Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract
Abstract
:1. Introduction
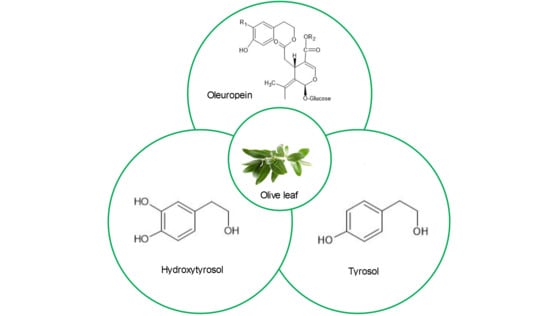
2. Main Chemical Compounds of Olive Leaves and Their Biological Effects
- simple phenols (the most common and important low-molecular weight phenolic compounds);
- flavonoids (flavones, flavanones, flavonols, and flavan-3-ols);
- secoiridoids.
Antimicrobial and Enzymatic Activity of Phenolic Compounds
3. Methods of Isolation, Characterization, and Determination of Antimicrobial Potentials of Main Chemical Compounds in Olive Leaves
3.1. Isolation of Main Chemical Compounds from Olive Leaves
- acid-base properties;
- charge;
- molecular size;
- solubility (hydrophobicity or hydrophilicity);
- stability.
3.1.1. Traditional Extraction Techniques
Solid-Liquid Extraction Technique (SLE)
Soxhlet Extraction Technique
- by assisting extraction with auxiliary energies;
- by automating extraction with different approaches;
- by increasing the pressure in the sample cartridge.
- lack of versatility;
- limited solvent choice;
- long extraction time;
- possible degradations of the target compounds due to local overheating;
- relatively high costs due to solvent consumption.
3.1.2. Non-Conventional Extraction Techniques
- microwave-assisted extraction;
- ultrasound-assisted extraction;
- supercritical fluid extraction (the most used are subcritical water extraction and supercritical carbon dioxide extraction).
Microwave-Assisted Extraction (MAE)
- separation of the solutes from the active sites of the sample matrix under increased pressure and temperature;
- diffusion of solvent across the matrix of sample;
- release of the solutes from the sample matrix to the solvent.
- high extraction selectivity, which make it a desirable technique in extraction of phenolic compounds from olive leaves;
- higher extraction efficiency;
- less working time;
- shortened extraction time.
Ultrasound-Assisted Extraction (UAE)
- low-temperature levels;
- high yields;
- short process time.
Supercritical Fluid Extraction (SFE)
- less solvent consuming (environmentally friendly);
- very cleaner extracts;
- extraction of nonpolar compounds by this procedure has very low energy costs.
3.2. Analytical Methods
3.3. Methods for Determination of Antimicrobial Potential
3.4. Methods for Determination of Antioxidant Activity
3.4.1. N,N-Dimethyl-p-phenylenediamine Dihydrochloride (DMPD) Method
3.4.2. 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Method
3.4.3. 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Method
3.4.4. Ferric Ion Reducing Antioxidant Power (FRAP) Method
3.4.5. Primary and Secondary Oxidation Methods
4. Table Review of Phenolic Compounds Isolation Methods, Presence of Enzymes and Antimicrobial and Antioxidant Activity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | acetone |
| CHCl3 | chloroform |
| CO2 | Carbon dioxide |
| DAD | diode array detector |
| EtAc | ethyl acetate |
| EtOH | Ethanol |
| GC | gas chromatography |
| GC/MS | gas chromatography/mass spectrometry |
| H2O | Water |
| HCl | hydrochloric acid |
| HPLC | high performance liquid chromatography |
| LC | liquid chromatography |
| LC/MS | liquid chromatography/mass spectrometry |
| MAE | microwave-assisted extraction |
| MBC | minimum bacterial concentration |
| MeOH | Methano |
| MFC | minimum fungicidal concentration |
| MIC | minimum inhibitory concentration |
| MS | mass spectrometry |
| n-Hex | n-hexane |
| NMR | nuclear magnetic resonance |
| OLE | Olive leaf extract |
| SC-CO2 | supercritical carbon dioxide extraction |
| SFE | supercritical fluid extraction |
| SLE | Solid-liquid extraction |
| UAE | Ultrasound-assisted extraction |
References
- Soler-Rivas, C.; Espin, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2020, 80, 1013–1023. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Janićijević-Hudomal, S.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Piperski, V.; Mitrović, D.M. Attenuation of cold restraint stress-induced gastric lesions by an olive leaf extract. Gen. Physiol. Biophys. 2009, 28, 135–142. [Google Scholar]
- What Is Olive Leaf Extract. Available online: https://www.superpharmacy.com.au/blog/the-many-benefits-of-olive-leaf-extract (accessed on 18 June 2020).
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Erdohan, Z.Ö.; Turhan, K.N. Olive leaf extract and usage for development of antimicrobial food packaging. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex: Norristown, PA, USA, 2012; pp. 1094–1101. [Google Scholar]
- Dekanski, D.; Selaković, V.; Piperski, V.; Željka, R.; Korenić, A.; Radenović, L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine 2011, 18, 1137–1143. [Google Scholar] [CrossRef]
- Goreishi, S.M.; Gholami Shahrestani, R. Subcritical water extraction of mannitol from olive leaves. J. Food Eng. 2009, 93, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Şahin, S.; Şamli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochemistry 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M.; Dramur, M.U. Investigation of oleuropein content in olive leaf extract obtained by supercritical fluid extraction and Soxhlet methods. Sep. Sci. Technol. 2011, 46, 1829–1837. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic hydrolysis of oleuropein from Olea europea (Olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.-Y.; Cho, J. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a Bioflavonoid Inhibits Colorectal Cancer through Modulation of Multiple Signaling Pathways: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Rodríguez, G.; Rodríguez, R.; Fernández-Bolaños, J.; Guillén, R.; Jimenez-Araujo, A. Antioxidant activity of effluents during the purification of hydroxytyrosol and 3,4-dihydroxyphenyl glycol from olive oil waste. Eur. Food Res. Technol. 2006, 224, 733–741. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; de Roos, B.; Duthie, G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015, 54, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- De La Puerta, R.; Gutierrez, V.R.; Hoult, J.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Lee-Huang, S.; Zhang, L.; Huang, P.L.; Chang, Y.-T.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef]
- Bayçin, D.; Altiok, E.; Ülkü, S.; Bayraktar, O.; Baycin, D. Adsorption of olive leaf (Olea europaea L.) antioxidants on silk fibroin. J. Agric. Food Chem. 2007, 55, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-level antimicrobial efficacy of representative mediterranean natural plant extracts against oral microorganisms. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ayana, B.; Turhan, K.N. Use of antimicrobial methylcellulose films to control Staphylococcus aureus during storage of Kasar cheese. Packag. Technol. Sci. 2009, 22, 461–469. [Google Scholar] [CrossRef]
- Brahmi, F.; Flamini, G.; Issaoui, M.; Dhibi, M.; Dabbou, S.; Mastouri, M.; Hammami, M. Chemical composition and biological activities of volatile fractions from three Tunisian cultivars of olive leaves. Med. Chem. Res. 2012, 21, 2863–2872. [Google Scholar] [CrossRef]
- Markin, D.; Duek, L.; Berdicevsky, I. In vitro antimicrobial activity of olive leaves. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Veter. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Španinger, E.; Hrnčič, M.K.; Škerget, M.; Željko, K.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Ghanbari, V.; Dehnad, D.; Ganje, M. Neural networks modeling of Aspergillus flavus growth in tomato paste containing microencapsulated olive leaf extract. J. Food Saf. 2017, 38, 12396. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Moneim, A.E.A. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed. Pharmacother. 2017, 91, 338–349. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Pirkovic-Cabarkapa, A.; Živković, L.; Žukovec, D.; Djelić, N.; Bajić, V.P.; Dekanski, D.; Spremo-Potparevic, B. Protective effect of dry olive leaf extract in adrenaline induced DNA damage evaluated using in vitro comet assay with human peripheral leukocytes. Toxicol. Vitr. 2014, 28, 451–456. [Google Scholar] [CrossRef]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J. Agric. Food Chem. 2007, 55, 4139–4143. [Google Scholar] [CrossRef]
- Türkez, H.; Toğar, B.; Polat, E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology 2012, 64, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Altiok, E.; Bayçın, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef] [Green Version]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Seddik, L.; Bah, T.M.; Aoues, A.; Slimani, M.; Benderdour, M. Elucidation of mechanisms underlying the protective effects of olive leaf extract against lead-induced neurotoxicity in Wistar rats. J. Toxicol. Sci. 2011, 36, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felizón, B.; Fernández-Bolaños, J.; Heredia, A.; Guillen, R. Steam-explosion pretreatment of olive cake. J. Am. Oil Chem. Soc. 2000, 77, 15–22. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Prior, Á.F.; Rodríguez-Gutiérrez, G. The use of industrial thermal techniques to improve the bioactive compounds extraction and the olive oil solid waste utilization. Innov. Food Sci. Emerg. Technol. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Valavanidis, A.; Nisiotou, C.; Papageorgiou, Y.; Kremli, I.; Satravelas, N.; Zinieris, N.; Zygalaki, H. Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatment. J. Agric. Food Chem. 2004, 52, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Madej, K. Microwave-assisted and cloud-point extraction in determination of drugs and other bioactive compounds. TrAC Trends Anal. Chem. 2009, 28, 436–446. [Google Scholar] [CrossRef]
- Sánchez-Avila, N.; Priego-Capote, F.; Ruiz-Jiménez, J.; Luquedecastro, M. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging technology to develop novel red winemaking practices: An overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Çoban, J.; Öztezcan, S.; Doğru-Abbasoğlu, S.; Bingül, I.; Yeşil-Mizrak, K.; Uysal, M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr. Gerontol. Int. 2014, 14, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloë odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyrás, S.; Moreno-Millán, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A.; de Castro, M.L. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 723, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Nivetha, C.V.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.; Fernandes, F.A. Ultrasound-assisted extraction. Stewart Postharvest Rev. 2009, 5, 1–11. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Cör, D.; Željko, K. Subcritical extraction of oil from black and white chia seeds with n-propane and comparison with conventional techniques. J. Supercrit. Fluids 2018, 140, 182–187. [Google Scholar] [CrossRef]
- Žitek, T.; Leitgeb, M.; Golle, A.; Dariš, B.; Željko, K.; Hrnčič, M.K. The influence of hemp extract in combination with ginger on the metabolic activity of metastatic cells and microorganisms. Molecules 2020, 25, 4992. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Ivanovski, M.; Cör, D.; Željko, K. Chia seeds (Salvia Hispanica L.): An Overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, P.; Masson, L.; Barriga, A.; Chávez, J.; Robert, P. Oxidative stability of oils containing olive leaf extracts obtained by pressure, supercritical and solvent-extraction. Eur. J. Lipid Sci. Technol. 2010, 113, 497–505. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Parada-Alonso, F.; Ibáñez, E.; Cifuentes, A. Recent applications of on-line supercritical fluid extraction coupled to advanced analytical techniques for compounds extraction and identification. J. Sep. Sci. 2019, 42, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2006, 70, S270–S276. [Google Scholar] [CrossRef]
- Islam, M.N.; Jo, Y.-T.; Jung, S.-K.; Park, J.-H. Thermodynamic and kinetic study for subcritical water extraction of PAHs. J. Ind. Eng. Chem. 2013, 19, 129–136. [Google Scholar] [CrossRef]
- Željko, K.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and value added compounds from biomass using sub- and supercritical water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Španinger, E.; Košir, I.J.; Željko, K.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- De Nino, A.; Lombardo, N.; Perri, E.; Procopio, A.; Raffaelli, A.; Sindona, G. Direct identification of phenolic glucosides from olive leaf extracts by atmospheric pressure ionization tandem mass spectrometry. J. Mass Spectrom. 1997, 32, 533–541. [Google Scholar] [CrossRef]
- Salta, F.; Mylona, A.; Chiou, A.; Boskou, G.; Andrikopoulos, N. Oxidative stability of edible vegetable oils enriched in polyphenols with olive leaf extract. Food Sci. Technol. Int. 2007, 13, 413–421. [Google Scholar] [CrossRef]
- Bouaziz, M.; Sayadi, S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005, 107, 497–504. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaziz, M.; Fki, I.; Jemai, H.; Ayadi, M.; Sayadi, S. Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008, 108, 253–262. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, M.; Feki, I.; Ayadi, M.; Jemai, H.; Sayadi, S. Stability of refined olive oil and olive-pomace oil added by phenolic compounds from olive leaves. Eur. J. Lipid Sci. Technol. 2010, 112, 894–905. [Google Scholar] [CrossRef]
- Coni, E.; Di Benedetto, R.; Di Pasquale, M.; Masella, R.; Modesti, D.; Mattei, R.; Carlini, E.A. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45–54. [Google Scholar] [CrossRef]
- Abaza, L.; Talorete, T.P.N.; Yamada, P.; Kurita, Y.; Zarrouk, M.; Isoda, H. Induction of growth inhibition and differentiation of human leukemia HL-60 cells by a Tunisian gerboui olive leaf extract. Biosci. Biotechnol. Biochem. 2007, 71, 1306–1312. [Google Scholar] [CrossRef]
- Flemmig, J.; Kuchta, K.; Arnhold, J.; Rauwald, H. Olea europaea leaf (Ph.Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine 2011, 18, 561–566. [Google Scholar] [CrossRef]
- Dekanski, D.; Janicijevic-Hudomal, S.; Tadic, V.M.; Marković, G.; Arsić, I.; Mitrovic, D. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serbian Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- Mohagheghi, F.; Reza Bigdeli, M.; Rasoulian, B.; Hashemi, P.; Rashidi Pour, M. The neuroprotective effect of olive leaf extract is related to improved blood–brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine 2011, 18, 170–175. [Google Scholar] [CrossRef]
- Japón-Luján, R.; de Castro, M.D.L. Liquid–liquid extraction for the enrichment of edible oils with phenols from olive leaf extracts. J. Agric. Food Chem. 2008, 56, 2505–2511. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Briante, R.; Patumi, M.; Terenziani, S.; Bismuto, E.; Febbraio, F.; Nucci, R. Olea europaea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002, 50, 4934–4940. [Google Scholar] [CrossRef] [PubMed]
- Briante, R.; La Cara, F.; Febbraio, F.; Patumi, M.; Nucci, R. Bioactive derivatives from oleuropein by a biotransformation on Olea europaea leaf extracts. J. Biotechnol. 2002, 93, 109–119. [Google Scholar] [CrossRef]
- Lu, X.; Rasco, B.A. Determination of antioxidant content and antioxidant activity in foods using infrared spectroscopy and chemometrics: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 853–875. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Bonoli-Carbognin, M.; Cerretani, L.; Bendini, A.; Almajano, M.P.; Gordon, M.H. Bovine serum albumin produces a synergistic increase in the antioxidant activity of virgin olive oil phenolic compounds in oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 7076–7081. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Vecchi, S.; Carrasco-Pancorbo, A.; Lercker, G. Protective effects of extra virgin olive oil phenolics on oxidative stability in the presence or absence of copper ions. J. Agric. Food Chem. 2006, 54, 4880–4887. [Google Scholar] [CrossRef]
- Hamza, M.; Khoufi, S.; Sayadi, S. Fungal enzymes as a powerful tool to release antioxidants from olive mill wastewater. Food Chem. 2012, 131, 1430–1436. [Google Scholar] [CrossRef]
- Micol, V.; Caturla, N.; Pérez-Fons, L.; Más, V.; Pérez, L.; Estepa, A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antivir. Res. 2005, 66, 129–136. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, D.W.; Zhang, J.Z.H.; Lee Huang, P.; Lin Huang, P.; Lee-Huang, S. Computational study of bindings of olive leaf extract (OLE) to HIV-1 fusion protein gp41. FEBS Lett. 2007, 581, 2737–2742. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili-Mahani, S.; Rezaeezadeh-Roukerd, M.; Esmaeilpour, K.; Abbasnejad, M.; Rasoulian, B.; Sheibani, V.; Kaeidi, A.; Hajializadeh, Z. Olive (Olea europaea L.) leaf extract elicits antinociceptive activity, potentiates morphine analgesia and suppresses morphine hyperalgesia in rats. J. Ethnopharmacol. 2010, 132, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumral, A.; Giriş, M.; Soluk-Tekkeşin, M.; Olgaç, V.; Doğru-Abbasoğlu, S.; Türkoğlu, Ü.; Uysal, M. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology 2015, 22, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Miljković, D.; Dekanski, D.; Miljković, Z.; Momčilović, M.; Mostarica-Stojkovic, M. Dry olive leaf extract ameliorates experimental autoimmune encephalomyelitis. Clin. Nutr. 2009, 28, 346–350. [Google Scholar] [CrossRef]
- Pasban-Aliabadi, H.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M.; Mehdizadeh, A.; Yaghoobi, M.M. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Res. 2013, 16, 134–142. [Google Scholar] [CrossRef]
- Dekanski, D.; Ristić, S.; Mitrović, D.M. Antioxidant effect of dry olive (Olea europaea L.) leaf extract on ethanol-induced gastric lesions in rats. Mediterr. J. Nutr. Metab. 2009, 2, 205–211. [Google Scholar] [CrossRef]
- Fakhraei, N.; Abdolghaffari, A.H.; Delfan, B.; Abbasi, A.; Rahimi, N.; Khansari, A.; Rahimian, R.; Dehpour, A.R. Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: Involvement of nitrergic and opioidergic systems. Phytother. Res. 2014, 28, 1367–1373. [Google Scholar] [CrossRef]



| Extraction, Isolation | Compounds | Enzymes | Antioxidant | Microbes | References |
|---|---|---|---|---|---|
| Extraction with boiling H2O for 30 min | Caffeic acid, verbascoside, oleuropein, luteolin 7-O-glucoside, rutin, apigenin 7-O-glucoside, luteolin 4′-O-glucoside | / | / | Bacillus cereus, Bacillus subtilis, Staphylococcus aureus (Gram+), Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae (Gram−) bacteria, and Candida albicans and C. neoformans (fungi) | [6] |
| Extraction with H2O/EtOH (1:1, v/v) shaken for 15 min at 4 °C | Oleuropein, oleouroside, oleuropein aglycone, tyrosol, hydroxytyrosol, syringic acid, gallic acid, ferulic acid | + | DMPD method | / | [84] |
| Extraction with n-Hex and with EtAc | Hydroxytyrosol, tyrosol, hydroxytyrosol acetate, 3,4-DHPEA-EDA, oleuropein, 3,4-DHPEA-EA, 4-HPEA-EDA | / | / | / | [40] |
| Extraction with Ac/HCl and with EtAc | Oleuropein, caffeic acid, luteolin, luteolin-7-O-glucoside, apigenin-7-O-glucoside, quercetin and chryseriol | Inhibition of antioxidative enzyme activity | / | / | [80] |
| Extraction with boiled H2O for 15 min | / | Respiratory enzymes of bacterial cells | / | Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli | [22] |
| Liquid/liquid extraction with EtAc | Oleuropein | Fungal enzyme (the β-glucosidase activity) | DPPH radical method | / | [90] |
| Extraction with H2O/MeOH (1:4, v/v) and left to stand overnight under agitation in the dark | BHT, hydroxytyrosol, hydrolysate extract, oleuropein, ethyl acetate extract and CH3OH/H2O leaf extract | The production of hydroxytyrosol by enzymatic hydrolysis | Using the β-carotene linoleate model system | / | [72] |
| Microwave-assisted extraction with magnetic stirring (6 min irradiation) | Phenolic compounds | / | Thermal oxidative stability analysis | / | [12] |
| Extraction with H2O/EtOH at different volume ratios | Oleuropein | Enzyme immobilization | DMPD method | / | [85] |
| Extraction with H2O/EtOH (3:7, v/v) for 2 h at 25 °C | Oleuropein, rutin | / | With aqueous ABTS solution | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa | [24] |
| Extraction with H2O/EtOH (1:1, v/v) shaken for 15 min at 4 °C | Oleuropein, tyrosol, vanillic acid, hydroxytyrosol, 4-hydroxy, 3-methoxyphenyl acetic acid, 3,4-dihydroxy-benzoic acid, 3,4-dihydroxy phenyl acetic acid, syringic acid, gentisic acid, gallic acid, ferulic acid, caffeic acid, sinapic acid, oleuropein aglycon | Enzyme immobilization | DMPD method | / | [77] |
| Extraction with MeOH in a shaker at room temperature | Gallic acid, hydroxytyrosol, chlorogenic acid, protocatechuic acid, hydroxyphenylacetic acid, 4-hydroxybenzoic acid, catechin, oleuropeine, p-coumaric acid, ferrulic acid, rosmarinic acid, vanillic acid, m-coumaric acid, o-coumaric acid, phenylacetic acid, cinnamic acid, luteolin, apigenin, 3-hydroxybenzoic acid | / | DPPH radical scavenging assay; ABTS+ radical cation scavenging | / | [15] |
| Hydrodistillation for 3 h using a Clevenger-type apparatus | Furfural, (E,Z)-2,4-hexadienal, (E)-2-hexenol, (E)-3-hexenol, 1-hexanol, (Z)-4-heptenal, heptanal, (E,E)-2,4-hexadienal, 2-acetylfurane, α-Pinene, (Z)-2-heptenal, benzaldehyde, 3-ethenylpyridine, phenol, hexanoic acid, 3-octanone, 6-methyl-5-hepten-2-one, octanal, (E,E)-2,4-heptadienal, (E,Z)-2,4-heptadienal, benzyl alcohol, phenylacetaldehyde, (E)-2-octenal, 1-octanol, cis-linalool oxide, trans-linalool oxide, linalool, nonanal, phenylethyl alcohol, methyl nicotinate, 4-ketoisophorone, (E,Z)-2,6-nonadienal, (E)-2-nonenal, 1-nonanol, trans-linalool oxide (pyranoid), p-cymen-8-ol, α-terpineol, methyl salicylate, (Z)-4-decenal, decanal, 2-ethylbenzaldehyde, benzothiazole, geraniol, (E)-2-decenal, salicylic alcohol, p-menth-1-en-7-al, 1-tridecene, (E,Z)-2,4-decadienal, 4-vinylguaiacol, (E,E)-2,4-decadienal, eugenol, (E)-β-damascenone, cis-α-bergamotene, (Z,E)-2,6-dodecadienal, trans-α-bergamotene, (E)-isoeugenol, (E)-geranylacetone, (E)-β-ionone, caryophyllene oxide | / | DPPH radical scavenging assay; ABTS+ radical cation scavenging | Enterococcus faecalis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa | [27] |
| Extraction with Ac by mechanical stirring for 12 h | Oleuropein, hydroxytyrosol | / | / | Streptococcus mutans, Streptococcus sobrinus, Streptococcus oralis, Enterococcus faecalis, Candida albicans, Escherichia coli, Staphylococcus aureus, Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Parvimonas micra | [25] |
| Extraction with 20% H2O and autoclaved for 20 min at 121 °C | Oleuropein, tyrosol, hydroxytyrosol, quercetin, p-hydroxybenzoic, vanillic, verbascoside and p-coumaric acids | Deglucosidation by the enzyme β-glucosidase to produce an aglycone structure of oleuropein | / | Escherichia coli and Staphylococcus aureus | [26] |
| Microwave-assisted extraction (8 min of microwave irradiation at 200 W) with H2O/EtOH (1:4, v/v) | Oleuropein and luteolin | / | / | / | [55] |
| Extraction twice with distilled H2O for 12 h at 80 °C | Rutin, verbascoside, luteolin7-glucoside, apigenin7-glucoside, flavonoid x, oleuropein and oleuroside | / | / | / | [23] |
| * High strength olive leaf extract was obtained from Spain | Oleuropein | / | / | / | [91] |
| Extraction with EtOH for 2 weeks at room temperature | Oleoside, hydroxytyrosol, tyrosol, aesculin, hydroxypinoresinol-glycoside, luteolin glucoside derivative, oleuropein and luteolin 7-glucoside | Plasma enzymatic activity; decreasing liver enzymes | / | / | [39] |
| Ultrasound-assisted extraction in an ultrasonic bath at 25 °C | / | / | DPPH radical method | / | [10] |
| * High strength olive leaf extract was purchased from a local health food store | Oleuropein | / | / | Acinetobacter calcoaceticus, Bacillus cereus, Bacillus subtilis, Campylobacter jejuni, Candida albicans, Candida glabrata, Candida parapsilosis, Enterococcus faecalis, Escherichia coli, Helicobacter pylori, Klebsiella pneumoniae, Kocuria rhizophila, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus spp, Listeria innocua, Listeria monocytogenes, Micrococcus luteus, Pseudomonas aeruginosa, Salmonella enterica, Serratia marcescens, MSSA, MRSA, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus xylosus and Streptococcus pyogenes | [3] |
| / | Oleuropein, tyrosol, hydoxytyrosol and caffeic acid | / | / | / | [81] |
| Extraction with H2O/EtOH (3:7, v/v) (was allowed to stand for at least one week at room temperature) | Apigenin 7-glucoside and oleuropein | / | / | / | [78] |
| Extraction in 20% H2O and autoclaved for 20 min at 121 °C | Oleuropein and hydroxytyrosol | / | / | Bacteria: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Klebsiella pneumoniae; Dermatophytes –Trichophyton mentagrophytes, Microsporum canis and T. rubrum; Yeast –Candida albicans | [28] |
| / | Oleuropein, oleuropein aglycone, elenolic acid and hydroxytyrosol | / | / | HIV-1 | [92] |
| Extraction with EtAc | Vanillin, cinnamic acid, tyrosol, p-hydroxy-benzoic acid, p-hydroxy—phenylacetic acid, vannilic acid, hydroxy-tyrosol, protocatechuic acid, p-coumaric acid and ferulic acid | / | DPPH radical method | / | [71] |
| Extraction with H2O/EtOH (1:4, v/v) | Caffeic acid, vanilin, rutin, luteolin-7-O-glucoside, apigenin-7-O-glucoside, oleuropein, quercetin, luteolin, apigenin and chryseriol | / | DPPH radical method | / | [8] |
| Supercritical fluid extraction with CO2 and Soxhlet methods for 24 h | Oleuropein | / | / | / | [11] |
| Extraction with MeOH for 7 days in the dark at room temperature | Oleuropein, luteolin-7-O-glucoside, luteolin-4′-O-glucoside, luteolin and hydroxytyrosol acetate | / | DPPH radical method | / | [34] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein, caffeic acid, luteolin-7-O-glycoside, apigenine7-O-glycoside, quercetin and tannins | Antioxidative enzymes activity was compared with effects of i.g. pretreatment of reference drug, ranitidine | / | / | [4] |
| Extraction with H2O/EtOH (1:4, v/v) twice | Oleuropein, luteolin, apigenin, rutin, diosmetin, oleasterol, leine and glycoside oleoside | / | / | / | [93] |
| * Commercially available extract | Oleuropein, oleoside, hydroxytyrosol, luteolin-7-O-glucoside, tyrosol, verbascoside, apigenin-7-O-glucoside, rutin, vanillic acid, vanillin and luteolin | Inhibition angiotensin-converting enzyme in vitro and decreasing the activities of key cholesterol-regulatory enzymes | / | / | [94] |
| Extraction with H2O/EtOH (3:7, v/v) for 24 h at room temperature using a shaking incubator | Oleuropein | Antioxidant enzymes | / | / | [95] |
| Extraction with EtAc over-night at room temperature with constant stirring | Hydroxytyrosol, oleuropein, secoiridoids, flavonoids and triterpenes | / | / | / | [41] |
| * Standardized dry olive leaf extract was purchased | Oleuropein, luteoline-7-O-glycoside, apigenine-7-O-glycoside, quercetin, tannins and caffeic acid | / | / | / | [96] |
| Extraction with H2O/EtOH (1:4, v/v) twice | Oleuropein, tyrosol, hydroxy-tyrosol and caffeic acid | / | / | / | [97] |
| Extraction with distilled H2O in Soxhlet apparatus for 1 h at 60 °C | Oleuropein and flavonoids | Measuring of total activities of hippocampal enzymes, including glutathione-S-transferase and NADP-isocitrate dehydrogenase | / | / | [44] |
| Extraction with EtAc | Oleuropein and hydroxytyrosol | Enzymatic hydrolysis with different enzymes (β-glucosidase, hemicellulase, tannase, neutral protease, cellulase, glucoamylase, papain, alkaline protease, amylase, β-glucanase) | DPPH radical method | / | [13] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein, luteolin-7-O-glucoside, apigenine-7-O-glucoside, quercetin and caffeic acid | Antioxidant enzymes | / | / | [38] |
| Extraction with 70% EtOH for 24 h at room temperature by a shaking incubator | Oleuropein | / | / | / | [53] |
| Extraction with MeOH | Oleuropein | / | / | / | [70] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein, luteoline-7-O-glucoside, apigenine-7-O-glucoside, quercetin, tannins and caffeic acid | / | / | / | [98] |
| Extraction with H2O/EtOH carried out under magnetic stirring at 400 rpm and at room temperature (22 ± 2 °C) for predetermined time periods | Luteolin diglucoside, rutin (quercetin 3-O-rutinoside), luteolin glucoside, luteolin rutinoside, apigenin rutinoside and oleuropein | / | / | / | [75] |
| Extraction with H2O/MeOH and left to stand overnight under agitation in the dark | Oleuropein and hydroxytyrosol | Dehydrogenase enzyme | / | / | [37] |
| Quartz extraction with EtOH (8 min of microwave irradiation at 200 W) | Apigenin-7-glucoside, luteolin-7-glucoside and verbascoside | / | / | / | [82] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein, caffeic acid, hydroxytyrosol and tyrosol | Enzyme linked dimmunosorbent | / | / | [99] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein, hydroxytyrosol, caffeic acid, tyrosol, apigenin, apigenin-7-O-β-d-glucoside, luteolin-7-O-β-d-glucoside, luteolin and verbascoside | Catalyzed by the enzyme superoxide dismutase | / | / | [79] |
| Extraction with MeOH for 7 days in the dark at room temperature | Hydroxytyrosol glucoside, oleoside, hydroxytyrosol, secologanoside, oleuropein aglycon, 10-hydroxyoleuropein, verbascoside, hydroxytyrosol acetate, luteolin-7-O-rutinoside, 10-hydroxyoleuropein isomer, luteolin-7-O-b-d-glucopyranoside, oleuropein aglycon decarboxymethyl dialdehyde form, luteolin-40 -O-b-d-glucopyranoside, oleuropein, oleuropein aglucon, oleuropein isomer, oleuroside, ligstroside, luteolin and oleuropein aglycon | / | / | / | [73] |
| Extraction with different solvents (Ac, EtOH and their aqueous forms) for 24 h | Hydroxytyrosol, tyrosol, catechin, caffeic acid, vanillic acid, vanillin, rutin, luteolin-7-glucoside, verbascoside, apigenin-7-glucoside, diosmetin-7-glucoside, oleuropein and luteolin | / | ABTS/K2S8O2 method | / | [42] |
| Extraction with EtAc three times | Oleuropein, hydroxytyrosol and oleuropein aglycone | Enzymatic hydrolysis using β-glucosidase | ABTS assay | / | [16] |
| Extraction with H2O/MeOH and left to stand overnight under agitation in the dark | Oleuropein and hydroxytyrosol | / | DPPH radical method | / | [74] |
| Extraction with different solvents (H2O, EtOH, MeOH, CHCl3, CHCl3/EtOH and CHCl3/MeOH) | Hydroxytyrosol, tyrosol, catechin, caffeic acid, vanilic acid, vanilin, rutin, luteolin-7-glucoside, verbascoside, apigenin-7- glucoside, diosmetin-7- glucoside, oleuropein and luteolin | / | / | Staphylococcus aureus, Escherichia coli, Salmonella enteritidis, Salmonella typhimurium and Listeria monocytogenes | [7] |
| Extraction with absolute EtOH for 48 h | Caffeic acid, verbascoside, oleuropein, luteolin 7-O-glucoside, rutin, apigenin 7-O-glucoside and luteolin 4′-O-glucoside | / | / | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus cereus, Salmonella Typhi and Vibrio parahaemolyticus | [29] |
| Extraction with H2O/EtOH in the dark at room temperature | Oleuropein, oleuropein aglycone, hydroxytyrosol and triacetylhydroxytyrosol | Enzymatic hydrolysis was carried out using β-glucosidase from almond | DPPH radical method | / | [76] |
| Microwave-assisted extraction with different solvents (MeOH, EtOH and their aqueous forms) | Oleuropein aglycone, luteolin diglucoside, luteolin glucoside, luteolin, quercetin, apigenin, apigenin-7-O-glucoside, oleuropein and rutin | / | / | / | [56] |
| Extraction with H2O/EtOH for 24 h at room temperature, by hydraulic laboratory press and supercritical-CO2 extraction | Hydroxytyrosol, vanillic acid, hydroxytyrosol glycoside, vanillic hexoside acid, caffeic hexoside acid, vanillin, oleoside, chlorogenic acid, oleuropein aglycon, pinoresinol, caffeic acid, elenolic acid, p-coumaric acid, ferulic acid, verbacoside, ligstroside aglycon decarboxymethyl, luteolin-0-rutinoside, acetoxypinoresinol, luteolin-7-glucoside, hesperitin-3-rutinoside, quercetin-3-0-galactoside, apigenin-7-rutinoside, oleuropein, oleuroside acid-10-carboxilic, apigenin-7-glucoside, oleuroside, ligstroside, luteolin-3′-7-diglucoside, luteolin-7-rutinoside, oleuropein diglucoside, oleuropein aglycon aldehyde and quercetin | Diphenol oxidase | DPPH method | / | [64] |
| Extraction with H2O/EtOH (1:4, v/v) | Oleuropein and verbascoside | / | / | Listeria monocytogenes, Escherichia coli and Salmonella enteritidis | [30] |
| Extraction with H2O/MeOH (3:7, v/v) in the dark for 48 h at 4 °C with periodic mixing | Tyrosol, oleuropein, caffeic acid, rutin, luteolin derivatives and vanillin | Superoxide dismutase and myeloperoxidase | DPPH radical method | / | [32] |
| Extraction with H2O/MeOH (1:4, v/v) and carried out by a household microwave (microwave irradiation was performed for 10 min) | Oleuropein, caffeic acid, tyrosol and hydroxytyrosol | / | / | Aspergillus flavus | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. https://doi.org/10.3390/molecules25245946
Borjan D, Leitgeb M, Knez Ž, Hrnčič MK. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules. 2020; 25(24):5946. https://doi.org/10.3390/molecules25245946
Chicago/Turabian StyleBorjan, Dragana, Maja Leitgeb, Željko Knez, and Maša Knez Hrnčič. 2020. "Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract" Molecules 25, no. 24: 5946. https://doi.org/10.3390/molecules25245946