Antioxidant and Cell Proliferation Properties of the Vietnamese Traditional Medicinal Plant Peltophorum pterocarpum
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plants Material
4.2. Extraction and Isolation
4.3. LDL Preparation
4.4. Measurement of Conjugated Diene Formation
4.5. Measurement of TBARS
4.6. Cell Cultures and Cell Proliferation Assay
4.7. Caspase-3 Activity
4.8. Western Blotting Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandal, J.; Roy, I.; Chatterjee, S.; Gupta-Bhattacharya, S. Aerobiological investigation and in vitro studies of pollen grains from 2 dominant avenue trees in Kolkata, India. J. Investig. Allergol. Clin. Immunol. 2008, 18, 22–30. [Google Scholar]
- Loi, D.T. Vietnamese Medicinal Plants and Remedies, 8th ed.; Ha Noi Medicine Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Li, Y.-C.; Kuo, P.-C.; Yang, M.-L.; Chen, T.-Y.; Hwang, T.-L.; Chiang, C.-C.; Thang, T.D.; Tuan, N.N.; Tzen, J. Chemical Constituents of the Leaves of Peltophorum pterocarpum and Their Bioactivity. Molecules 2019, 24, 240. [Google Scholar] [CrossRef] [Green Version]
- Klaywong, K.; Khutrakul, G.; Choowongkomon, K.; Lekcharoensuk, C.; Petcharat, N.; Leckcharoensuk, P.; Ramasoota, P. Screening for lead compounds and herbal extracts with potential anti-influenza viral activity. Southeast Asian J. Trop. Med. Public Health 2014, 45, 62–74. [Google Scholar]
- Voravuthikunchai, S.; Lortheeranuwat, A.; Jeeju, W.; Sririrak, T.; Phongpaichit, S.; Supawita, T. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157:H7. J. Ethnopharmacol. 2004, 94, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.T.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants. Molecules 2010, 15, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.L.; Chan, E.W.L.; Tan, P.L.; Lim, Y.; Stanslas, J.; Goh, J.K. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complement. Altern. Med. 2011, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukumaran, S.; Kiruba, S.; Mahesh, M.; Nisha, S.R.; Miller, P.Z.; Ben, C.P.; Jeeva, S. Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.) Baker ex Heyne. Asian Pac. J. Trop. Med. 2011, 4, 735–738. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Rizwani, G.H.; Shareef, H.; Ćavar, S.; Zia-Ul-Haq, M. Assessment of total phenolic content and antioxidant potential of methanol extract of Peltophorum pterocarpum (DC.) Backer ex K. Heyne. Pak. J. Pharm. Sci. 2013, 26, 967–972. [Google Scholar]
- Biswas, K.; Kumar, A.; Babaria, B.A.; Prabhu, K.; Setty, R.S. Hepatoprotective effect of leaves of Peltophorum pterocarpum against paracetamol Induced acute liver damage in rats. J. Basic Clin. Pharm. 2010, 1, 10–15. [Google Scholar]
- Elufioye, T.O.; Hameed, H.A. Cognitive enhancing properties of morinda lucida (Rubiaceae) and Peltophorum pterocarpum (Fabaceae) in scopolamine induced amnesic mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Nishimoto, Y.; Tokuda, H.; Suzuki, N.; Yasukawa, K.; Kitdamrongtham, W.; Akazawa, H.; Manosroi, A.; Manosroi, J.; Akihisa, T. Cancer Chemopreventive Effect of Bergenin from Peltophorum pterocarpum Wood. Chem. Biodivers. 2013, 10, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.P.H.; Nguyen, Y.N.; Tran, M.H.; Le, T.H.V.; Giang, T.K.L. Antiproliferative activity of ethanol extracts from medicinal plants collected in Quang Nam, Da Nang using human leukemia cell lines. J. Med. Mater.-Hanoi 2020, 25, 3–11. [Google Scholar]
- Truman, J.-P.; Al Gadban, M.M.; Smith, K.J.; Jenkins, R.W.; Mayroo, N.; Virella, G.; Lopes-Virella, M.F.; Bielawska, A.; Hannun, Y.A.; Hammad, S.M. Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: Role in phagocytosis and cytokine release. Immunology 2012, 136, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2014, 22, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Qadir, M.I.; Perveen, N.; Ahmad, B.; Saleem, U.; Irshad, T. Inhibitors of Apoptotic Proteins: New Targets for Anticancer Therapy. Chem. Biol. Drug Des. 2013, 82, 243–251. [Google Scholar] [CrossRef]
- Virág, L.; Robaszkiewicz, A.; Rodriguez-Vargas, J.M.; Oliver, F.J. Poly(ADP-ribose) signaling in cell death. Mol. Asp. Med. 2013, 34, 1153–1167. [Google Scholar] [CrossRef]
- Hou, W.-H.; Chen, S.-H.; Yu, X. Poly-ADP ribosylation in DNA damage response and cancer therapy. Mutat. Res. Rev. Mutat. Res. 2019, 780, 82–91. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; E Puig, P.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Walensky, L.D. Targeting BAX to drug death directly. Nat. Chem. Biol. 2019, 15, 657–665. [Google Scholar] [CrossRef]
- Manaharan, T.; Teng, L.L.; Appleton, D.; Ming, C.H.; Masilamani, T.; Palanisamy, U.D. Antioxidant and antiglycemic potential of Peltophorum pterocarpum plant parts. Food Chem. 2011, 129, 1355–1361. [Google Scholar] [CrossRef]
- Subramanian, R.; Subbramaniyan, P.; Raj, V. Isolation of bergenin from Peltophorum pterocarpum flowers and its bioactivity. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.T.; To, D.-C.; Vo, P.H.T.; Tran, T.H.; Nguyen, P.-H.; Nguyen, H.M.; Tran, M.H. Cassaine Diterpenoid Amide from Stem Bark of Erythrophleum fordii Suppresses Cytotoxic and Induces Apoptosis of Human Leukemia Cells. Molecules 2020, 25, 3304. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Bayir, A.; Bayir, H.; Stoyanovsky, D.A.; Borisenko, G.G.; Tyurina, Y.Y.; Wipf, P.; Atkinson, J.; Greenberger, J.S.; Chapkin, R.S.; et al. Mitochondria-targeted disruptors and inhibitors of cytochromec/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol. Nutr. Food Res. 2009, 53, 104–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoye, E.; Langlois, M. Circulating oxidized low-density lipoprotein: A biomarker of atherosclerosis and cardiovascular risk? Clin. Chem. Lab. Med. 2009, 47, 128–137. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
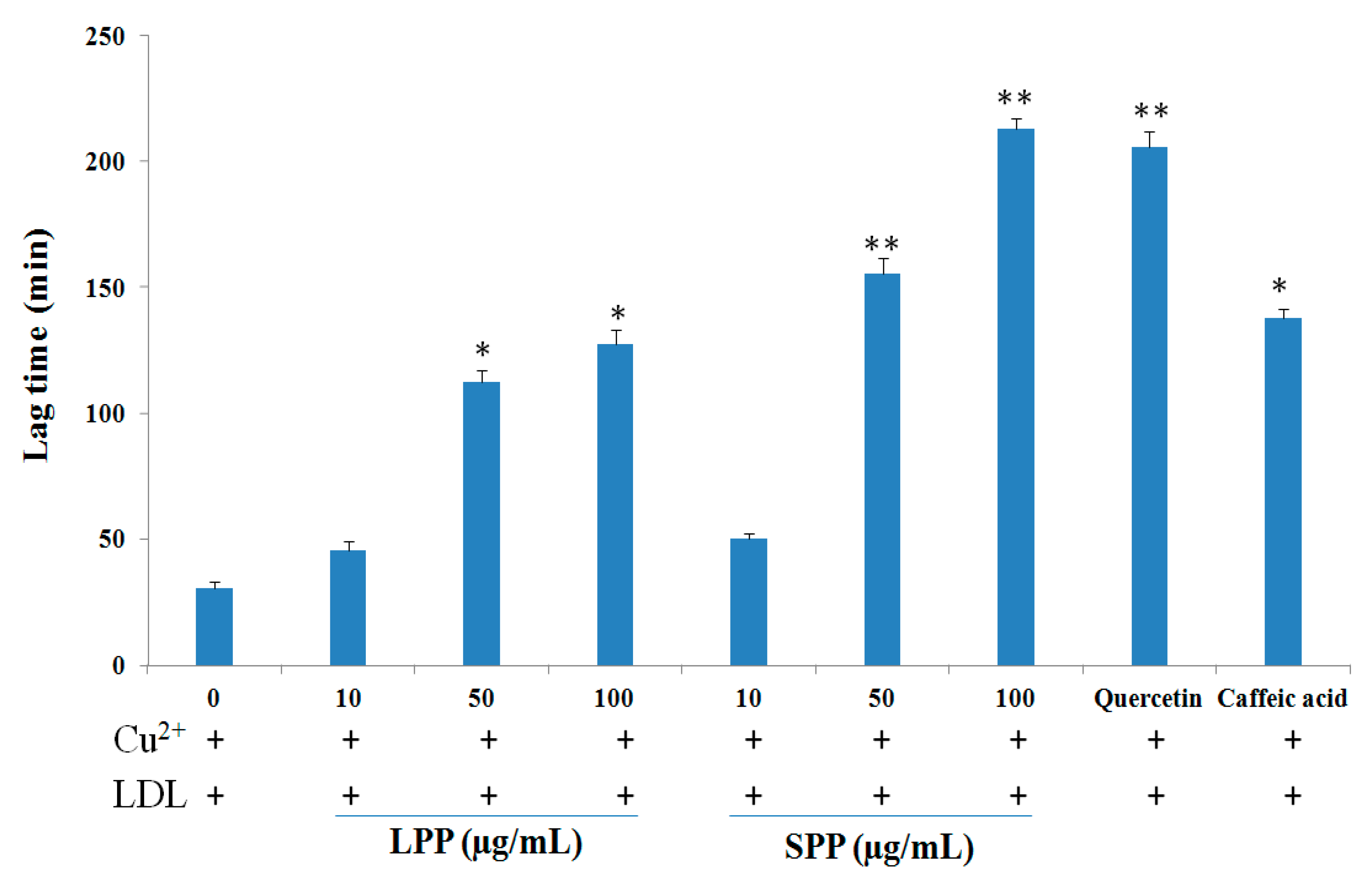
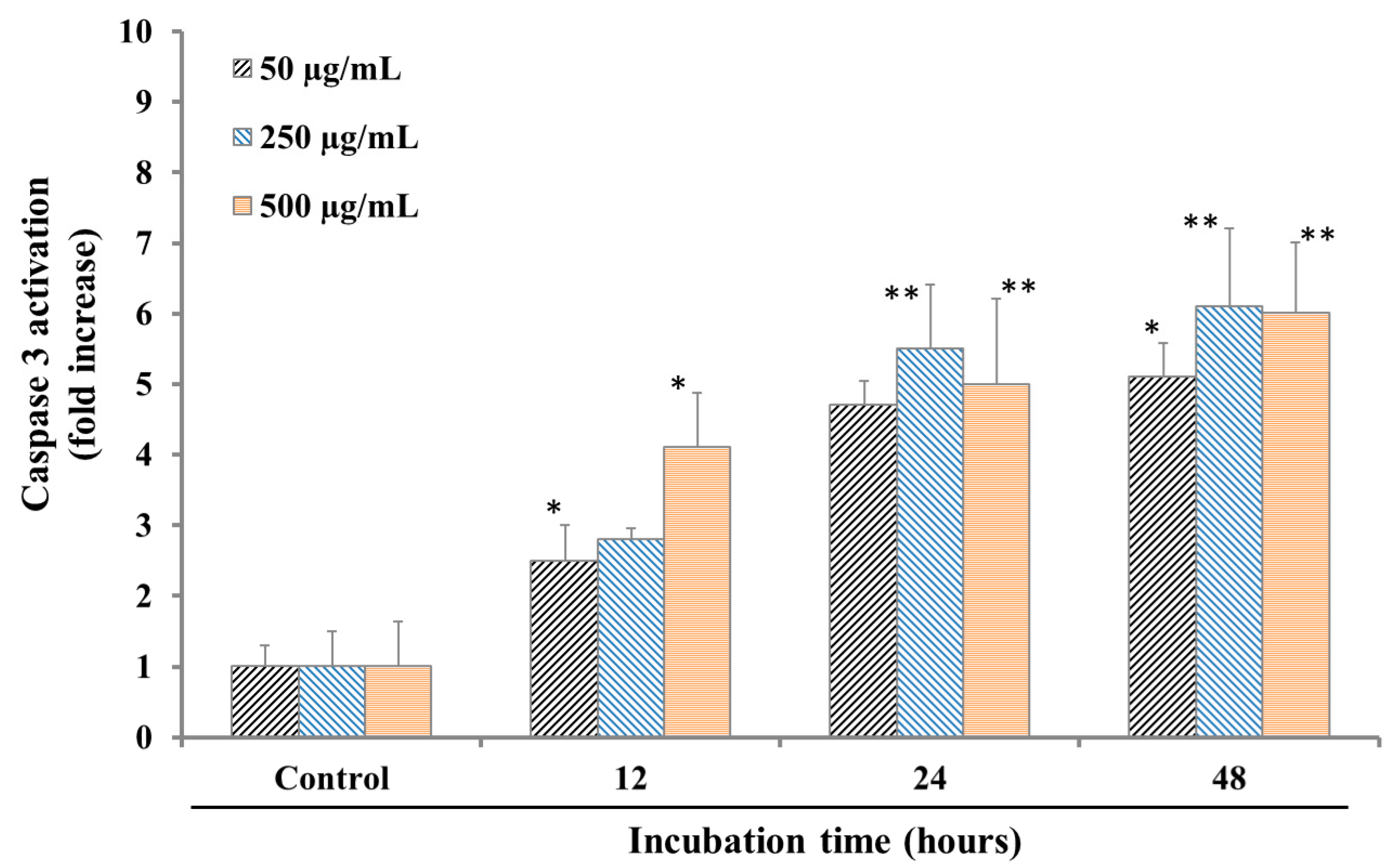
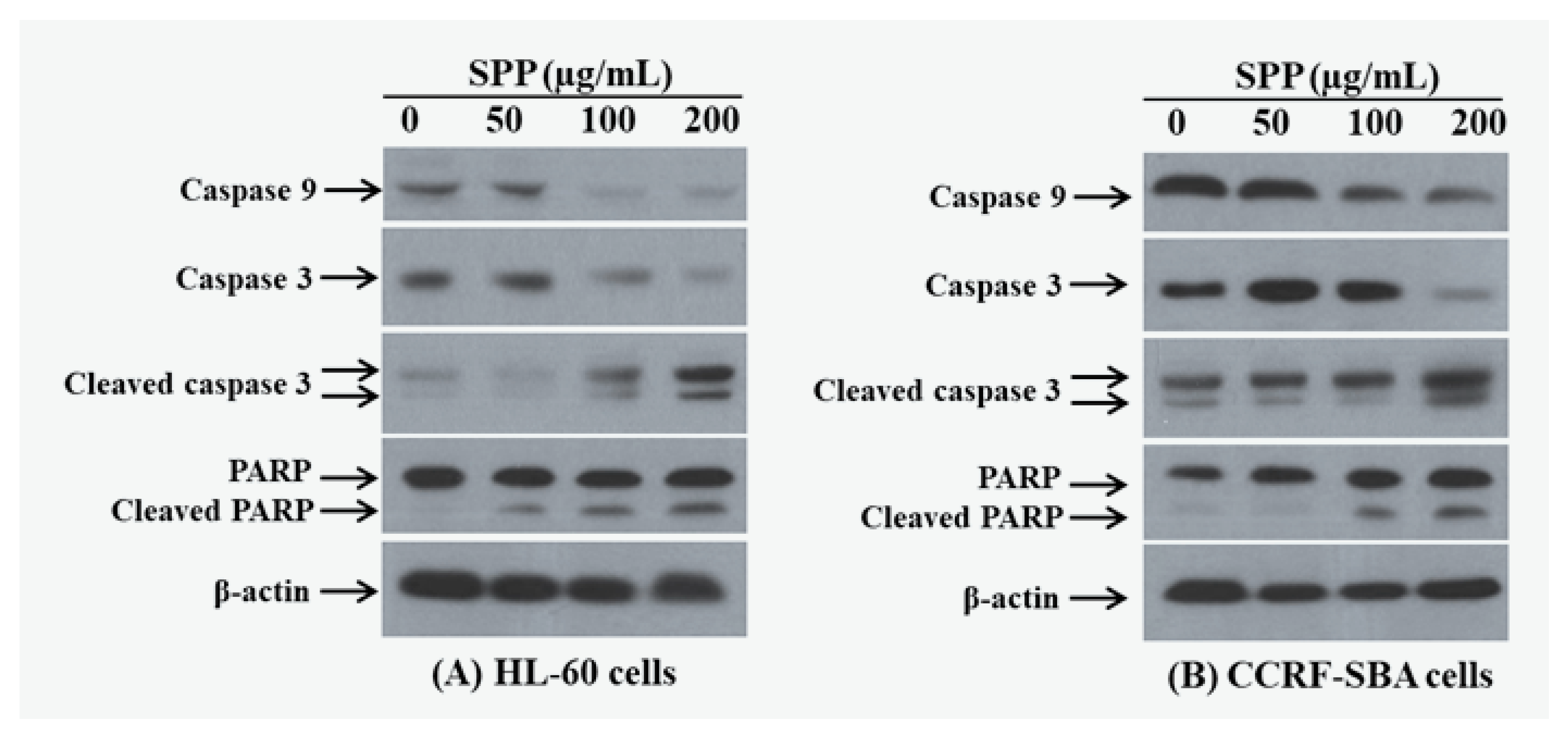

| Extracts/Compounds | Inhibition of LDL Oxidation IC50 (μg/mL) a | |
|---|---|---|
| Cu2+-Mediation | AAPH-Mediation | |
| LPP | 67.5 ± 3.6 | >100 |
| SPP | 48.0 ± 5.2 | 40.5 ± 3.2 |
| Quercetin b | 5.3 ± 0.8 | 10.5 ± 1.0 |
| Caffeic acid b | 5.7 ± 1.4 | 40.7 ± 1.8 |
| α-Tocopherol b | 25.8 ± 2.5 | 20.4 ± 1.0 |
| Samples | Human cancer cells, IC50 μg/mL a | ||||||
|---|---|---|---|---|---|---|---|
| MIA PACA2 | PANC-1 | A549 | OVCAR8 | KG | HL-60 | CCRF-SBA | |
| LPP | >500 | >500 | >300 | >300 | 228.1 ± 6.8 | 146.0 ± 5.5 | 157.2 ± 10.4 |
| SPP | 167.5 ± 5.6 | >300 | 244.1 ± 11.5 | >300 | 255.0 ± 8.1 | 118.5 ± 3.8 | 120.7 ± 6.0 |
| Camptothecin b | 3.2 ± 0.7 | 6.5 ± 0.8 | 2.05 ± 0.2 | 1.9 ± 0.1 | 0.8 ± 0.1 | 1.7 ± 0.4 | 11.7 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-R.; Cuong To, D.; Nguyen, P.H.; Nguyen, Y.N.; Cho, B.-J.; Tran, M.H. Antioxidant and Cell Proliferation Properties of the Vietnamese Traditional Medicinal Plant Peltophorum pterocarpum. Molecules 2020, 25, 4800. https://doi.org/10.3390/molecules25204800
Kim S-R, Cuong To D, Nguyen PH, Nguyen YN, Cho B-J, Tran MH. Antioxidant and Cell Proliferation Properties of the Vietnamese Traditional Medicinal Plant Peltophorum pterocarpum. Molecules. 2020; 25(20):4800. https://doi.org/10.3390/molecules25204800
Chicago/Turabian StyleKim, Seon-Rye, Dao Cuong To, Phi Hung Nguyen, Yen Nhi Nguyen, Byung-Jun Cho, and Manh Hung Tran. 2020. "Antioxidant and Cell Proliferation Properties of the Vietnamese Traditional Medicinal Plant Peltophorum pterocarpum" Molecules 25, no. 20: 4800. https://doi.org/10.3390/molecules25204800






