Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2
Abstract
:1. Introduction
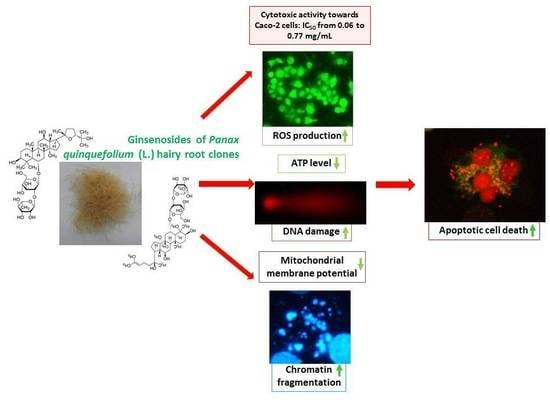
2. Results and Discussion
2.1. Ginsenoside Content in Studied Clones of Hairy Root Cultures of P. quinquefolium
2.2. Cytotoxic Activity of P. quinquefolium Extracts
2.3. Estimation of Half Maximal Inhibitory Concentration (IC50)
2.4. Basal Endogenous DNA Damage Induced by P. quinquefolium Extracts
2.5. Effect of P. quinquefolium Extracts on Colony Formation
2.6. The Effect of P. quinquefolium Extracts on Intracellular ATP Level, Mitochondrial Membrane Potential, Intracellular Oxidative Stress and Apoptosis Induction
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. P. quinquefolium Hairy Root Culture
3.3. Preparation of P. quinquefolium Roots’ Extracts
3.4. Determination of Ginsenoside Content Using HPLC Method
3.5. Caco-2 Cell Culture
3.6. MTT and PB Assays
3.7. Genotoxicity Testing (Comet Assay)
3.8. Clonogenic Assay
3.9. Measurement of ATP Production and Mitochondrial Membrane Potential (MMP)
F530/620 and F485/530 for the control cells) × 100
3.10. Detection of Intracellular Reactive Oxygen Species (ROS) Generation
control cells × 100
3.11. Phosphatidylserine Externalisation and Membrane Permeabilization
3.12. Detection of Mono- and Oligonucleosomes Release (Apoptotic DNA Degradation and Necrosis Detection)
cells × 100
3.13. Fluorescent Microscopic Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pengelly, A.K.; Bennett, K. Appalachian Plant Monographs: Panax quinquefolius L., American Ginseng. Available online: http://www.frostburg.edu/aces/appalachian-plants/ (accessed on 5 January 2019).
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [Green Version]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American Ginseng (Panax quinquefolium L.) as a Source of Bioactive Phytochemicals with Pro-Health Properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef] [Green Version]
- Quan, K.; Liu, Q.; Wan, J.-Y.; Zhao, Y.-J.; Guo, R.-Z.; Alolga, R.N.; Li, P.; Qi, L.-W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015, 5, 8598. [Google Scholar] [CrossRef] [Green Version]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef]
- Guo, D.; Cheng, L.; Zhang, Y.; Zheng, H.; Ma, H.; Li, L. An improved method for the preparation of Ginsenoside Rg5 from ginseng fibrous root powder. Heliyon 2019, 5, e02694. [Google Scholar] [CrossRef] [Green Version]
- An, K.S.; Choi, Y.O.; Lee, S.M.; Ryu, H.Y.; Kang, S.J.; Yeon, Y.; Kim, Y.R.; Lee, J.G.; Kim, C.J.; Lee, Y.J.; et al. Ginsenosides Rg5 and Rk1 Enriched Cultured Wild Ginseng Root Extract Bioconversion of Pediococcus pentosaceus HLJG0702: Effect on Scopolamine-Induced Memory Dysfunction in Mice. Nutrients 2019, 11, 1120. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.; Wong, A. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruse-Sanders, J.M.; Hamrick, J.L. Genetic diversity in harvested and protected populations of wild American ginseng, Panax quinquefolius L. (Araliaceae). Am. J. Bot. 2004, 91, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, J.T.A.; Shelp, B.J. Effect of boron nutrition on American ginseng in field and in nutrient cultures. J. Ginseng Res. 2014, 38, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Zhao, Y. Current Evaluation of the Millennium Phytomedicine- Ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and Regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures. In Food Biotechnology; Stahl, U., Donalies, U.E.B., Nevoigt, E., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 187–228. ISBN 978-3-540-70535-2. [Google Scholar]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Bioreactor Technology for Hairy Roots Cultivation. In Bioprocessing of Plant In Vitro Systems; Pavlov, A., Bley, T., Eds.; Springer International Publishing: Cham, Switzerlan, 2016; pp. 1–25. ISBN 978-3-319-32004-5. [Google Scholar]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G. Nitrogen and phosphorus as the factors affecting ginsenoside production in hairy root cultures of Panax quinquefolium cultivated in shake flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2016, 38, 149. [Google Scholar] [CrossRef]
- Kochan, E.; Balcerczak, E.; Lipert, A.; Szymańska, G.; Szymczyk, P. Methyl jasmonate as a control factor of the synthase squalene gene promoter and ginsenoside production in American ginseng hairy root cultured in shake flasks and a nutrient sprinkle bioreactor. Ind. Crops Prod. 2018, 115, 182–193. [Google Scholar] [CrossRef]
- Kochan, E.; Królicka, A.; Chmiel, A. Growth and ginsenoside production in Panax quinquefolium hairy roots cultivated in flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2012, 34, 1513–1518. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant. Cell Tissue Organ. Cult. PCTOC 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Hayta, S.; Gurel, A.; Akgun, I.; Altan, F.; Ganzera, M.; Tanyolac, B.; Bedir, E. Induction of Gentiana cruciata hairy roots and their secondary metabolites. Biologia (Bratisl.) 2011, 66, 618–625. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; da Silva, J.A.T.; Ma, G. Agrobacterium rhizogenes-mediated genetic transformation of Psammosilene tunicoides and identification of high saponin-yielding clones. Environ. Exp. Biol. 2015, 13, 19–23. [Google Scholar]
- Bulgakov, V.P. Functions of rol genes in plant secondary metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef]
- Sujatha, M.; Vijay, S.; Vasavi, S.; Veera Reddy, P.; Chander Rao, S. Agrobacterium-mediated transformation of cotyledons of mature seeds of multiple genotypes of sunflower (Helianthus annuus L.). Plant Cell Tissue Organ. Cult. PCTOC 2012, 110, 275–287. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. Individual and combined effects of the rol A,B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol. Bioeng. 2008, 100, 118–125. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Ku, S.; You, H.J.; Ji, G.E. A stereo-selective growth inhibition profile of ginsenoside Rh2 on human colon cancer cells. CyTA-J. Food 2019, 17, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.-J.; Kim, J.-S. Comparison of Ginsenoside Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Z.; Anderson, S.; Du, W.; He, T.-C.; Yuan, C.-S. Red ginseng and cancer treatment. Chin. J. Nat. Med. 2016, 14, 7–16. [Google Scholar] [PubMed]
- Luo, X.; Wang, C.-Z.; Chen, J.; Song, W.-X.; Luo, J.; Tang, N.; He, B.-C.; Kang, Q.; Wang, Y.; Du, W.; et al. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. Int. J. Oncol. 2008, 32, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Wang, Z.; Zhao, F.; Liu, J.; Zhang, W.; Li, J.; Song, Z.; Xu, H. AFM-detected apoptosis of hepatocellular carcinoma cells induced by American ginseng root water extract. Micron 2018, 104, 1–7. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, Y.C.; Lee, J.W.; Choi, J.S.; Bae, S.H. Effects of ginsenoside Rg3 on apoptosis in A375.S2 melanoma cells. Transl. Cancer Res. 2019, 8, 357–366. [Google Scholar] [CrossRef]
- Li, T.; Sun, W.; Dong, X.; Yu, W.; Cai, J.; Yuan, Q.; Shan, L.; Efferth, T. Total ginsenosides of Chinese ginseng induces cell cycle arrest and apoptosis in colorectal carcinoma HT-29 cells. Oncol. Lett. 2018, 16, 4640–4648. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-H.; Li, H.-D.; Li, B.; Jiang, S.-D.; Jiang, L.-S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncol. Rep. 2014, 31, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskic, D.; Popovic, S.; Ristic, P.; Arsenijevic, N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Salim, L.; Mohan, S.; Othman, R.; Abdelwahab, S.; Kamalidehghan, B.; Sheikh, B.; Ibrahim, M. Thymoquinone Induces Mitochondria-Mediated Apoptosis in Acute Lymphoblastic Leukaemia in Vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, Y.; Zheng, S.-L.; Qin, Y.; Julian McClements, D.; Hu, J.-N.; Deng, Z.-Y. Antitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing mice. J. Funct. Foods 2017, 32, 382–390. [Google Scholar] [CrossRef]
- Duan, Z.; Deng, J.; Dong, Y.; Zhu, C.; Li, W.; Fan, D. Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: In vitro and in vivo. Food Funct. 2017, 8, 3723–3736. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Khan, M.N.; Zhang, L.; Chen, Q.; Zhao, Y.; Yan, Q.; Fu, L.; Liu, J. Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial–mesenchymal transition and stemness. Cancer Lett. 2018, 415, 73–85. [Google Scholar] [CrossRef]
- Choi, J.-S.; Chun, K.-S.; Kundu, J.; Kundu, J.K. Biochemical basis of cancer chemoprevention and/or chemotherapy with ginsenosides (Review). Int. J. Mol. Med. 2013, 32, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-M.; Kim, E.; Chun, S. Ginsenoside Compound K Induces Ros-Mediated Apoptosis and Autophagic Inhibition in Human Neuroblastoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 4279. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fan, D. Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model. Food Funct. 2018, 9, 5513–5527. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Wang, C.-Z.; Searle, J.; He, T.-C.; Yuan, C.-S.; Du, W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011, 301, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Hong, S.; Hwang, H.-J.; Kim, J.W.; Kim, J.A.; Lee, Y.B.; Roh, E.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Ginsenoside compound-Mc1 attenuates oxidative stress and apoptosis in cardiomyocytes through an AMP-activated protein kinase–dependent mechanism. J. Ginseng Res. 2019, S1226845318304202. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium. Molecules 2018, 23, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, A.; Sójka, M.; Klewicka, E.; Lipińska, L.; Klewicki, R.; Kołodziejczyk, K. Ellagitannins from Rubus idaeus L. Exert Geno- and Cytotoxic Effects against Human Colon Adenocarcinoma Cell Line Caco-2. J. Agric. Food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- OECD/OCDE TG 442D Adopted: February 2015 OECD Guideline For The Testing Of Chemicals In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method 2015. Available online: https://www.oecd-ilibrary.org/environment/test-no-442d-in-vitro-skin-sensitisation_9789264229822-en (accessed on 13 January 2020).
- Choi, Y.H. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed. Pharmacother. 2018, 106, 902–909. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Metabolite | Saponin Content [mg/g d.w.] ± S.E.M. | ||||||
|---|---|---|---|---|---|---|---|
| A | B | G | Ae | Be | Ge | ||
| Rg group | Rg1 | 1.28 ± 0.113 a | 1.04 ± 0.064 a | 1.42 ± 0.037 a | 0.79 ± 0.053 b | 1.41 ± 0.038 a | 2.00 ± 0.063 c |
| Re | 2.57 ± 0.253 a | 0.86 ± 0.037 b | 1.27 ± 0.035 c | 1.49 ± 0.030 d | 1.22 ± 0.021 c | 1.21 ± 0.028 c | |
| Sum | 3.85 ± 0.359 a | 1.9 ± 0.101 b | 2.68 ± 0.072 c | 2.28 ± 0.083 b | 2.63 ± 0.048 c | 3.21 ± 0.089 a | |
| Rb group | Rb1 | 4.74 ± 0.162 a | 1.56 ± 0.021 b | 1.64 ± 0.034 b | 9.91 ± 0.200 c | 8.48 ± 0.061 d | 9.10 ± 0.282 c,d |
| Rc | 4.51 ± 0.150 a | 1.63 ± 0.194 b | 1.35 ± 0.016 b | 6.35 ± 0.080 c | 2.67 ± 0.041 d | 1.83 ± 0.086 b | |
| Rb2 | 1.28 ± 0.039 a | 0.34 ± 0.014 b | 0.22 ± 0.005 c | 3.13 ± 0.001 d | 0.96 ± 0.038 e | 1.10 ± 0.042 a,e | |
| Rb3 | 0.76 ± 0.022 a | 0.14 ± 0.014 b | 0.08 ± 0.002 c | 1.83 ± 0.011 d | 0.48 ± 0.020 e | 0.49 ± 0.018 e | |
| Rd | 1.88 ± 0.114 a | 0.56 ± 0.094 b | 0.31 ± 0.012 b | 10.81 ± 0.141 c | 4.94 ± 0.085 d | 5.11 ± 0.189 d | |
| Sum | 13.19 ± 0.311 a | 4.22 ± 0.245 b | 3.72 ± 0.046 b | 31.99 ± 0.194 c | 17.53 ± 0.217 d | 17.64 ± 0.611 d | |
| Total | Rg + Rb group | 17.04 ± 0.646 a | 6.12 ± 0.194 b | 6.27 ± 0.112 b | 34.96 ± 0.278 c | 20.16 ± 0.240 d | 20.85 ± 0.603 d |
| Extract | IC50 [mg/mL] | |
|---|---|---|
| MTT | PrestoBlue | |
| A | 0.35 | 0.40 |
| Ae | 0.29 | 0.31 |
| B | 0.19 | 0.33 |
| Be | 0.06 | 0.21 |
| G | 0.64 | 0.77 |
| Ge | 0.42 | 0.43 |
| Extract | Concentration [mg/mL] | DNA in the Tail [%] ± S.E.M. |
|---|---|---|
| A | 0.017 0.068 0.270 | 10.2 ± 0.6 a,b 6.6 ± 1.1 a,c 63.5 ± 1.9 b,c |
| Ae | 0.017 0.068 0.270 | 8.1 ±1.4 j 9.5 ± 1.5 k 25.6 ± 2.3 j,k |
| B | 0.009 0.035 0.137 | 10.2 ± 0.7 e,f 7.6 ± 1.1 e,g 34.0 ± 3.3 f,g |
| Be | 0.009 0.035 0.137 | 9.9 ± 0.6 l,m 7.6 ± 0.8 l,n 40.9 ± 2.4 m |
| G | 0.032 0.128 0.510 | 7.6 ± 0.7 h 8.5 ± 1.7 i 66.6 ± 1.8 h,i |
| Ge | 0.016 0.064 0.255 | 12.1 ± 0.7 o,q 8.4 ± 1.3 o,r 41.6 ± 2.7 q,r |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, E.; Nowak, A.; Zakłos-Szyda, M.; Szczuka, D.; Szymańska, G.; Motyl, I. Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 2262. https://doi.org/10.3390/molecules25092262
Kochan E, Nowak A, Zakłos-Szyda M, Szczuka D, Szymańska G, Motyl I. Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2. Molecules. 2020; 25(9):2262. https://doi.org/10.3390/molecules25092262
Chicago/Turabian StyleKochan, Ewa, Adriana Nowak, Małgorzata Zakłos-Szyda, Daria Szczuka, Grażyna Szymańska, and Ilona Motyl. 2020. "Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2" Molecules 25, no. 9: 2262. https://doi.org/10.3390/molecules25092262