Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Lyophilized Hydro-Alcoholic Extract of the Total Fruit, Pulp and Seed of Euterpe oleracea Mart
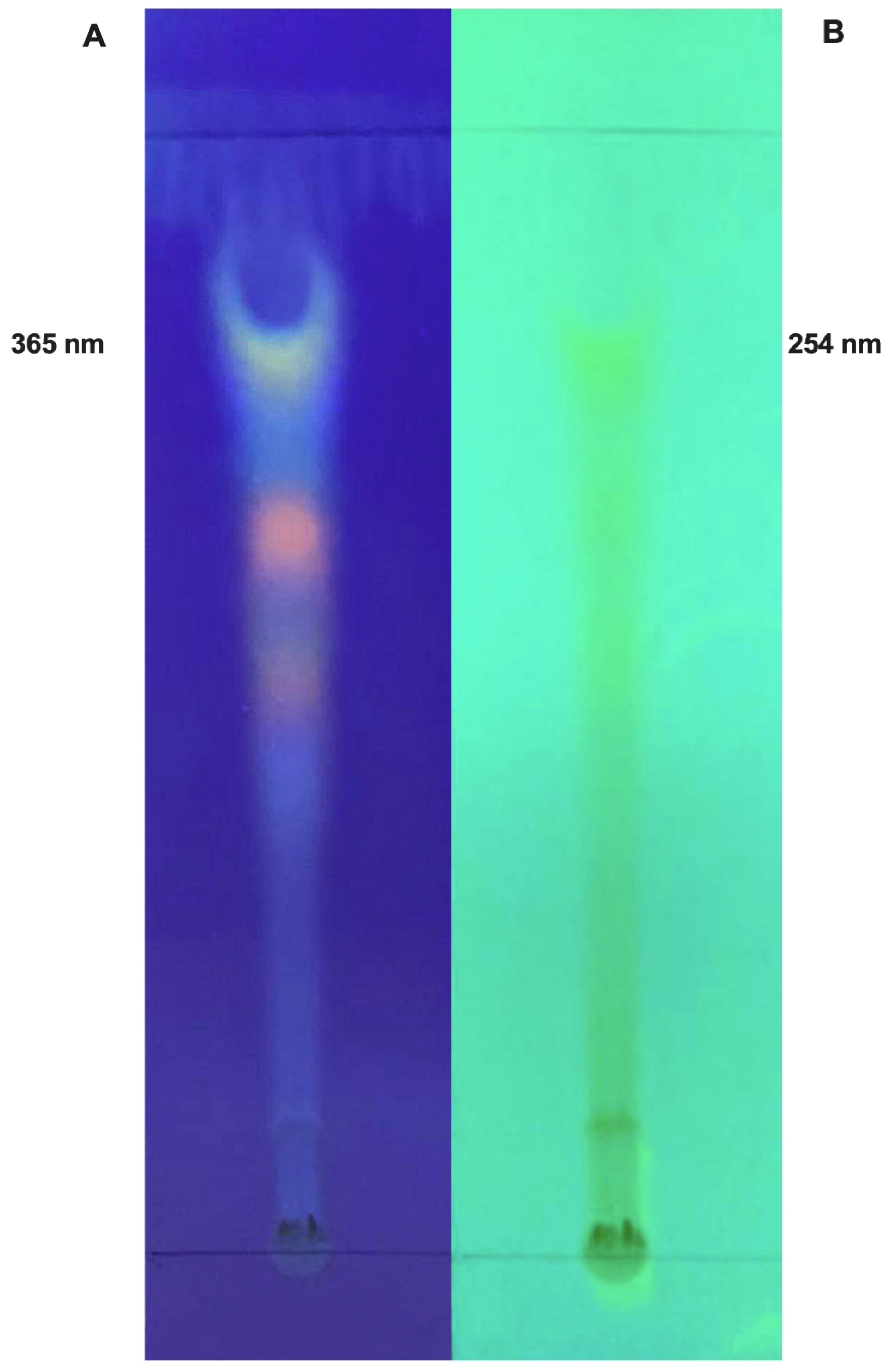
2.2. Thin Layer Chromatography (TLC) Analysis
2.3. MS/MS Analysis
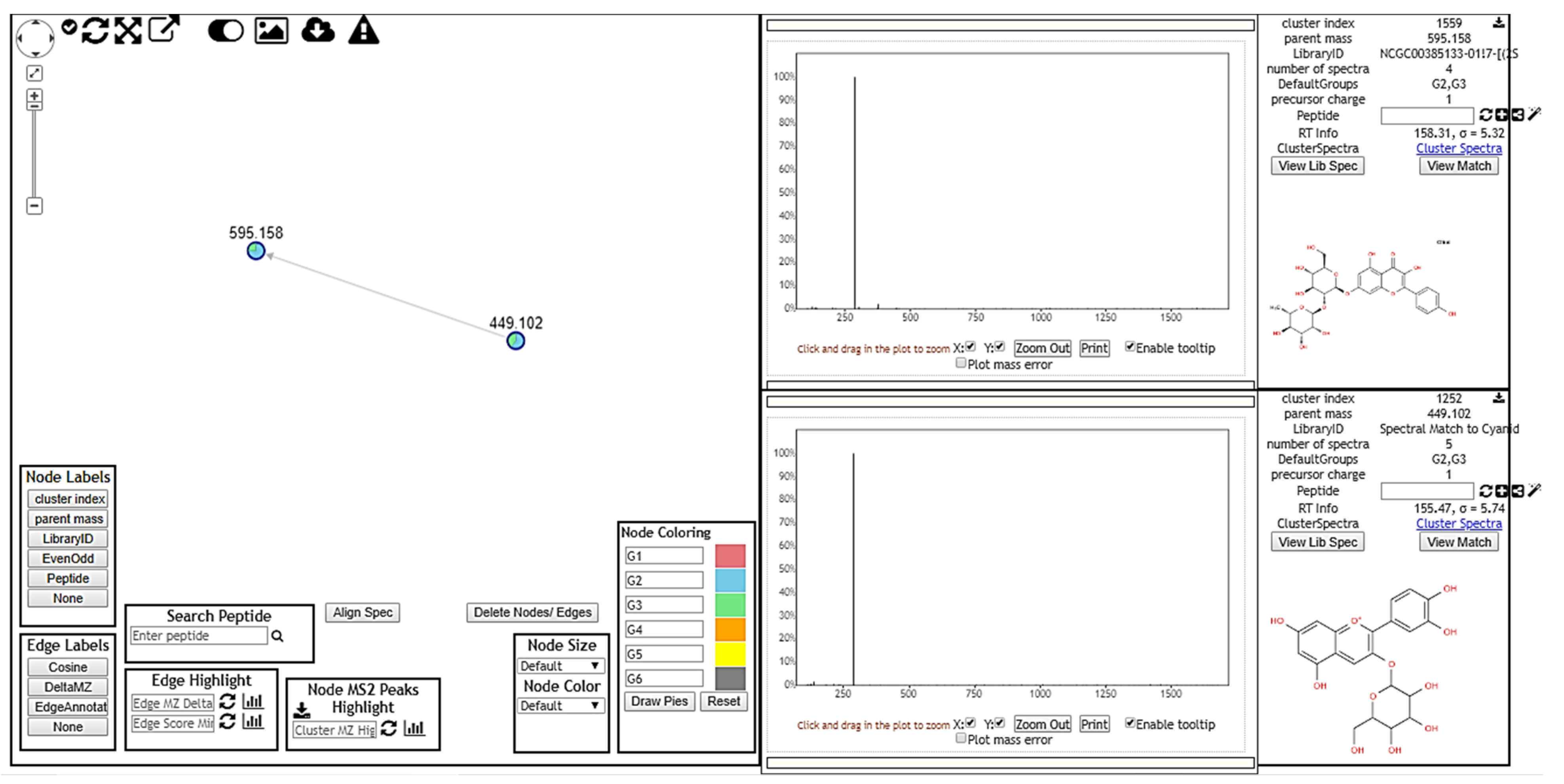
2.4. MS/MS Molecular Networking
2.5. Antioxidant Activity Assay by DPPH (2,2-Diphenyl-1-picrylhydrazly)
2.6. Cell Viability Assay
2.7. Clonogenicity Assay
2.8. Quantification of Apoptosis by Flow Cytometry
2.9. DAF-2DA Diacetate Analysis for Reactive Oxygen Species (ROS) Production by Flow Cytometry
2.10. Time-Lapse Microscopy
2.11. Acridine Orange Staining
2.12. Statistical Analysis
3. Results
3.1. Açai Seed Extract Is Rich in Flavonoids
3.1.1. Thin-Layer Chromatography (TLC) Analysis
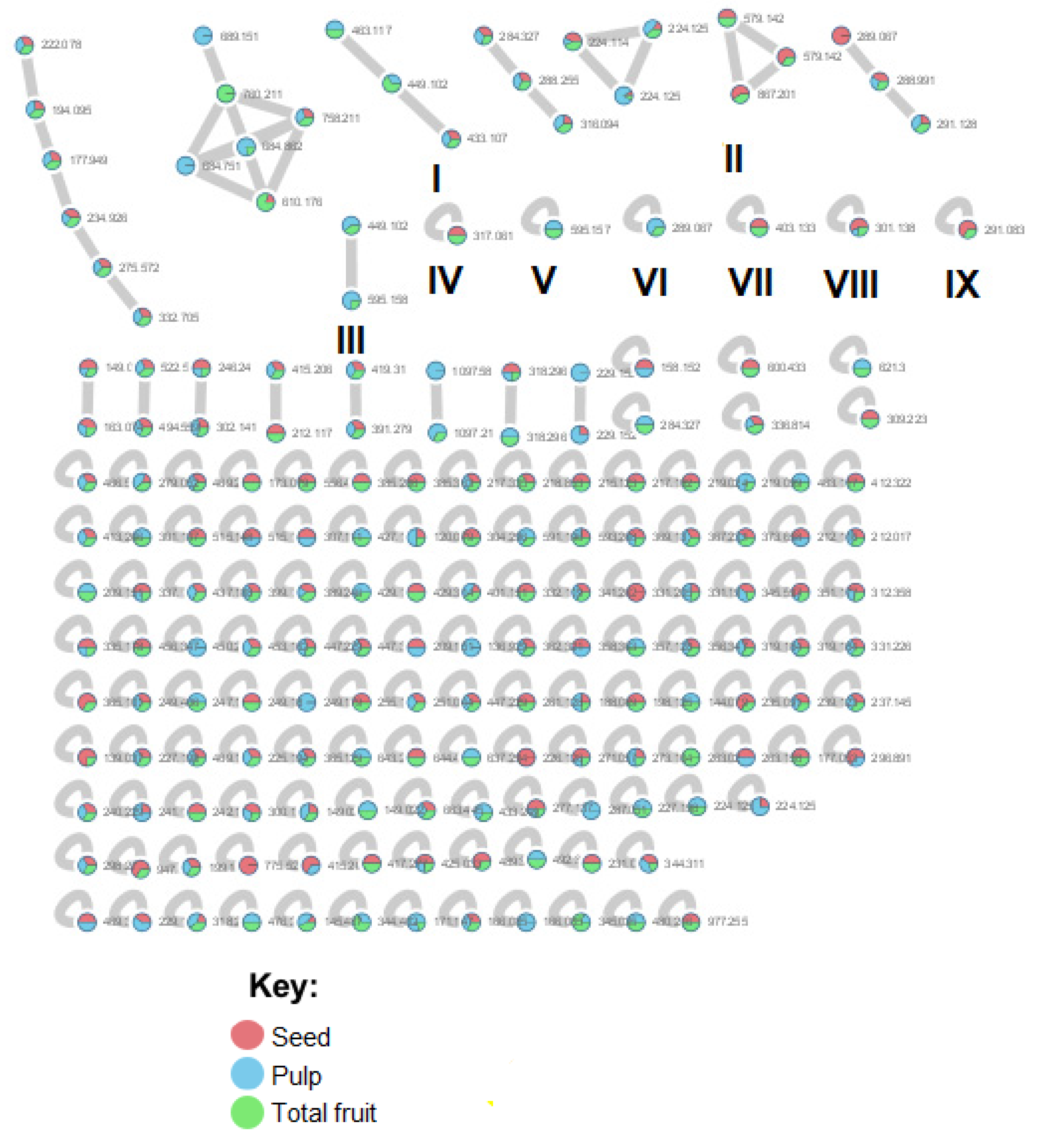
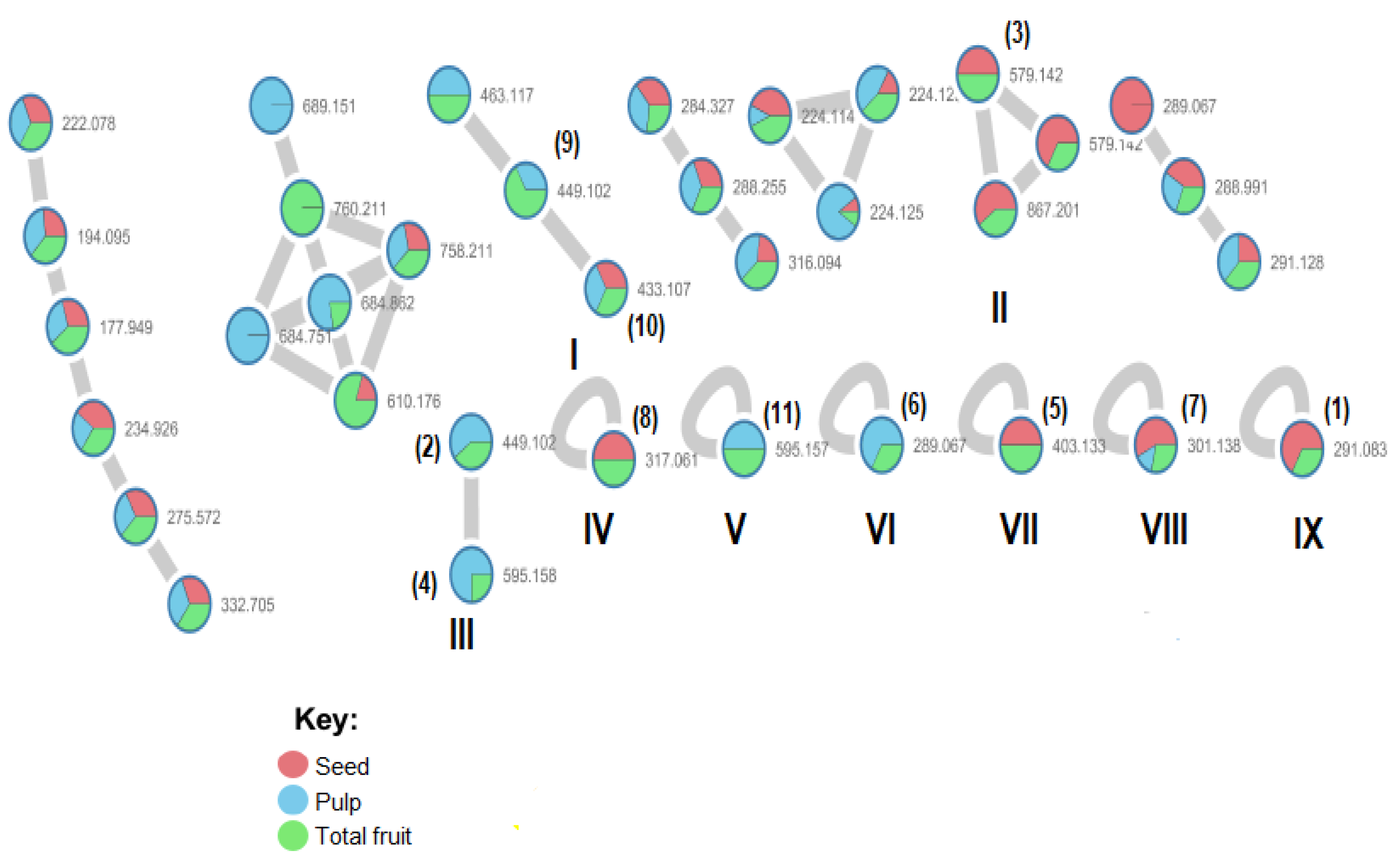
3.1.2. MS/MS Analysis and Molecular Networking
3.2. Chemical Compounds Identified by GNPS
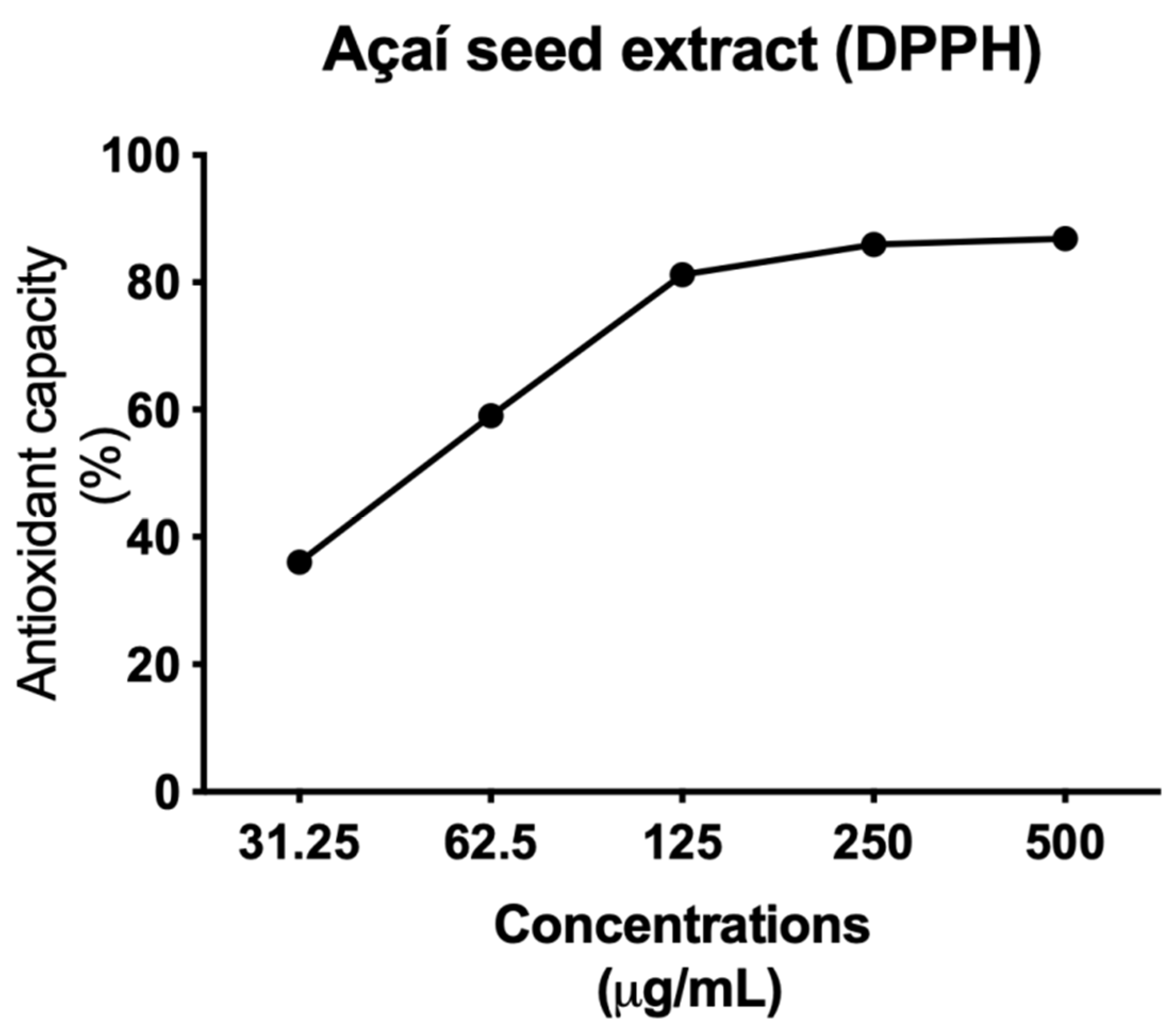
3.3. Açai Seed Extract Has Antioxidant Activity
3.4. Açai Seed Extract Reduces Cell Viability of MCF-7 Breast Cancer Cell Line
3.5. Açai Seed Extract Reduces the Clonogenic Capacity in MCF-7 Cells
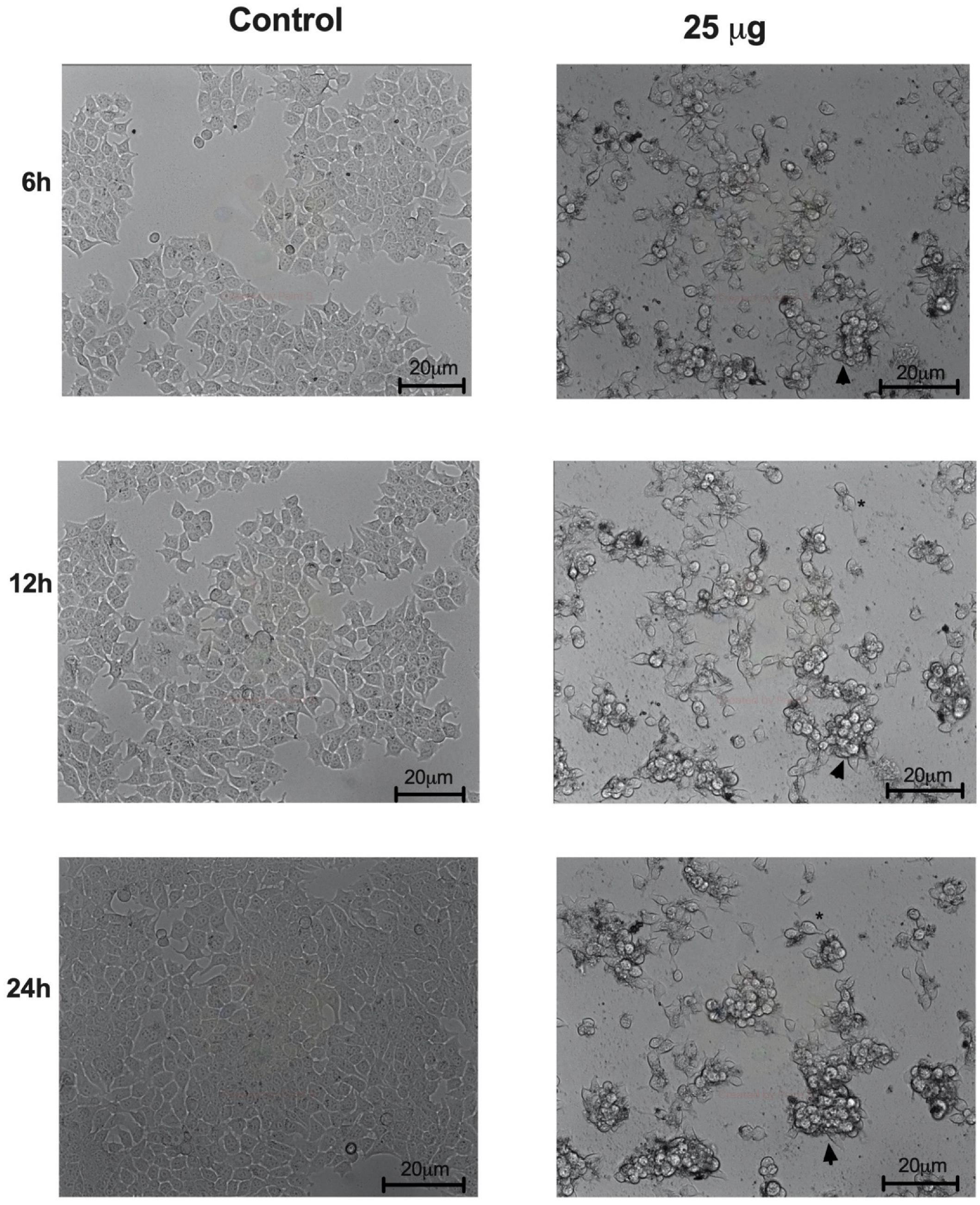
3.6. Açai Seed Extract Induces Morphologic Changes in MCF-7 Breast Cancer Cell Line
3.7. Açai Seed Extract Induces Cell Death by Autophagy in MCF-7 Breast Cancer Cell Line
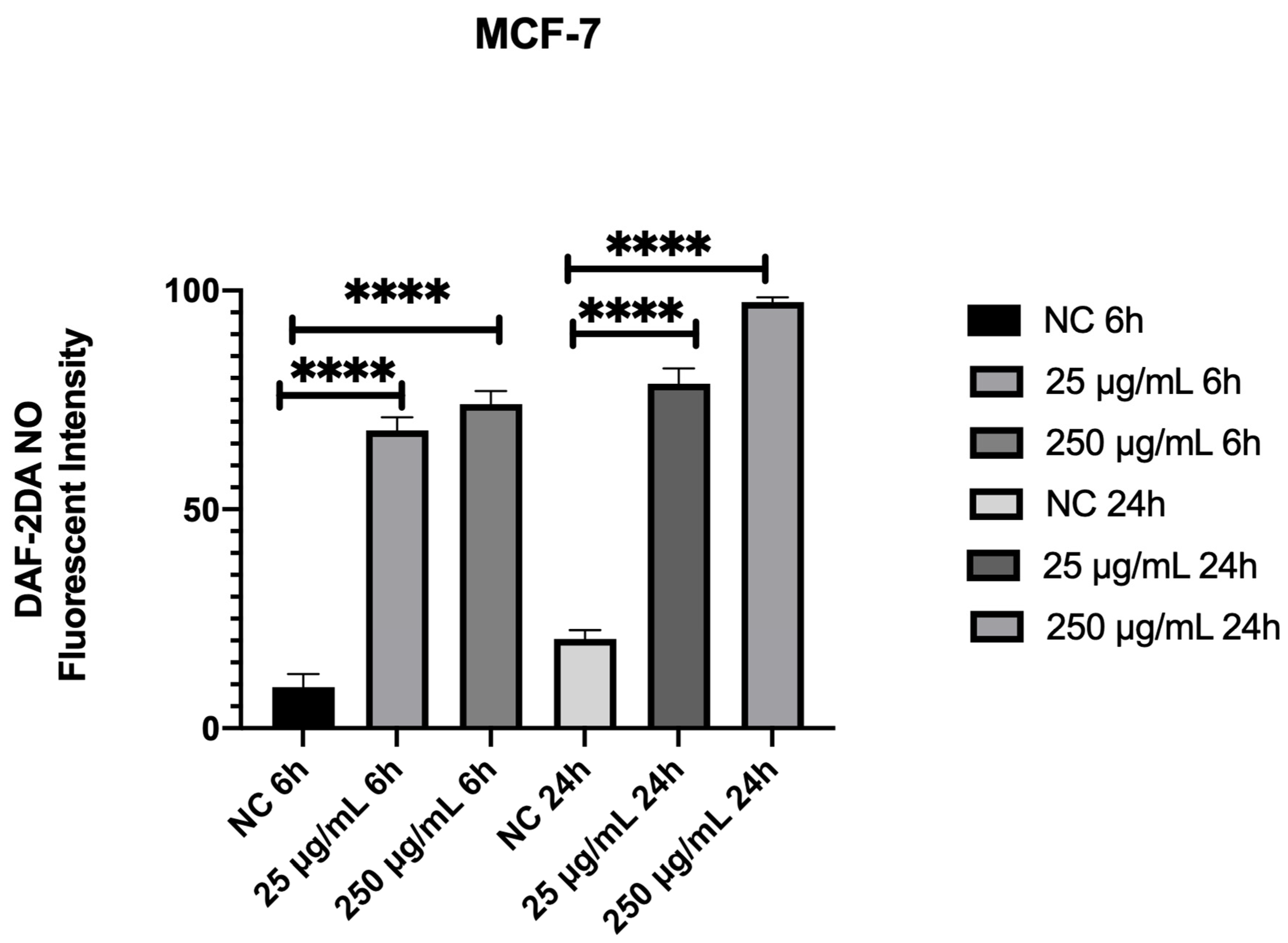
3.8. Açai Seed Extract Increases ROS Production in MCF-7 Breast Cancer Cell Line
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Baker, W.J.; Dransfield, J. Beyond Genera Palmarum: Progress and prospects in palm systematics. Bot. J. Linn. Soc. 2016, 182, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A. The Palms of the Amazon; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Yamaguchi, K.K.; Pereira, L.F.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; Van Damme, P. Effect of a health claim and personal characteristics on consumer acceptance of fruit juices with different concentrations of acai (Euterpe oleracea Mart). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef]
- Costa, A.G.V.; García-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red-black berries. J. Funct. Food 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Menezes, E.; Deliza, R.; Chan, H.L.; Guinard, J.X. Preferences and attitudes towards açai-based products among North American consumers. Food Res. Int. 2011, 44, 1997–2008. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Palencia, L.A.; Talcott, S.T. Chemical stability of açai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010, 118, 17–25. [Google Scholar] [CrossRef]
- Lichtenthaler, R.; Belandrino, R.; Maia, J.; Papaiannopoulos, M.; Fabricius, H.; Marx, F. Total antioxidant scavenging capacities of Euterpe oleracea Mart. (açai). Int. J. Food Sci. Nutr. 2005, 56, 68–75. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and nutrient composition of the freeze dried amazonian palm berry, Euterpe Oleraceae Mart. (Açai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidante effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef]
- Chin, Y.W.; Chai, H.B.; Keller, W.J.; Kinghorn, A.D. Lignans and other constituents of the fruits of Euterpe oleracea (Açai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef]
- Gallori, S.; Bilia, A.R.; Bergonzi, M.C.; Barbosa, W.L.R.; Vincieri, F.F. Polyphenolic constituents of anthocyanins from the açai fruit (Euterpe oleracea) Mart. Cienc. Tecnol. Aliment. 2004, 20, 388–390. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Sistema de Produção do Açai. Available online: http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Acai/SistemaProducaoAcai_2ed/index.htm (accessed on 8 August 2018).
- Pacheco-Palencia, L.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleracea Mart. (açai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Antunes, L.M.; Aissa, A.F.; Darin, J.; Vera de Rosso, V.; Mercadante, A.Z.; Bianchi, M. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using erythrocytes micronucleus test and the comet assay. Mutat. Res. 2010, 695, 22–28. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcoot, S.T. Phytochemical composition and pigment stability of açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.; Rodrigues-Baptista, K.C.; Machado, D.E.; Nasciutti, L.E.; Perini, J.A. Anticancer potential, molecular mechanisms and toxicity of Euterpe oleracea extract (açai): A systematic review. PLoS ONE 2018, 13, e0200101. [Google Scholar]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Freitas, D.D.S.; Morgado-Díaz, J.A.; Gehren, A.S.; Vidal, F.C.B.; Fernandes, R.M.T.; Romão, W.; Tose, L.V.; Frazão, F.N.S.; Costa, M.C.P.; Silva, D.F.; et al. Cytotoxic analysis and chemical characterization of fractions of the hydroalcoholic extract of the Euterpe oleracea Mart. seed in the MCF-7 cell line. J. Pharm. Pharmacol. 2017, 69, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Vidal, F.C.B.; Santos, D.; Costa, M.C.P.; Morgado-Díaz, J.A.; Nascimento, M.D.S.B.; De Moura, R.S. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement. Altern. Med. 2014, 14, 175. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.M.D.S.; Noratto, G.; Martino, H.S.D.; Arbizu, S.; Peluzio, M.D.C.G.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S. Pro-apoptotic activities of polyphenolics from açai (Euterpe oleracea Martius.) in human SW-480 colon cancer cells. Nutr. Cancer 2014, 66, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Guimarães, D.A.B.; Berniz, C.R.; Abreu, J.P.; Rocha, A.P.M.; De Moura, R.S.; Resende, A.C.; Teodoro, A.J. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Stoner, G.D.; Wang, L.S.; Seguin, C.; Rocha, C.; Stoner, K.; Chiu, S.; Kinghorn, A.D. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm. Res. 2010, 27, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, M.F.; Prado, M.G.; Barbosa, L.; Rocha, N.S.; Barbisan, L.F. Inhibition of mouse urinary bladder carcinogenesis by açai fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum. Nutr. 2012, 67, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.F.; Romualdo, G.R.; Ribeiro, D.A.; Barbisan, L.F. Açai (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem. Toxicol. 2013, 58, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Açai berries inhibit colon tumorigenesis in azoxymethane/dextran sulfate sodium-treated mice. Gut Liver 2017, 11, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by açai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. 2017, 166, 301–310. [Google Scholar] [CrossRef]
- Nascimento, V.H.; Lima, C.D.; Paixão, J.T.; Freitas, J.J.; Kietzer, K.S. Antioxidant effects of açai seed (Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir. Bras. 2016, 31, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidebottom, A.M.; Johnson, A.R.; Karty, J.A.; Trader, D.J.; Carlson, E.E. Integrated metabolomics approach facilitates discovery of an unpredicted natural product suite from Streptomyces coelicolor M145. ACS Chem. Biol. 2013, 8, 2009–2016. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.E.; Heycke, N.; Schmitt, S.; et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Engel, P.; Vizcaino, M.I.; Crawford, J.M. Gut symbionts from distinct hosts exhibit genotoxic activity via divergent colibactin biosynthesis pathways. Appl. Environ. Microbiol. 2015, 81, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, N.; Tsur, D.; Frank, A.; Zevzner, P. Protein identification by spectral networks analysis. Proc. Natl. Acad. Sci. USA 2007, 104, 6140–6145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (ACAI) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Bladt, S.; Zgainski, E.M. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Eom, S.A.; Kim, D.W.; Shin, M.J.; Ahn, E.H.; Chung, S.Y.; Sohn, E.J.; Jo, H.S.; Jeon, S.-J.; Kim, D.-S.; Kwon, H.Y.; et al. Protective effects of PEP-1-Catalase on stress-induced cellular toxicity and MPTP-induced Parkinson’s disease. BMB Rep. 2015, 48, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Kanzawa, T.; Kondo, Y.; Ito, H.; Kondo, S.; Germano, I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003, 63, 2103–2108. [Google Scholar]
- Traganos, F.; Darzynkiewicz, Z. Lysosomal Proton Pump Activity: Supravital Cell Staining with Acridine Orange Differentiates Leukocytes Subpopulations; Academic Press: New York, NY, USA, 1994; Volume 41, pp. 185–194. [Google Scholar]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)—A phytochemical and pharmacological assessment of the species’ health claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Yoon, H.S.; Moon, S.C.; Kim, N.D.; Park, B.S.; Jeong, M.H.; Yoo, Y.H. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP. Biochem. Biophys. Res. Commun. 2000, 276, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Antosiak, A.; Milowska, K.; Maczynska, K.; Rozalska, S.; Gabryelak, T. Cytotoxic activity of genistein-8-C-glucoside form Lupinus luteus L. and genistein against human SK-OV-3 ovarian carcinoma cell line. Med. Chem. Res. 2017, 26, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Kim, T.; Lee, M. Pro-apoptotic effect and cytotoxicity of genistein and genistin in Human ovarian cancer SK-OV-3 cells. Life Sci. 2007, 80, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogez, H. Açai: Preparo, Composição e Melhoramento da Conservação, 1st ed.; UFPA: Belém, Brazil, 2000. [Google Scholar]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, T.; Thorburn, A. Autophagy and cell death. Essays Biochem. 2013, 55, 105–117. [Google Scholar] [PubMed] [Green Version]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.D.; Kim, K.-Y.; Park, K.I.; Kim, S.-H.; Park, S.-G.; Yu, S.-N.; Kim, Y.-W.; Kim, D.S.; Chung, K.T.; Ahn, S.-C. Dual role of reactive oxygen species in autophagy and apoptosis induced by compound PN in prostate cancer cells. Mol. Cell. Toxicol. 2020, 17, 41–50. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, Y.; Cho, D.H.; Jeong, S.Y.; Kim, S.B.; Suh, N.; Lee, J.S.; Choi, E.K.; Koh, J.-Y.; Hwang, J.J.; et al. Raloxifene induces autophagy-dependent cell death in breast cancer cells via the activation of AMP-activated protein kinase. Mol. Cells 2015, 38, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.L.; Chen, J.; Wang, Y.P.; Zheng, H. Autophagy protects breast cancer cells from epirubicin-induced apoptosis and facilitates epirubicin-resistance development. Autophagy 2011, 7, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zava, D.T.; Duwe, G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr. Cancer 1997, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, C.; Salvadori, S.; Valacchi, G.; Soucek, K.; Slabakova, E.; Muscettola, M.; Volpi, N.; Maioli, E. Alternative pathways of cancer cell death by rottlerin: Apoptosis versus autophagy. Evid. Based Complement. Altern. Med. 2012, 2012, 980658. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.J.; Chang, H.; Peng, X.L.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS ONE 2014, 9, e102535. [Google Scholar]
- Kuntz, L.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Ramalingam, J.; Long, L.H. Hydrogen peroxide: Ubiquitous in cell culture and in vivo? IUBMB Life 2000, 50, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active oxygen generation by flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Yokomizo, A.; Moriwaki, M. Effects of Uptake of Flavonoids on Oxidative Stress Induced by Hydrogen Peroxide in Human Intestinal Caco-2 Cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef]
- Habtemarium, S.; Dagne, E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010, 76, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Valdameri, G.; Trombetta-Lima, M.; Worfel, P.R.; Pires, A.R.; Martinez, G.R.; Noleto, G.R.; Cadena, S.M.S.C.; Sogayar, M.C.; Winnischofer, S.M.B.; Rocha, M.E.M. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem. Biol. Interact. 2011, 193, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Wang, J.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol. Appl. Pharm. 2012, 265, 83–92. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.F.; Yeh, W.L.; Huang, S.M.; Tan, T.W.; Lu, D.Y. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int. J. Mol. Sci. 2012, 13, 9877–9892. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifico, S.; Scognamiglio, M.; D’Abrosca, B.; Piccolella, S.; Tsafantakis, N.; Gallicchio, M.; Ricci, A.; Fiorentino, A. Spectroscopic characterization and antiproliferative activity on HepG2 human hepatoblastoma cells of flavonoid C-glycosides from Petrorhagia velutina. J. Nat. Prod. 2010, 73, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Benz, D.; Cadet, P.; Mantione, K.; Zhu, W.; Stefano, G. Tonal nitric oxide and health—A free radical and a scavenger of free radicals. Med. Sci. Monit. 2002, 8, RA1–RA4. [Google Scholar] [PubMed]
- Sessa, W.C. The nitric oxide synthase family of proteins. J. Vasc. Res. 1994, 31, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, R.H.; Evens, A.M. Oxidative stress and apoptosis: A new treatment paradigm in cancer. Front. Biosci. 2006, 11, 300–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherz-Shouval, R.; Shvets, E.; Elazar, Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy 2007, 3, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.N.; Chowdhury, R.; Trudel, L.J.; Tee, A.R.; Slack, R.S.; Walker, C.L.; Wogan, G.N. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2- mediated suppression of mTORC1. Proc. Natl. Acad. Sci. USA 2013, 110, E2950–E2957. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Korolchuk, V.I.; Renna, M.; Imarisio, S.; Fleming, A.; Williams, A.; Garcia-Arencibia, M.; Rose, C.; Luo, S.; Underwood, B.R.; et al. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell. 2011, 43, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Danzer, B.; Levenson, V.V.; Maki, C.G. Critical roles for Nitric oxide and ERK in the completion of prosurvival autophagy in 4OHTAM-treated estrogen receptor-positive breast cancer cells. Cancer Lett. 2014, 353, 290–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Compound | Structure | Calculated m/z | Observed m/z | Error (ppm) | Parts from Açai |
|---|---|---|---|---|---|
| 1 | 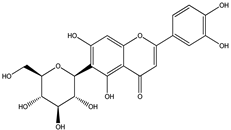 | 291.0863 | 291.0848 | −5.15 | seed and total fruit |
| 2 | 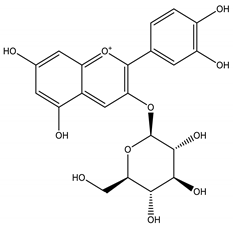 | 449.1078 | 449.1056 | −4.89 | pulp and total fruit |
| 3 | 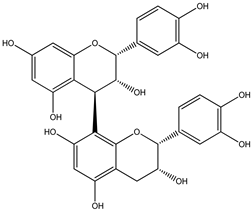 | 579.1497 | 579.1481 | −2.76 | seed and total fruit |
| 4 | 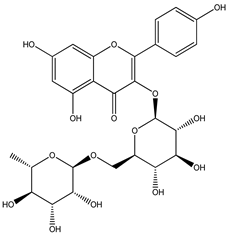 | 595.1657 | 595.1628 | −4.87 | pulp and total fruit |
| 5 | 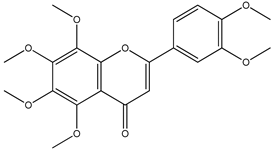 | 403.1387 | 403.1371 | −3.96 | seed and total fruit |
| 6 | 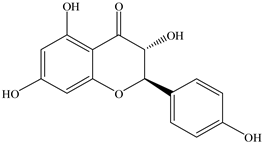 | 289.0707 | 289.0695 | −4.15 | pulp and total fruit |
| 7 | 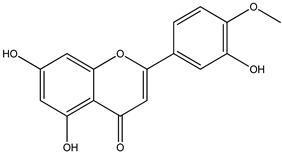 | 301.0707 | 301.0695 | −3.98 | seed and total fruit |
| 8 | 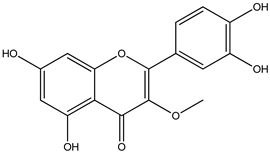 | 317.0656 | 317.0643 | −4.10 | seed and total fruit |
| 9 | 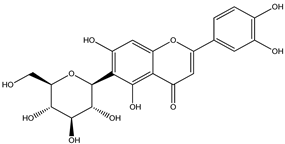 | 449.1078 | 449.1060 | −4.00 | pulp and total fruit |
| 10 | 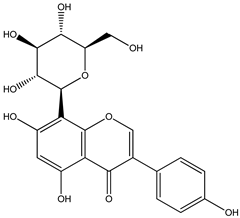 | 433.1129 | 433.1110 | −4.38 | seed, pulp and total fruit |
| 11 | 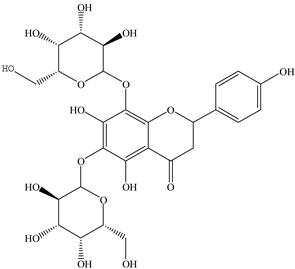 | 595.1657 | 595.1632 | −4.20 | pulp and total fruit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.A.C.N.d.; Costa, J.H.; Pacheco-Fill, T.; Ruiz, A.L.T.G.; Vidal, F.C.B.; Borges, K.R.A.; Guimarães, S.J.A.; Azevedo-Santos, A.P.S.d.; Buglio, K.E.; Foglio, M.A.; et al. Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line. Molecules 2021, 26, 3546. https://doi.org/10.3390/molecules26123546
Silva MACNd, Costa JH, Pacheco-Fill T, Ruiz ALTG, Vidal FCB, Borges KRA, Guimarães SJA, Azevedo-Santos APSd, Buglio KE, Foglio MA, et al. Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line. Molecules. 2021; 26(12):3546. https://doi.org/10.3390/molecules26123546
Chicago/Turabian StyleSilva, Marcos Antonio Custódio Neto da, Jonas Henrique Costa, Taícia Pacheco-Fill, Ana Lúcia Tasca Gois Ruiz, Flávia Castello Branco Vidal, Kátia Regina Assunção Borges, Sulayne Janaina Araújo Guimarães, Ana Paula Silva de Azevedo-Santos, Kaio Eduardo Buglio, Mary Ann Foglio, and et al. 2021. "Açai (Euterpe oleracea Mart.) Seed Extract Induces ROS Production and Cell Death in MCF-7 Breast Cancer Cell Line" Molecules 26, no. 12: 3546. https://doi.org/10.3390/molecules26123546






