Endosomal pH-Responsive Fe-Based Hyaluronate Nanoparticles for Doxorubicin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DOX-Loaded Fe-Based HA NPs
2.3. Characterization of Fe-Based HA NPs
2.4. In Vitro DOX Release Behavior
2.5. Cell Culture
2.6. In Vitro Cellular Uptake Experiments
2.7. In Vitro Cytotoxicity
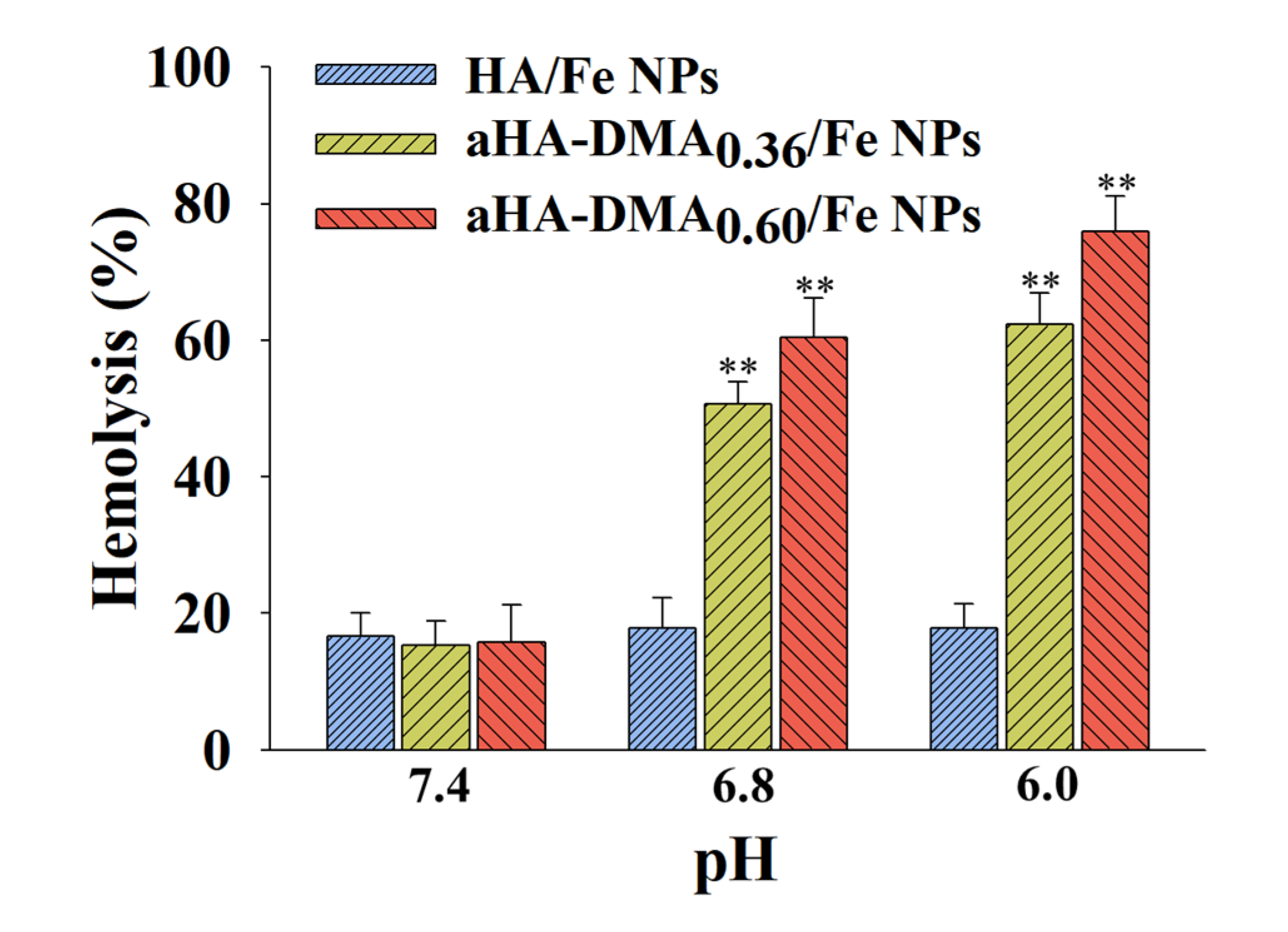
2.8. Hemolysis Test
2.9. Local Healing Assay
2.10. Statistical Evaluation
3. Results and Discussion
3.1. Synthesis of DOX-Loaded Fe-Based HA NPs
3.2. Characterization of DOX-Loaded Fe-Based HA NPs
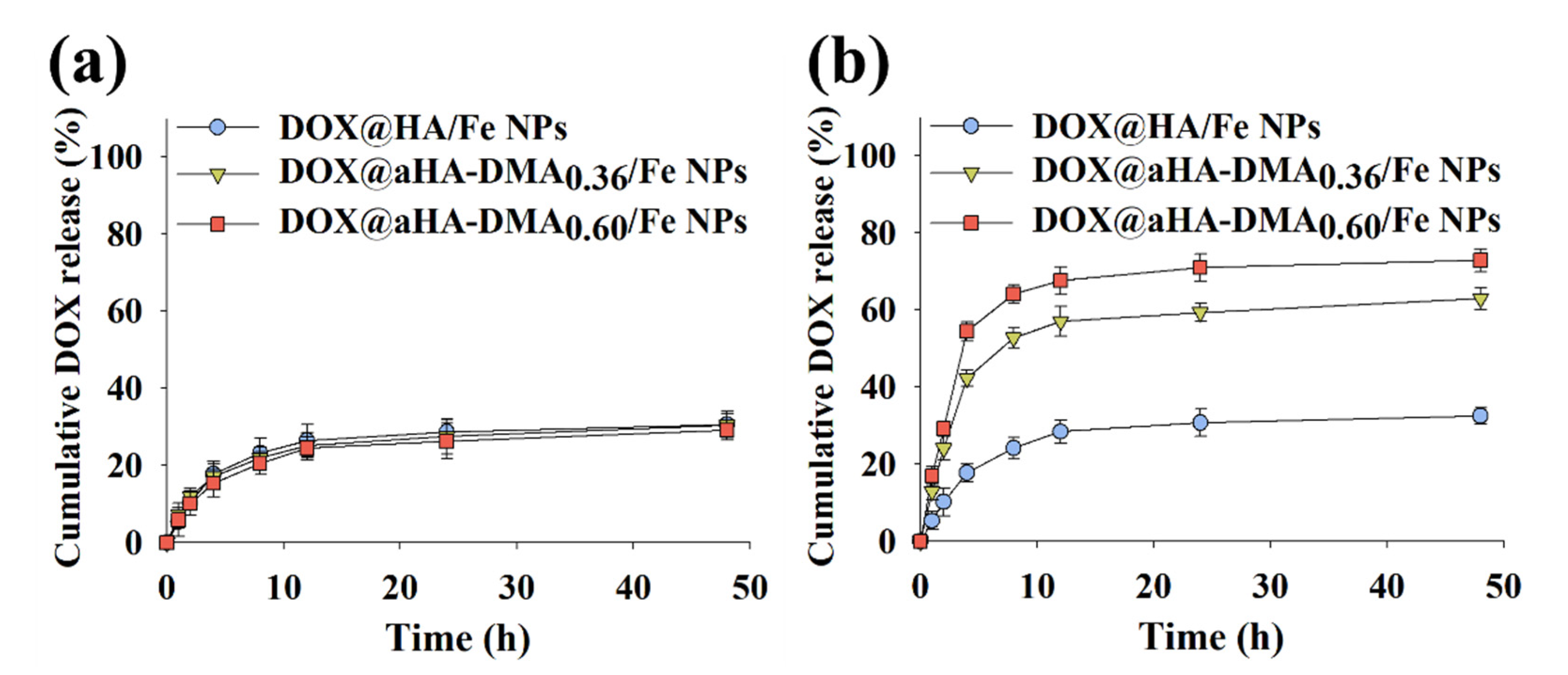
3.3. In Vitro DOX Release
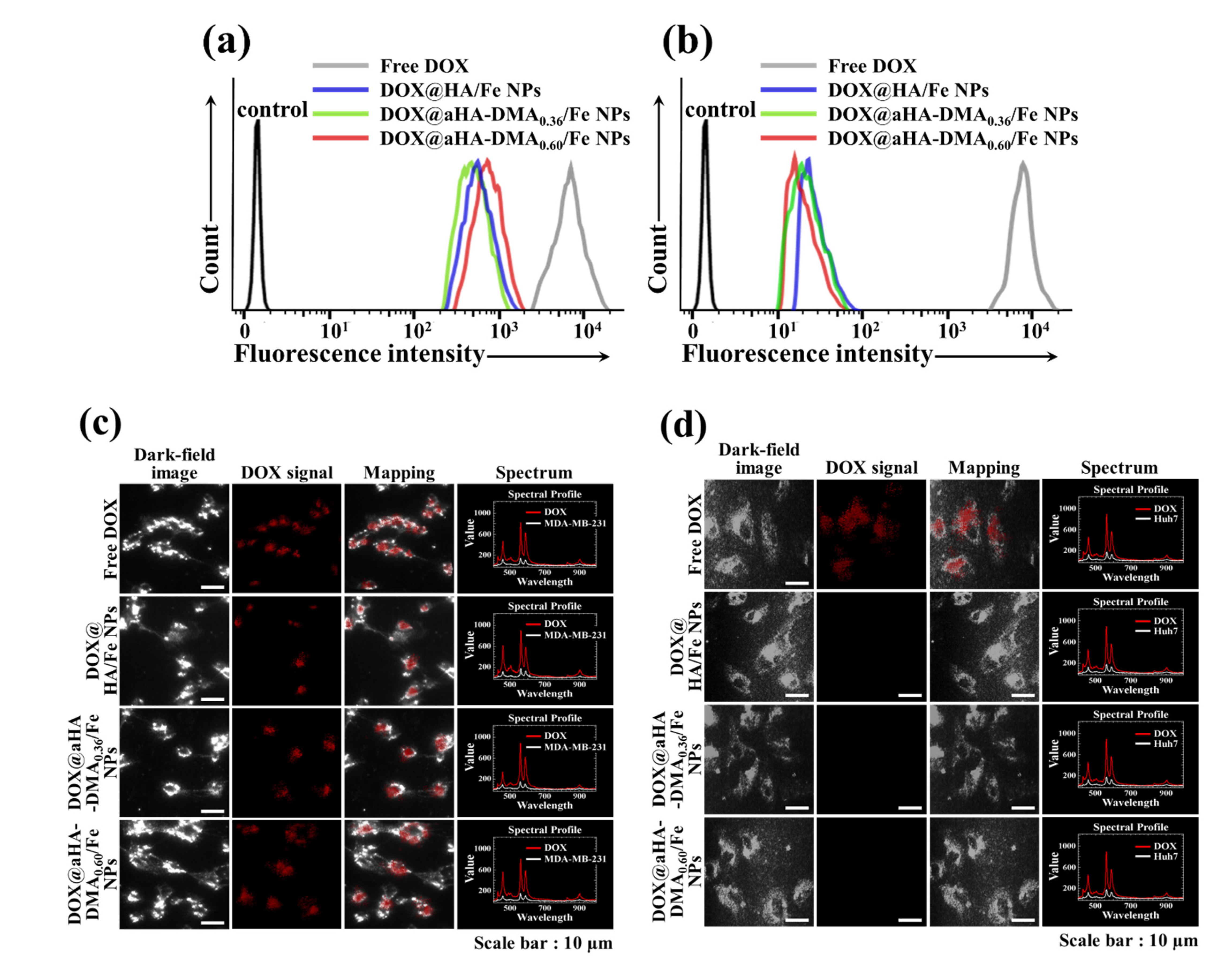
3.4. In Vitro Cellular Uptake of DOX-Loaded Fe-Based HA NPs
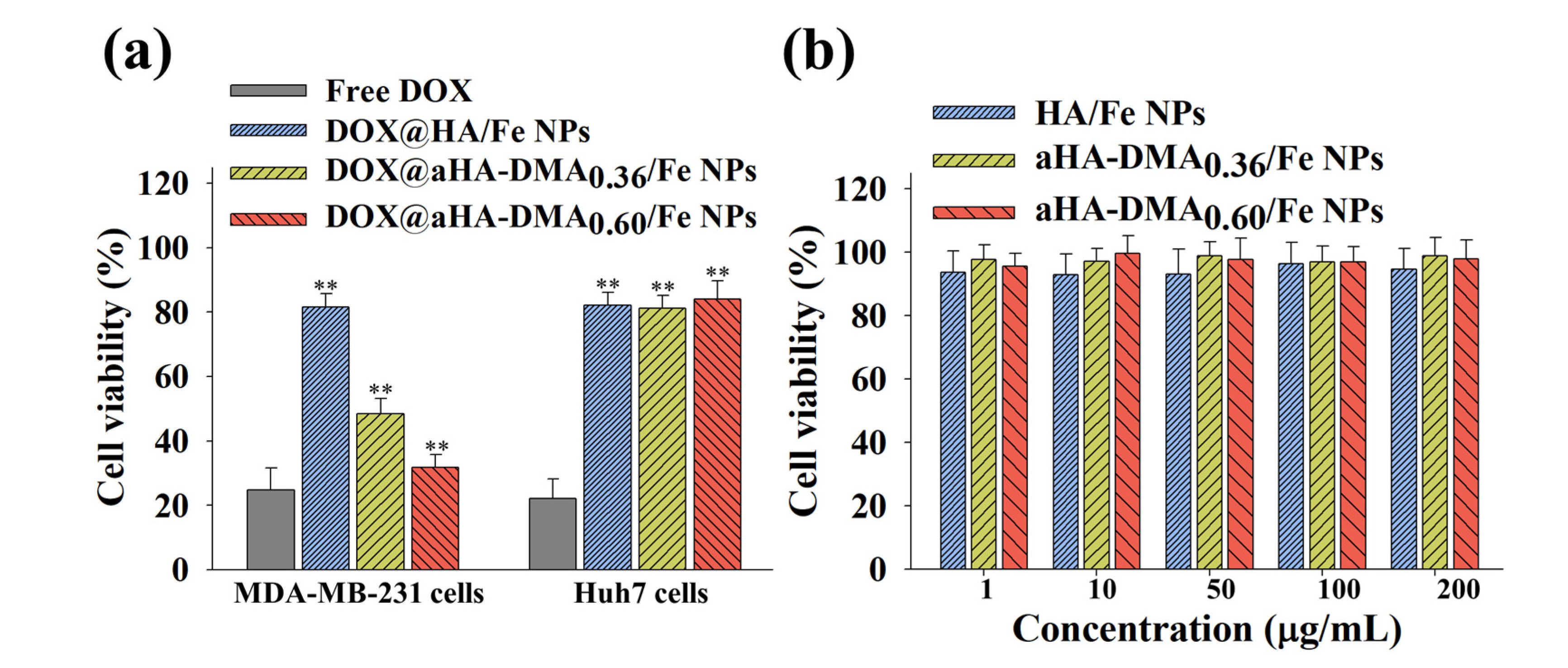
3.5. In Vitro Tumor Inhibition of DOX-Loaded Fe-Based HA NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Akhtar, M.J.; Alhadlaq, H.A.; Kumar, S.; Alrokayan, S.A.; Ahamed, M. Selective cancer-killing ability of metal-based nanoparticles: Implications for cancer therapy. Arch. Toxicol. 2015, 89, 1895–1907. [Google Scholar] [CrossRef] [PubMed]
- Dzhardimalieva, G.I.; Uflyand, I.E. Preparation of metal-polymer nanocomposites by chemical reduction of metal ions: Functions of polymer matrices. J. Polym. Res. 2018, 25, 255. [Google Scholar] [CrossRef]
- Malola, S.; Nieminen, P.; Pihlajamaki, A.; Hamalainen, J.; Karkkainen, T.; Hakkinen, H. A method for structure prediction of metal-ligand interfaces of hybrid nanoparticles. Nat. Commun. 2019, 10, 3973. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; He, Y.; Yu, D.G.; Li, W.; Wang, K. Drug-zein@lipid hybrid nanoparticles: Electrospraying preparation and drug extended release application. Colloids Surf. B 2021, 201, 111629. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hou, J.; Yu, D.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Herrera, S.E.; Agazzi, M.L.; Cortez, M.L.; Marmisolle, W.A.; Tagliazucchi, M.; Azzaroni, O. Multitasking polyamine/ferrioxalate nano-sized assemblies: Thermo-, photo-, and redox-responsive soft materials made easy. Chem. Commun. 2019, 55, 14653–14656. [Google Scholar] [CrossRef]
- Neumann, L.N.; Urban, D.A.; Lemal, P.; Ramani, S.; Petri-Fink, A.; Balog, S.; Weder, C.; Schrettl, S. Preparation of metallosupramolecular single-chain polymeric nanoparticles and their characterization by Taylor dispersion. Polym. Chem. 2020, 11, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Huo, Q.; Zhu, J.; Niu, Y.; Shi, H.; Gong, Y.; Li, Y.; Song, H.; Liu, Y. pH-triggered surface charge-switchable polymer micelles for the co-delivery of paclitaxel/disulfiram and overcoming multidrug resistance in cancer. Int. J. Nanomed. 2017, 12, 8631–8647. [Google Scholar] [CrossRef]
- Noh, G.J.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Cyclic RGD-conjugated hyaluronate dot bearing cleavable doxorubicin for multivalent tumor targeting. Biomacromolecules 2020, 21, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, Y.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Transferrin-conjugated pH-responsive gamma-cyclodextrin nanoparticles for antitumoral topotecan delivery. Pharmaceutics 2020, 12, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.H.; Ryu, J.H. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, N.M.; Kwag, D.S.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Electrostatic charge conversion processes in engineered tumor-identifying polypeptides for targeted chemotherapy. Biomaterials 2012, 33, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Lan, J.; Kang, Y.; Zhang, T.; Ding, Y.; Zhang, X.; Lu, L. Reduction-sensitive CD44 receptor-targeted hyaluronic acid derivative micelles for doxorubicin delivery. Int. J. Nanomed. 2018, 13, 4361–4378. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Ponce, N.T.; Banerjee, A.; Bandopadhyay, R.; Rajendran, S.; Lichtfouse, E. Green polymeric nanomaterials for the photocatalytic degradation of dyes: A review. Environ. Chem. Lett. 2020, 18, 1569–1580. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Youn, Y.S.; Oh, K.T.; Lee, E.S. Tumor-homing pH-sensitive extracellular vesicles for targeting heterogeneous tumors. Pharmaceutic 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, H.; Noh, G.J.; Lee, E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018, 202, 323–333. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Tumor-targeting liposomes with transient holes allowing intact rituximab internally. Biomacromolecules 2021, 22, 723–731. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, H.; Oh, K.T.; Lee, E.S. pH-responsive hyaluronated liposomes for docetaxel delivery. Int. J. Pharm. 2018, 547, 377–384. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, J.M.; Youn, Y.S.; Na, K.; Lee, E.S. Hyaluronate dots for highly efficient photodynamic therapy. Carbohydr. Polym. 2018, 181, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Koo, M.; Oh, K.T.; Noh, G.; Lee, E.S. Gold nanoparticles bearing a tumor pH-sensitive cyclodextrin cap. ACS Appl. Mater. Interfaces 2018, 10, 24450–24458. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Lee, J.M.; Youn, Y.S.; Oh, K.T.; Na, K.; Lee, E.S. Gamma-cyclodextrin-phenylacetic acid mesh as a drug trap. Carbohydr. Polym. 2018, 184, 390–400. [Google Scholar] [CrossRef]
- Lee, U.Y.; Oh, Y.T.; Kim, D.; Lee, E.S. Multimeric grain-marked micelles for highly efficient photodynamic therapy and magnetic resonance imaging of tumors. Int. J. Pharm. 2014, 471, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.Y.; Oh, N.M.; Kwag, D.S.; Oh, K.T.; Oh, Y.T.; Youn, Y.S.; Lee, E.S. Facile synthesis of multimeric micelles. Angew. Chem. Int. Ed. Engl. 2012, 51, 7287–7291. [Google Scholar] [CrossRef] [PubMed]
- Kwag, D.S.; Oh, K.T.; Lee, E.S. Facile synthesis of multilayered polysaccharidic vesicles. J. Control Release 2014, 187, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Park, H.; Tran, T.H.; Hwang, S.Y.; Na, K.; Lee, E.S.; Oh, K.T.; Oh, D.X.; Park, A.J. Poisonous caterpillar-inspired chitosan nanofiber enabling dual photothermal and photodynamic tumor ablation. Pharmaceutics 2019, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Lee, U.Y.; Youn, Y.S.; Park, J.; Lee, E.S. Y-shaped ligand-driven gold nanoparticles for highly efficient tumoral uptake and photothermal ablation. ACS Nano 2014, 8, 12858–12865. [Google Scholar] [CrossRef]
- Noh, G.; Youn, Y.S.; Lee, E.S. Preparation of iron oxide nanoparticles functionalized with Y-shaped ligands for brain tumor targeting. J. Mater. Chem. B 2016, 4, 6074–6080. [Google Scholar] [CrossRef]
- Lee, E.; Park, J.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Alendronate/cRGD-decorated ultrafine hyaluronate dot targeting bone metastasis. Biomedicines 2020, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Lee, E.S. Honeycomb-like pH-responsive gamma-cyclodextrin electrospun particles for highly efficient tumor therapy. Carbohydr. Polym. 2020, 230, 115563. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, Y.; Kim, Y.; Lee, E.S. Endosomal pH-Responsive Fe-Based Hyaluronate Nanoparticles for Doxorubicin Delivery. Molecules 2021, 26, 3547. https://doi.org/10.3390/molecules26123547
Bae Y, Kim Y, Lee ES. Endosomal pH-Responsive Fe-Based Hyaluronate Nanoparticles for Doxorubicin Delivery. Molecules. 2021; 26(12):3547. https://doi.org/10.3390/molecules26123547
Chicago/Turabian StyleBae, Yangmun, Yoonyoung Kim, and Eun Seong Lee. 2021. "Endosomal pH-Responsive Fe-Based Hyaluronate Nanoparticles for Doxorubicin Delivery" Molecules 26, no. 12: 3547. https://doi.org/10.3390/molecules26123547





