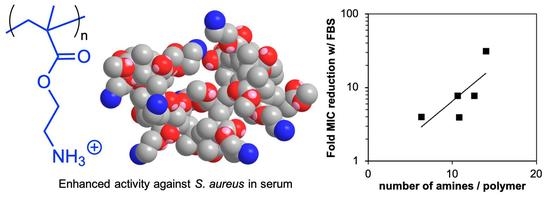
Unexpected Enhancement of Antimicrobial Polymer Activity against Staphylococcus aureus in the Presence of Fetal Bovine Serum
Abstract
:1. Introduction
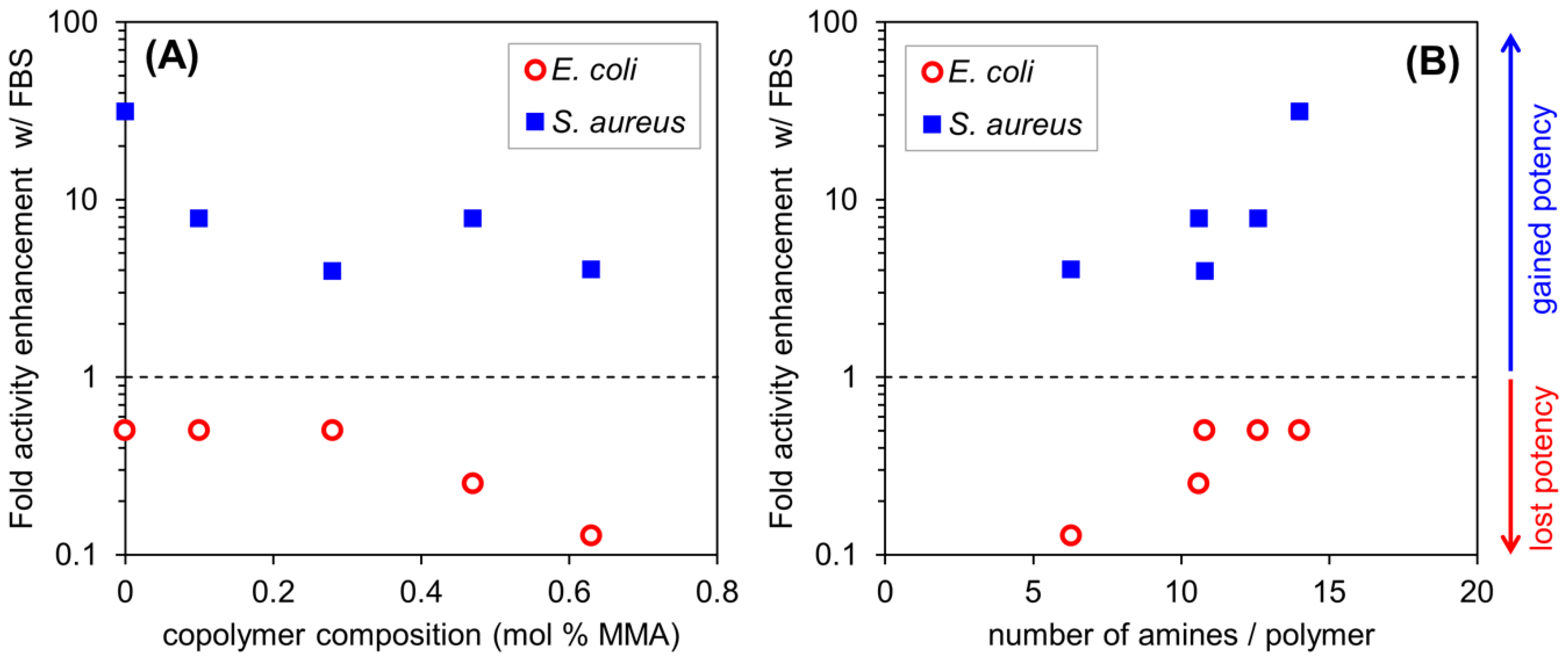
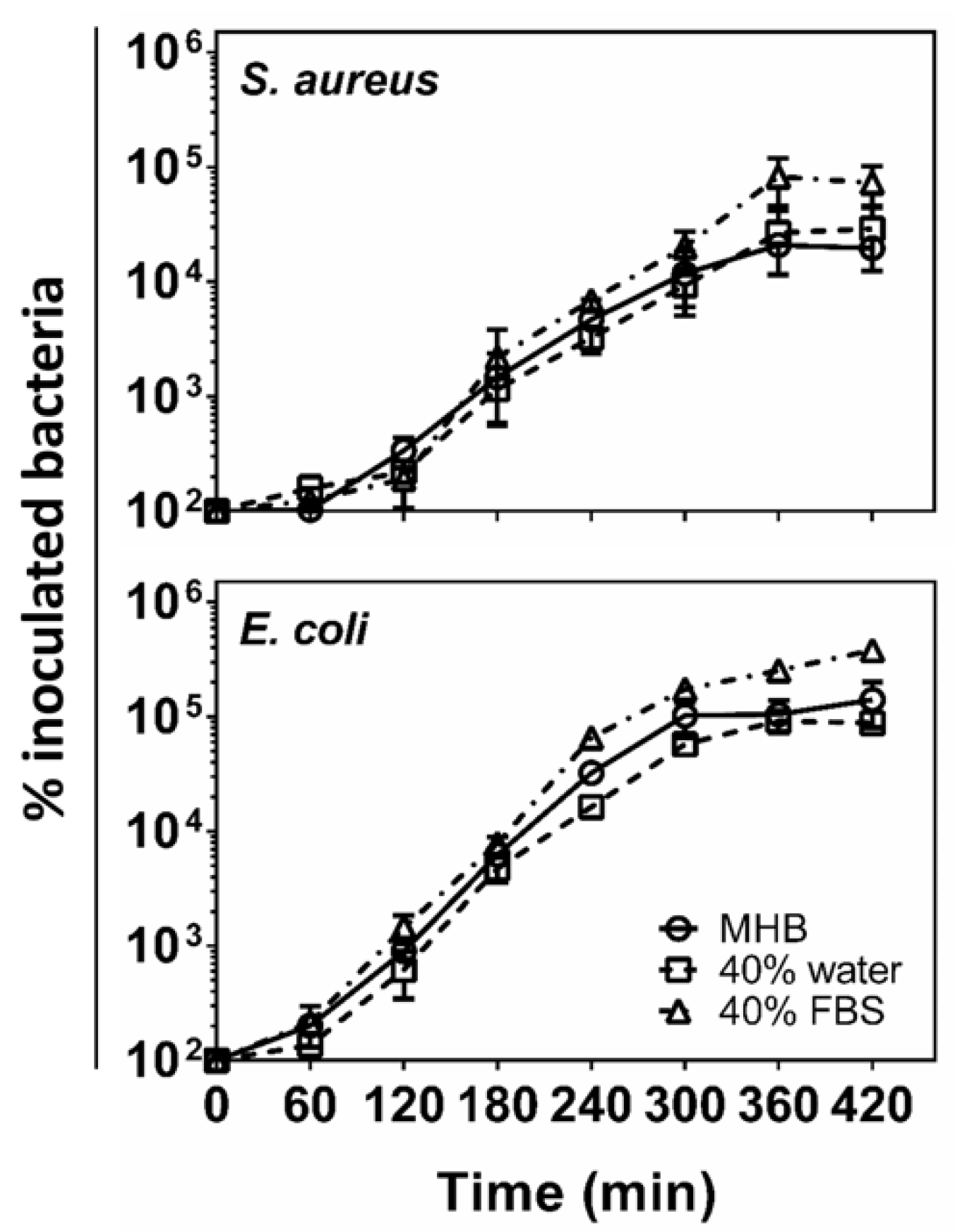
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Polymer Synthesis
4.3. Antimicrobial Assay
4.4. Bacterial Growth Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to Antibiotics—A Pipeline Portfolio Review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic Antimicrobial Polymers: Recent Advances in Molecular Design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Tew, G.N.; Liu, D.; Chen, B.; Doerksen, R.J.; Kaplan, J.; Carroll, P.J.; Klein, M.L.; Degrado, W.F. De novo Design of Biomimetic Antimicrobial Polymers. Proc. Natl. Acad. Sci. USA 2002, 99, 5110–5114. [Google Scholar] [CrossRef] [Green Version]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent Progress in Polymer Research to Tackle Infections and Antimicrobial Resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef]
- Sovadinova, I.; Palermo, E.F.; Urban, M.; Mpiga, P.; Caputo, G.A.; Kuroda, K. Activity and Mechanism of Antimicrobial Peptide-mimetic Amphiphilic Polymethacrylate Derivatives. Polymers 2011, 3, 1512–1532. [Google Scholar] [CrossRef]
- Thoma, L.M.; Boles, B.R.; Kuroda, K. Cationic Methacrylate Polymers as Topical Antimicrobial Agents Against Staphylococcus aureus Nasal Colonization. Biomacromolecules 2014, 15, 2933–2943. [Google Scholar] [CrossRef] [Green Version]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial Peptides for Therapeutic Use: Obstacles and Realistic Outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shockman, G.D.; Barrett, J.F. Structure, Function, and Assembly of Cell Walls of Gram-Positive Bacteria. Annu. Rev. Microbiol. 1983, 37, 501–527. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Foster, S.J.; Richards, R.G. An Introduction to Staphylococcus aureus, and Techniques for Identifying and Quantifying S. aureus Adhesins in Relation to Adhesion to Biomaterials: Review. Eur. Cell. Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular Design, Structures, and Activity of Antimicrobial Peptide-Mimetic Polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sovadinova, I.; Palermo, E.F.; Huang, R.; Thoma, L.M.; Kuroda, K. Mechanism of Polymer-Induced Hemolysis: Nanosized Pore Formation and Osmotic Lysis. Biomacromolecules 2011, 12, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. All About Albumin; Elsevier: Cambridge, MA, USA, 1995; ISBN 978-0-12-552110-9. [Google Scholar]
- Francis, G.L. Albumin and Mammalian Cell Culture: Implications for Biotechnology Applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, E.F.; Vemparala, S.; Kuroda, K. Cationic Spacer Arm Design Strategy for Control of Antimicrobial Activity and Conformation of Amphiphilic Methacrylate Random Copolymers. Biomacromolecules 2012, 13, 1632–1641. [Google Scholar] [CrossRef]
- Starr, C.G.; He, J.; Wimley, W.C. Host Cell Interactions Are a Significant Barrier to the Clinical Utility of Peptide Antibiotics. ACS Chem. Biol. 2016, 11, 3391–3399. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Maisetta, G.; Di Luca, M.; Esin, S.; Florio, W.; Brancatisano, F.L.; Bottai, D.; Campa, M.; Batoni, G. Evaluation of the Inhibitory Effects of Human Serum Components on Bactericidal Activity of Human Beta Defensin 3. Peptides 2008, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [Green Version]
- Hale, J.D.F.; Hancock, R.E.W. Alternative Mechanisms of Action of Cationic Antimicrobial Peptides on Bacteria. Expert Rev. Anti. Infect. Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef]
- Michl, T.D.; Hibbs, B.; Hyde, L.; Postma, A.; Tran, D.T.T.; Zhalgasbaikyzy, A.; Vasilev, K.; Meagher, L.; Griesser, H.J.; Locock, K.E.S. Bacterial Membrane Permeability of Antimicrobial Polymethacrylates: Evidence for a Complex Mechanism from Super-Resolution Fluorescence Imaging. Acta Biomater. 2020, 108, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sass, V.; Schneider, T.; Wilmes, M.; Körner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.G. Human β-defensin 3 Inhibits Cell Wall Biosynthesis in Staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergene, C.; Palermo, E.F. Cationic Poly(benzyl ether)s as Self-Immolative Antimicrobial Polymers. Biomacromolecules 2017, 18, 3400–3409. [Google Scholar] [CrossRef]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as Antimicrobial Agents with Selective Activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Finn, M.G. Thiabicyclononane-Based Antimicrobial Polycations. J. Am. Chem. Soc. 2017, 139, 15401–15406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, C.; Zhang, N.; Ding, X.; Yu, B.; Xu, F.J. Polycationic Synergistic Antibacterial Agents with Multiple Functional Components for Efficient Anti-Infective Therapy. Adv. Funct. Mater. 2018, 28, 1706709. [Google Scholar] [CrossRef]
- M07: Dilution AST for Aerobically Grown Bacteria-CLSI. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 14 June 2021).
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In Vitro Susceptibility Tests for Cationic Peptides: Comparison of Broth Microdilution Methods for Bacteria that Grow Aerobically. Antimicrob. Agents Chemother. 2000, 44, 1694–1696. [Google Scholar] [CrossRef] [Green Version]

| Polymer | R | %R | DP a | Mn b |
|---|---|---|---|---|
| P0 | - | 0 | 14 | 2320 |
| M10 | Me | 10 | 14 | 2200 |
| M28 | Me | 28 | 15 | 2170 |
| M47 | Me | 47 | 20 | 2640 |
| M63 | Me | 63 | 17 | 2070 |
| B27 | Bu | 27 | 16 | 2530 |
| MIC S. aureus (µg/mL) | MIC E. coli (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Polymer | MH a | MH/H2O b (60:40) | MH/BSA c (95:5) | MH/FBS b (60:40) | MH a | MH/ H2O (60:40) | MH/BSA (95:5) | MH/FBS (60:40) |
| P0 | 125 | 31 | 250 | 4 | 500 | 125 | 1000 | >1000 |
| M10 | 125 | 31 | 500 | 16 | 500 | 125 | >1000 | >1000 |
| M28 | 250 | 125 | 1000 | 63 | 500 | 125 | >1000 | >1000 |
| M47 | 125 | 63 | 1000 | 16 | 63 | 31 | 125 | 250 |
| M63 | 125 | 63 | 1000 | 31 | 16 | 8 | 31 | 125 |
| B27 | 16 | 16 | 125 | 8 | 16 | 8 | 63 | 63 |
| Melittin | 6 | 6 | 50 | 25 | 13 | 13 | 50 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sovadinová, I.; Kuroda, K.; Palermo, E.F. Unexpected Enhancement of Antimicrobial Polymer Activity against Staphylococcus aureus in the Presence of Fetal Bovine Serum. Molecules 2021, 26, 4512. https://doi.org/10.3390/molecules26154512
Sovadinová I, Kuroda K, Palermo EF. Unexpected Enhancement of Antimicrobial Polymer Activity against Staphylococcus aureus in the Presence of Fetal Bovine Serum. Molecules. 2021; 26(15):4512. https://doi.org/10.3390/molecules26154512
Chicago/Turabian StyleSovadinová, Iva, Kenichi Kuroda, and Edmund F. Palermo. 2021. "Unexpected Enhancement of Antimicrobial Polymer Activity against Staphylococcus aureus in the Presence of Fetal Bovine Serum" Molecules 26, no. 15: 4512. https://doi.org/10.3390/molecules26154512
APA StyleSovadinová, I., Kuroda, K., & Palermo, E. F. (2021). Unexpected Enhancement of Antimicrobial Polymer Activity against Staphylococcus aureus in the Presence of Fetal Bovine Serum. Molecules, 26(15), 4512. https://doi.org/10.3390/molecules26154512