leskinen, P.; Cardellini, G.; Gonzales-Garcia, S.; Hurmekoski, E.; Sathre, R.; Seppälä, J.; Smyth, C.; Stern, T.; Verkerk, P. Substitution Effects of Wood-Based Products in Climate Change Mitigation; Technical Report Science to Policy 7; European Forest Institute (EFI): Joensuu, Finland, 2018. [Google Scholar]
- Jabbar, A.; Tausif, M.; Tahir, H.; Basit, A.; Bhatti, M.; Abbas, G. Polylactic acid/lyocell fibre as an eco-friendly alternative to polyethylene terephthalate/cotton fibre blended yarns and knitted fabrics. J. Text. Inst. 2020, 111, 129–138. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, X.; Guan, J.; Gu, Y.; Su, Z.; Zhao, Y.; Tu, Y.; Li, X.; Li, Y.; Li, J. Ionic liquid-grafted polyamide 6 by radiation-induced grafting: New strategy to prepare covalently bonded ion-containing polymers and their application as functional fibers. ACS Appl. Mater. Interfaces 2019, 11, 5462–5475. [Google Scholar] [CrossRef]
- Haaparanta, A.M.; Järvinen, E.; Cengiz, I.; Ellä, V.; Kokkonen, H.; Kiviranta, I.; Kellomäki, M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, M.; Puolakka, A.; Takala, T.; Elert, A.; Mylläri, V.; Jönkkäri, I.; Sarlin, E.; Seitsonen, J.; Ruokolainen, J.; Saris, P.; et al. Antibacterial polymer fibres by rosin compounding and melt-spinning. Mater. Today Commun. 2019, 20, 100527. [Google Scholar] [CrossRef]
- Son, W.; Youk, J.; Park, W. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Rangari, V.; Mohammad, G.; Jeelani, S.; Hundley, A.; Vig, K.; Singh, S.; Pillai, S. Synthesis of Ag/CNT hybrid nanoparticles and fabrication of their Nylon-6 polymer nanocomposite fibers for antimicrobial applications. Nanotechnology 2010, 21, 095102. [Google Scholar] [CrossRef]
- Dobrovol’Skaya, I.; Yudin, V.; Drozdova, N.; Smirnova, V.; Gofman, I.; Popova, E.; Bochek, A.; Zabivalova, N.; Plugar‘, I.; Panarin, E. Structure and characteristics of film composites based on methyl cellulose, poviargol, and montmorillonite. Polym. Sci. Ser. A 2011, 53, 166–171. [Google Scholar] [CrossRef]
- Hwang, S.; Jeong, S. Electrospun nano composites of poly(vinyl pyrrolidone)/nano-silver for antibacterial materials. J. Nanosci. Nanotechnol. 2011, 11, 610–613. [Google Scholar] [CrossRef]
- Metreveli, G.; David, J.; Schneider, R.; Kurtz, S.; Schaumann, G. Morphology, structure, and composition of sulfidized silver nanoparticles and their aggregation dynamics in river water. Sci. Total Environ. 2020, 739, 139989. [Google Scholar] [CrossRef]
- Yaqoob, A.; Umar, K.; Ibrahim, M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Meireles Gouvâ, D.; Santos Mendonça, R.; Lopez Soto, M.; Souza Cruz, R. Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging. LWT Food Sci. Technol. 2015, 63, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gankov, N. Effect of aging of polycaproamide crumb on the quality of vidlon textile fibre. Fibre Chem. 2004, 36, 119–121. [Google Scholar] [CrossRef]
- Aouat, T.; Kaci, M.; Lopez-Cuesta, J.; Devaux, E. Investigation on the durability of PLA bionanocomposite fibers under hygrothermal conditions. Front. Mater. 2019, 6, 323. [Google Scholar] [CrossRef] [Green Version]
- Söderberg, T.; Gref, R.; Holm, S.; Elmros, T.; Hallmans, G. Antibacterial activity of rosin and resin acids in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Woo-il, B.; Navamathavan, R.; Kalpana, D.; Lee, Y.S.; Kim, H.Y. Influence of antimicrobial additives on the formation of rosin nanofibers via electrospinning. Colloids Surf. B Biointerfaces 2013, 104, 262–267. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry—Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 1993; ISBN 978-0-08-092589-9. [Google Scholar]
- Himejima, M.; Hobson, K.; Otsuka, T.; Wood, D.; Kubo, I. Antimicrobial terpenes from oleoresin of ponderosa pine tree Pinus ponderosa: A defense mechanism against microbial invasion. J. Chem. Ecol. 1992, 18, 1809–1818. [Google Scholar] [CrossRef]
- Ekeberg, D.; Flate, P.O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Hänninen, T.; Kyyhkynen, A. Effect of volatile organic compounds from Pinus sylvestris and Picea abies on Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae and Salmonella enterica serovar Typhimurium. Holzforschung 2017, 71, 905–912. [Google Scholar] [CrossRef]
- Vainio-Kaila, T.; Zhang, X.; Hänninen, T.; Kyyhkynen, A.; Johansson, L.S.; Willför, S.; Österberg, M.; Siitonen, A.; Rautkari, L. Antibacterial effects of wood structural components and extractives from Pinus sylvestris and Picea abies on Methicillin-resistant Staphylococcus aureus and Escherichia coli O157:H7. Bioresources 2017, 12, 7601–7614. [Google Scholar]
- Vainio-Kaila, T.; Kyyhkynen, A.; Rautkari, L.; Siitonen, A. Antibacterial effects of extracts of pinus sylvestris and picea abies against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Streptococcus pneumoniae. BioResources 2015, 10, 7763–7771. [Google Scholar] [CrossRef]
- Shi, Y.; Si, H.; Wang, P.; Chen, S.; Shang, S.; Song, Z.; Wang, Z.; Liao, S. Derivatization of natural compound β-pinene enhances its in vitro antifungal activity against plant pathogens. Molecules 2019, 24, 3144. [Google Scholar] [CrossRef] [Green Version]
- Rosu, L.; Mustafa, F.; Rosu, D.; Varganici, C.; Rosca, I.; Rusu, T. Bio-based coatings from epoxy resins crosslinked with a rosin acid derivative for wood thermal and anti–fungal protection. Prog. Org. Coat. 2020, 151, 106008. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, F.; Lu, M.; Sun, J. Inducible pine rosin defense mediates interactions between an invasive insect–fungal complex and newly acquired sympatric fungal associates. Integr. Zool. 2015, 10, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.; Markkanen, J.; Kivimäenpää, M.; Lyytikäinen-Saarenmaa, P.; Holopainen, J. Needle removal by pine sawfly larvae increases branch-level VOC emissions and reduces below-ground emissions of scots pine. Environ. Sci. Technol. 2013, 47, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, A.; Peltola, R.; Jokinen, J.J.; Laitinen, K.; Lohi, J.; Rautio, M.; Männistö, M.; Sipponen, P.; Lounatmaa, K. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct. Pathol. 2009, 33, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tikka, P.; Gullichsen, J. Chemical Pulping Part 2. Chapter Recovery of Chemicals and Energy; Papermaking Science and Technology: Espoo, Finland, 2000; pp. 378–388. ISBN 952-5216-06-3. [Google Scholar]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- ISO. Sample Preparation–Dispersing Procedures for Powders in Liquids; Standard No. 14887(E); International Organization for Standardization (ISO): Geneva, Switzerland, 2000. [Google Scholar]
- Siljander, S.; Keinänen, P.; Räty, A.; Ramakrishnan, K.; Tuukkanen, S.; Kunnari, V.; Harlin, A.; Vuorinen, J.; Kanerva, M. Effect of Surfactant Type and Sonication Energy on the Electrical Conductivity Properties of Nanocellulose-CNT Nanocomposite Films. Int. J. Mol. Sci. 2018, 19, 1819. [Google Scholar] [CrossRef] [Green Version]
- Kanerva, M.; Matrenichev, V.; Layek, R.; Takala, T.; Laurikainen, P.; Sarlin, E.; Elert, A.; Yudin, V.; Seitsonen, J.; Ruokolainen, J.; et al. Comparison of rosin and propolis antimicrobials in cellulose acetate fibers against Staphylococcus aureus. BioResources 2020, 15, 3756–3773. [Google Scholar]
- Piwowarek, K.; Lipińska, E.; Hać-Szymaćczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.–source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [Green Version]
- Pickett, O.; Peterson, J. Terpenes and terpene alcohols: I vapor pressure temperature relationships. Ind. Eng. Chem. 1929, 21, 325–326. [Google Scholar] [CrossRef]
- Sarria, S.; Wong, B.; Martín, H.; Keasling, J.; Peralta-Yahya, P. Microbial synthesis of pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef]
- Zhou, J.H.; Zhou, C.S.; Jiang, X.Y.; Xie, L.W. Extraction of essential oil from shaddock peel and analysis of its components by gas chromatography-mass spectrometry. J. Cent. South Univ. Technol. 2006, 13, 44–48. [Google Scholar] [CrossRef]
- Baiju, J.; Motokucho, S.; Kojio, K.; Furukawa, M. Polyamide 6 fibers with superior mechanical properties: TPU coating techniques. J. Soc. Fiber Sci. Technol. 2009, 65, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Dees, J.R.; Spruiell, J.E. Structure development during melt spinning of linear polyethylene fibers. J. Appl. Polym. Sci. 1974, 18, 1053–1078. [Google Scholar] [CrossRef]
- Wu, W.; Black, W.B. High-strength polyethylene. Polym. Eng. Sci. 1979, 19, 1163–1169. [Google Scholar] [CrossRef]
- Jönkkäri, I.; Poliakova, V.; Mylläri, V.; Anderson, R.; Andersson, M.; Vuorinen, J. Compounding and characterization of recycled multilayer plastic films. J. Appl. Polym. Sci. 2020, 137, 49101. [Google Scholar] [CrossRef]
- Yuan, X.; Mak, A.F.T.; Yao, K. In vitro degradation of poly(L- lactic acid) fibers in phosphate buffered saline. J. Appl. Polym. Sci. 2002, 85, 936–943. [Google Scholar] [CrossRef]





| Polymer Basis | Grade | Provider |
|---|---|---|
| High-density polyethylene (HDPE) | CG9620 | Borealis Polymers |
| Poly lactic acid | 2003D | Ingeo/NatureWorks |
| Polymer | Rosin % (w/w) | Temperature (°C) | Series Names |
|---|---|---|---|
| PE | 0, 10 | 160, 180 | fPE-160, fPE10-160, fPE10-180 |
| PLA | 0, 10 | 160–180 | fPLA, fPLA10 |
| Polymer | PF/Ro, Ro | Temperature (°C) | Series Names |
|---|---|---|---|
| PE | 0.01, 20% (w/w) | 160 | fPE1PF |
| PLA | 0.01, 20% (w/w) | 160 | fPLA1PF |
| Polymer | Ag % (w/w) | Temperature (°C) | Series Names |
|---|---|---|---|
| PE | 1 | 180 | fPE1Ag |
| PLA | 1 | 180 | fPLA1Ag |
| Fibre Series | Additive(s) | Ultimate Strength (MPa), Respectively | Strain (/L) at Break (%), Respectively |
|---|---|---|---|
| fPE-160, from [6] | Ro (0, 10 w-%f) | 63 ± 17, 61 ± 26 | 1130 ± 95, 1528 ± 210 |
| fPE-180, from [6] | Ro (0, 10 w-%) | 41 ± 20, 19 ± 11 | 2076 ± 242, 1118 ± 225 |
| fPE1PF | PF, Ro (20 w-%) | 38 ± 16 | 1234 ± 491 |
| fPE1Ag | Ag (1 w-%) | 53 ± 47 | 1407 ± 574 |
| fPLA, from [6] | Ro (0, 10 w-%) | 153 ± 47, 49 ± 5 | 294 ± 60, 3 ± 0 |
| fPLA1PF | PF, Ro (20 w-%) | 77 ± 30 | 4 ± 1 |
| fPLA1Ag | Ag (1 w-%) | 146 ± 47 | 231 ± 73 |
| Fibre Series | Temperature 25 °C | Temperature 60 °C | Temperature 105 °C |
|---|---|---|---|
| fPE | 381 (255–308) | 8386 (6252–10,520) | 160,092 (137,863–182,321) |
| fPE10 | 113 (113–114) | 6637 (6434–6840) | 214,205 (200,338–228,071) |
| fPLA | 40 (39–41) | 766 (532–1000) | 19,382 (18,531–20,233) |
| fPLA10 | 31 (0–31) | 1120 (691–1548) | 929,724 (568,861–1,290,588) |
| fPLA1Ag | 46 (30–61) | 1132 (442–1832) | 38,693 (33,419–43,967) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanerva, M.; Mensah-Attipoe, J.; Puolakka, A.; Takala, T.M.; Hyttinen, M.; Layek, R.; Palola, S.; Yudin, V.; Pasanen, P.; Saris, P. Weathering of Antibacterial Melt-Spun Polyfilaments Modified by Pine Rosin. Molecules 2021, 26, 876. https://doi.org/10.3390/molecules26040876
Kanerva M, Mensah-Attipoe J, Puolakka A, Takala TM, Hyttinen M, Layek R, Palola S, Yudin V, Pasanen P, Saris P. Weathering of Antibacterial Melt-Spun Polyfilaments Modified by Pine Rosin. Molecules. 2021; 26(4):876. https://doi.org/10.3390/molecules26040876
Chicago/Turabian StyleKanerva, Mikko, Jacob Mensah-Attipoe, Arja Puolakka, Timo M. Takala, Marko Hyttinen, Rama Layek, Sarianna Palola, Vladimir Yudin, Pertti Pasanen, and Per Saris. 2021. "Weathering of Antibacterial Melt-Spun Polyfilaments Modified by Pine Rosin" Molecules 26, no. 4: 876. https://doi.org/10.3390/molecules26040876