Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
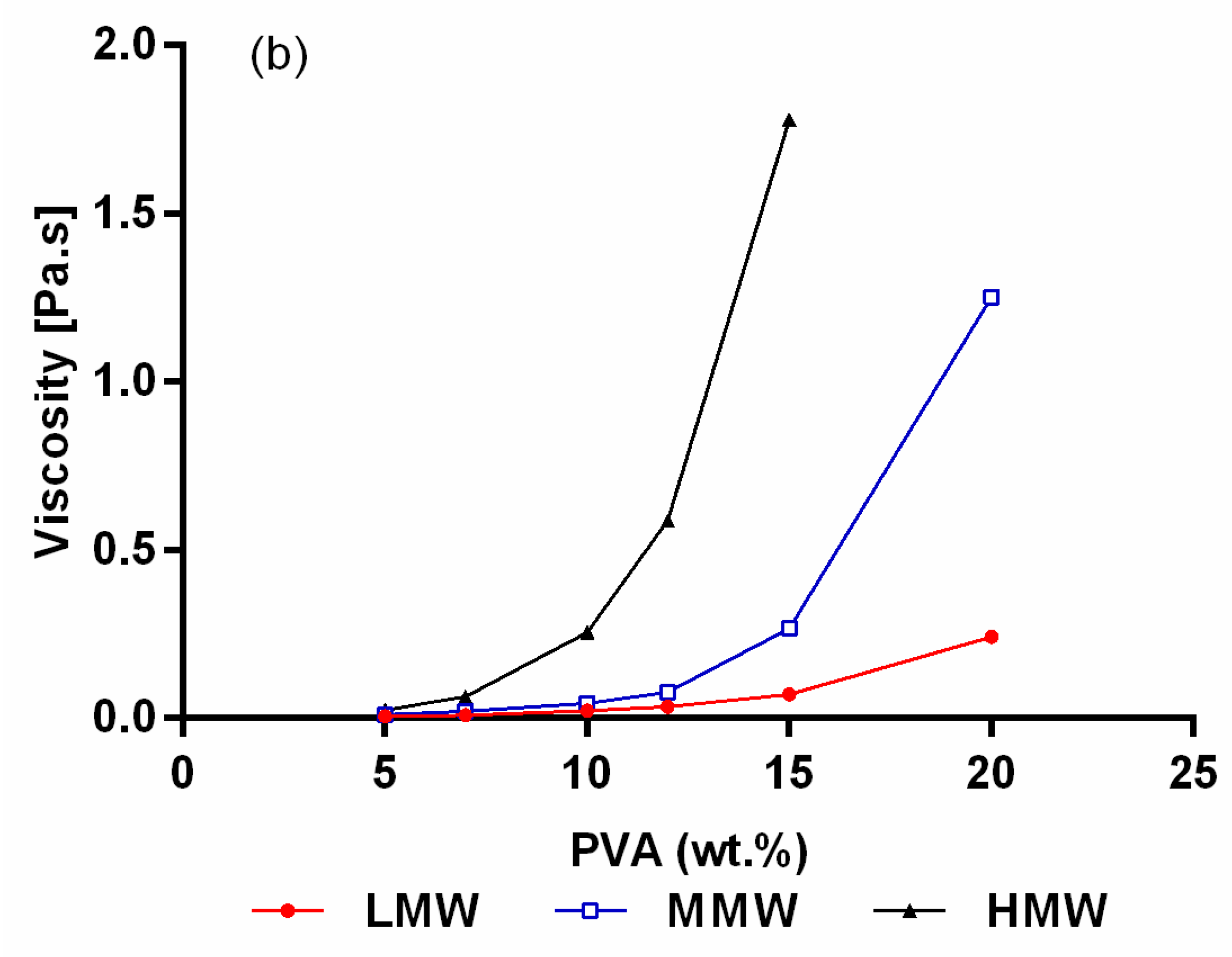
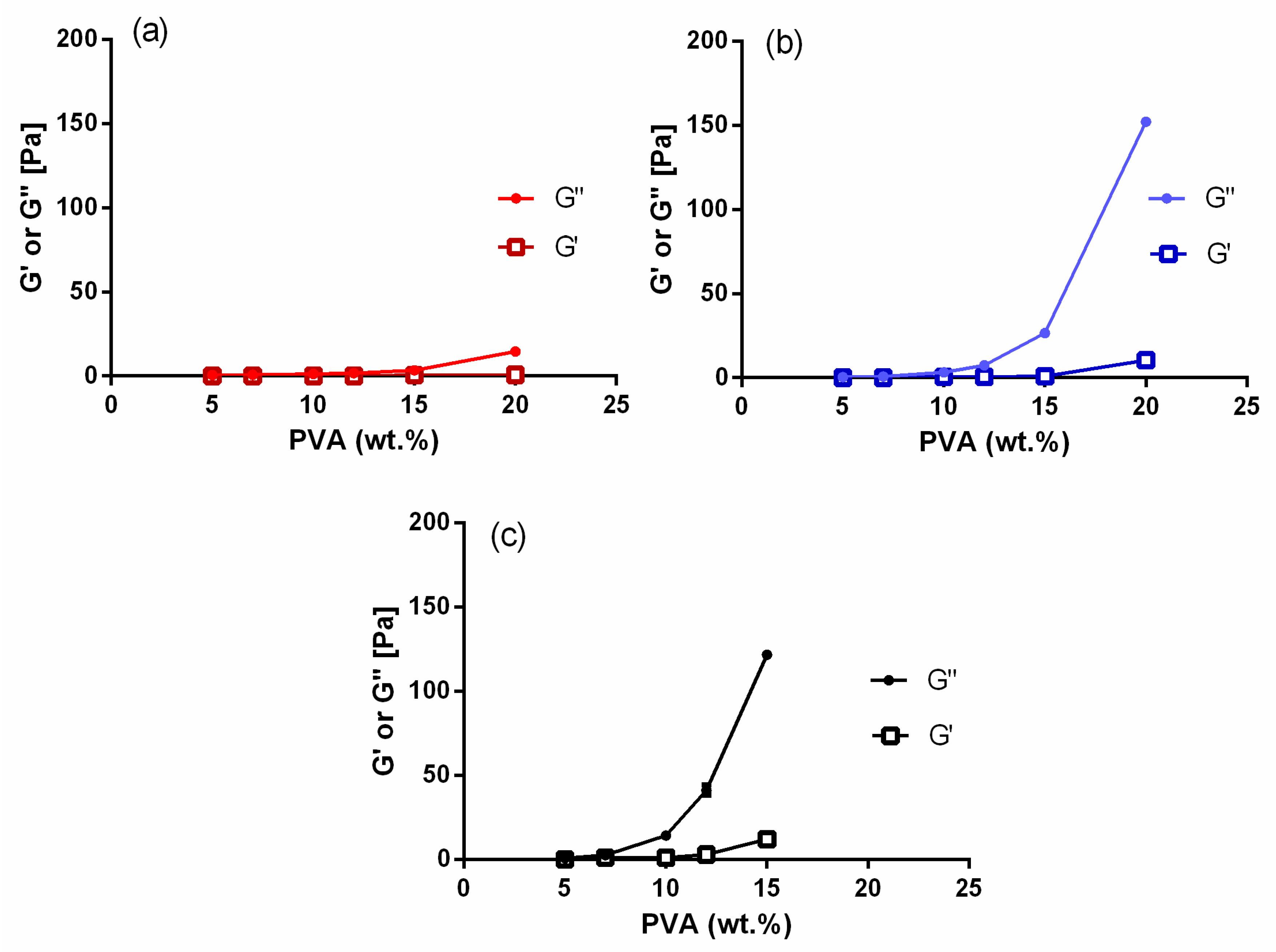
2.1. Rheological Characterization of the Electrospinning Solutions
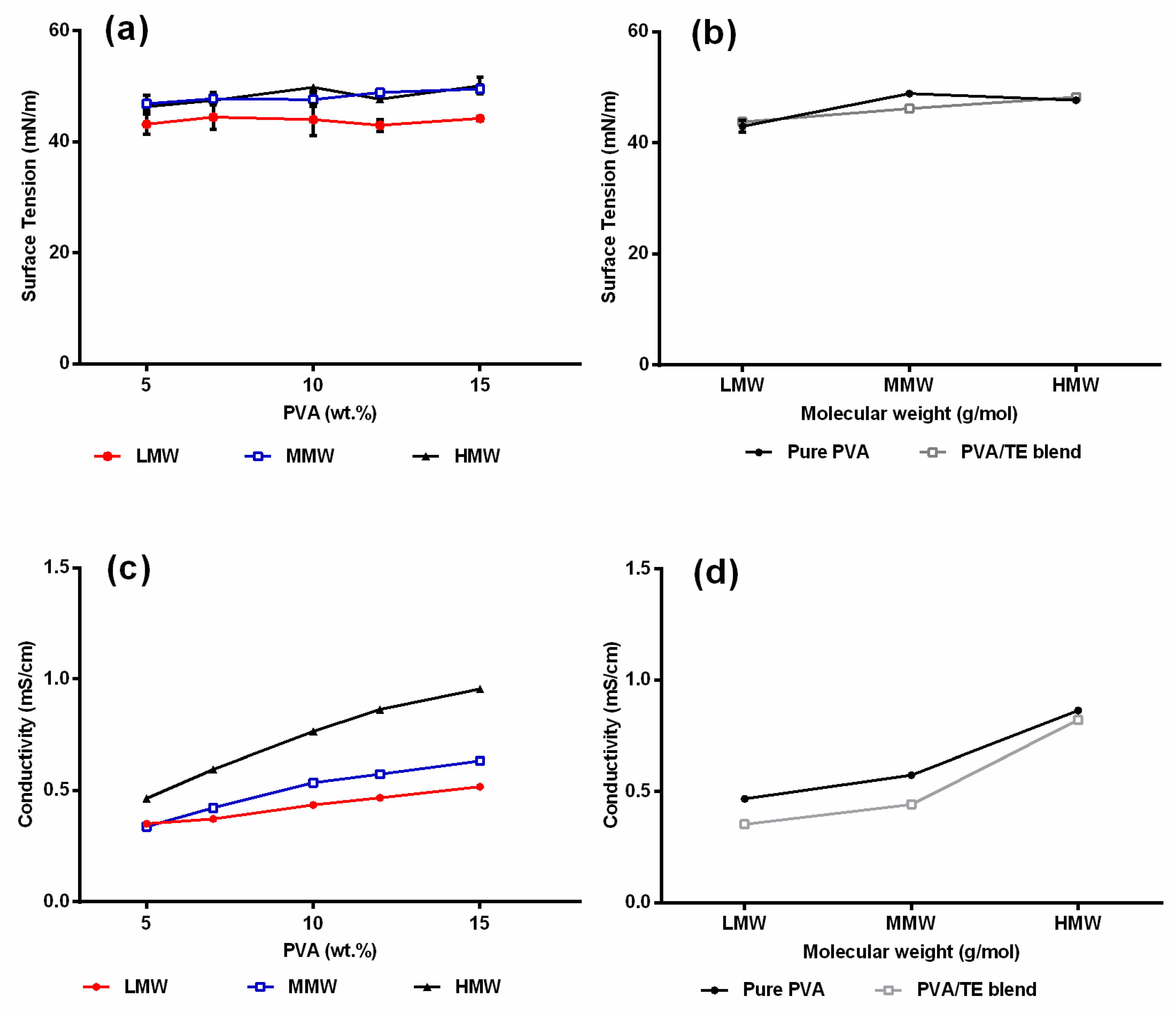
2.2. Surface Tension and Conductivity of Polymer Solutions
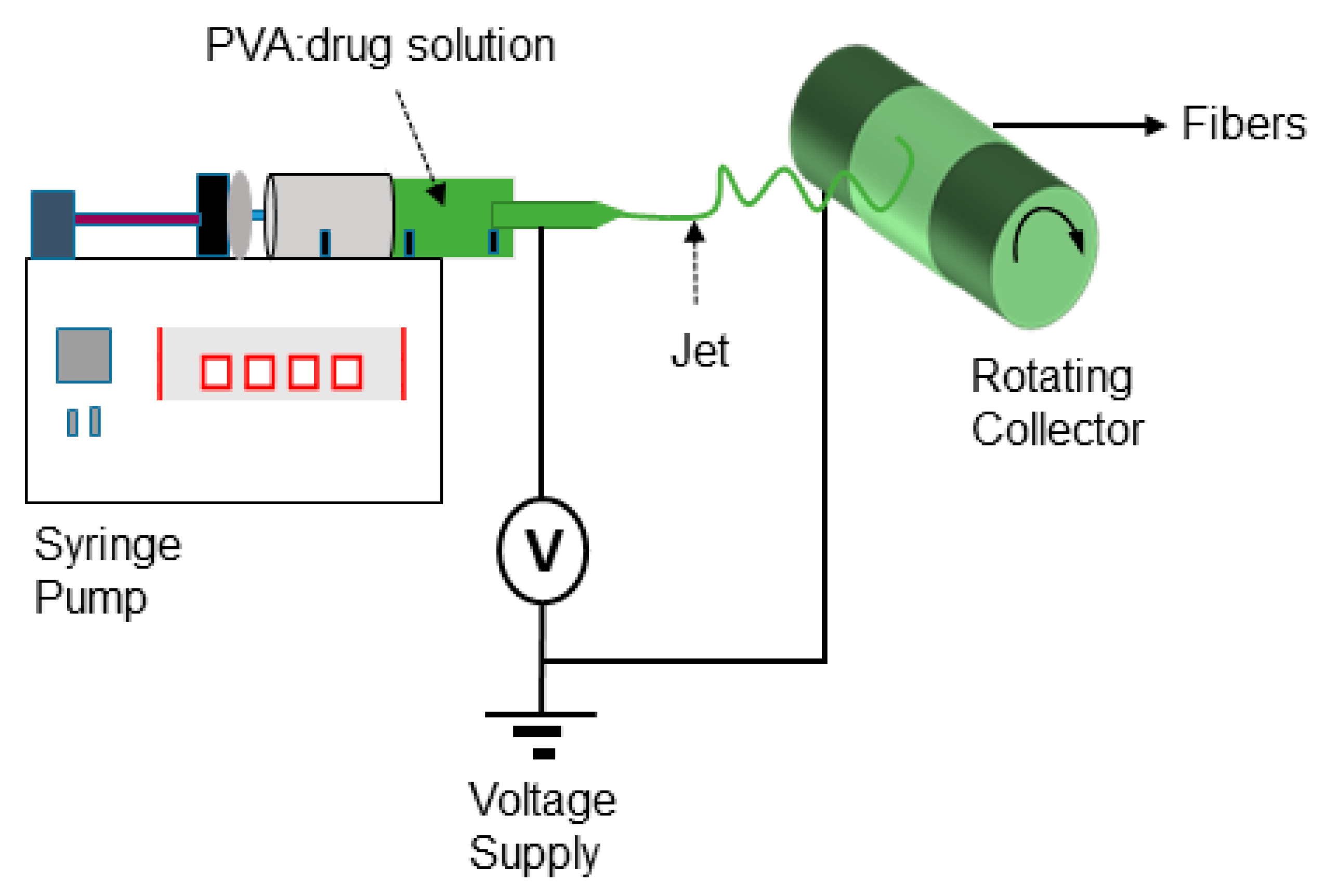
2.3. Qualitative Analysis of Electrospinning Process and Nanofiber Formation
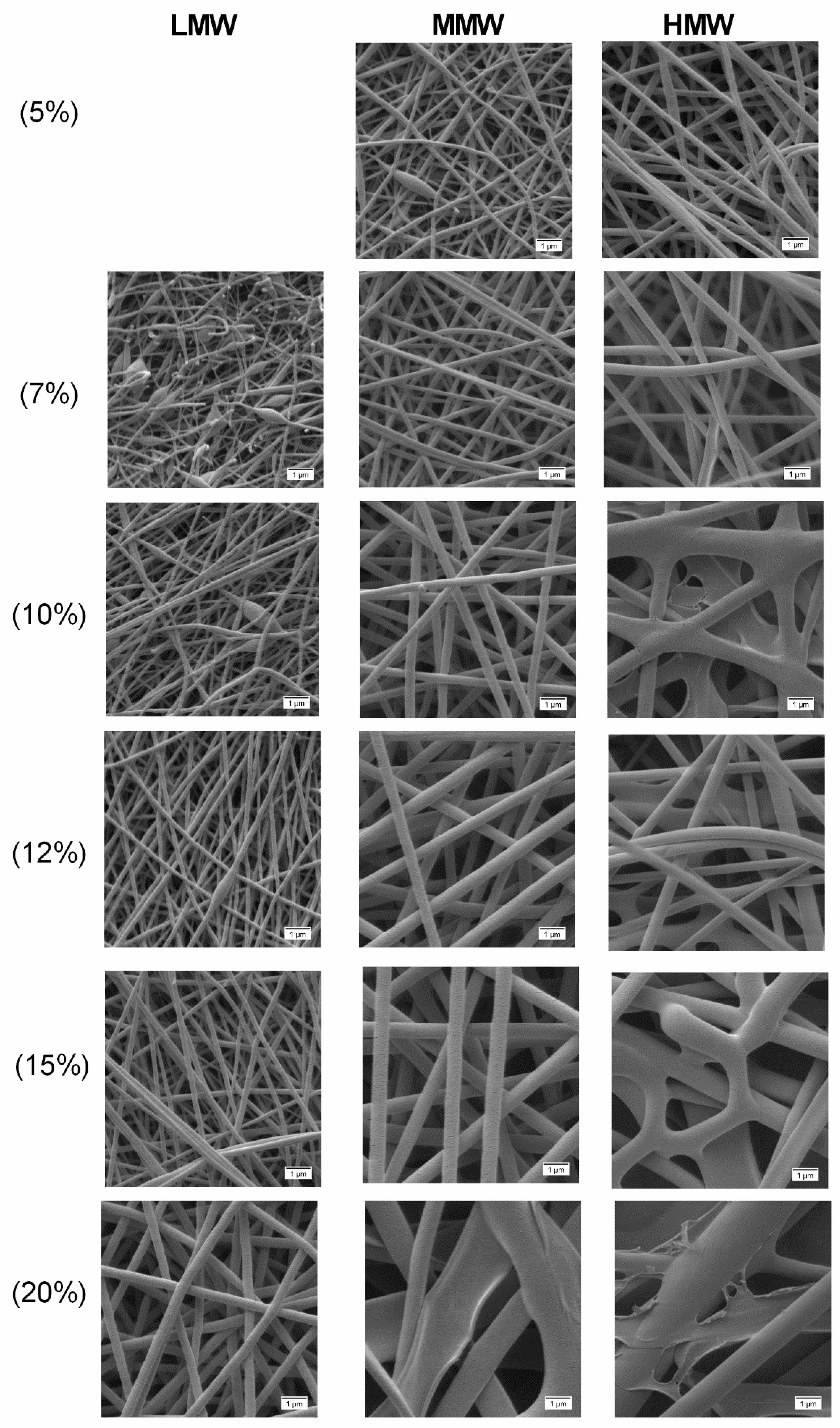
2.4. Effect of Concentration and Molecular Weight on Fiber Morphology
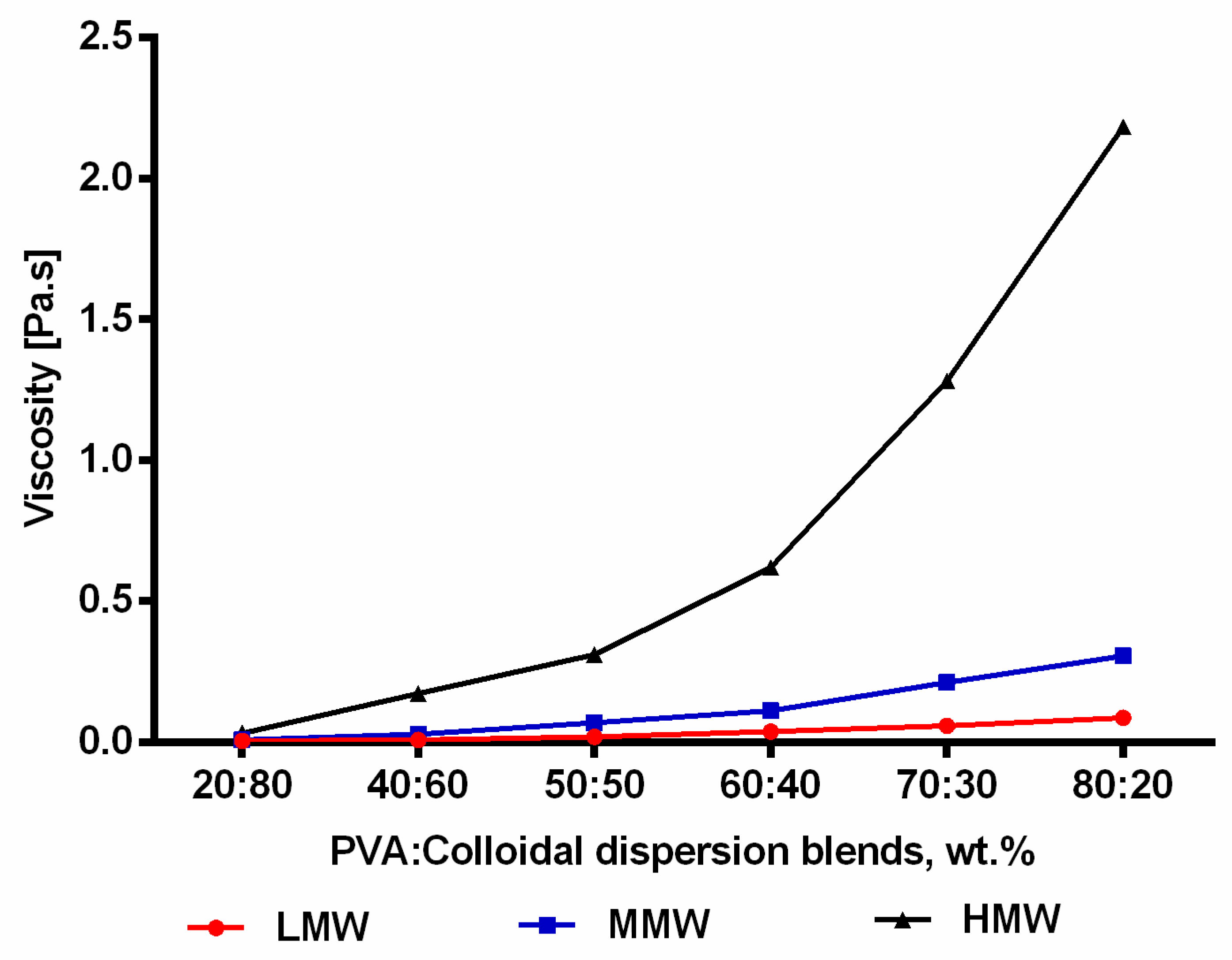
2.5. Incorporation of TE Colloidal Dispersions into Nanofibers
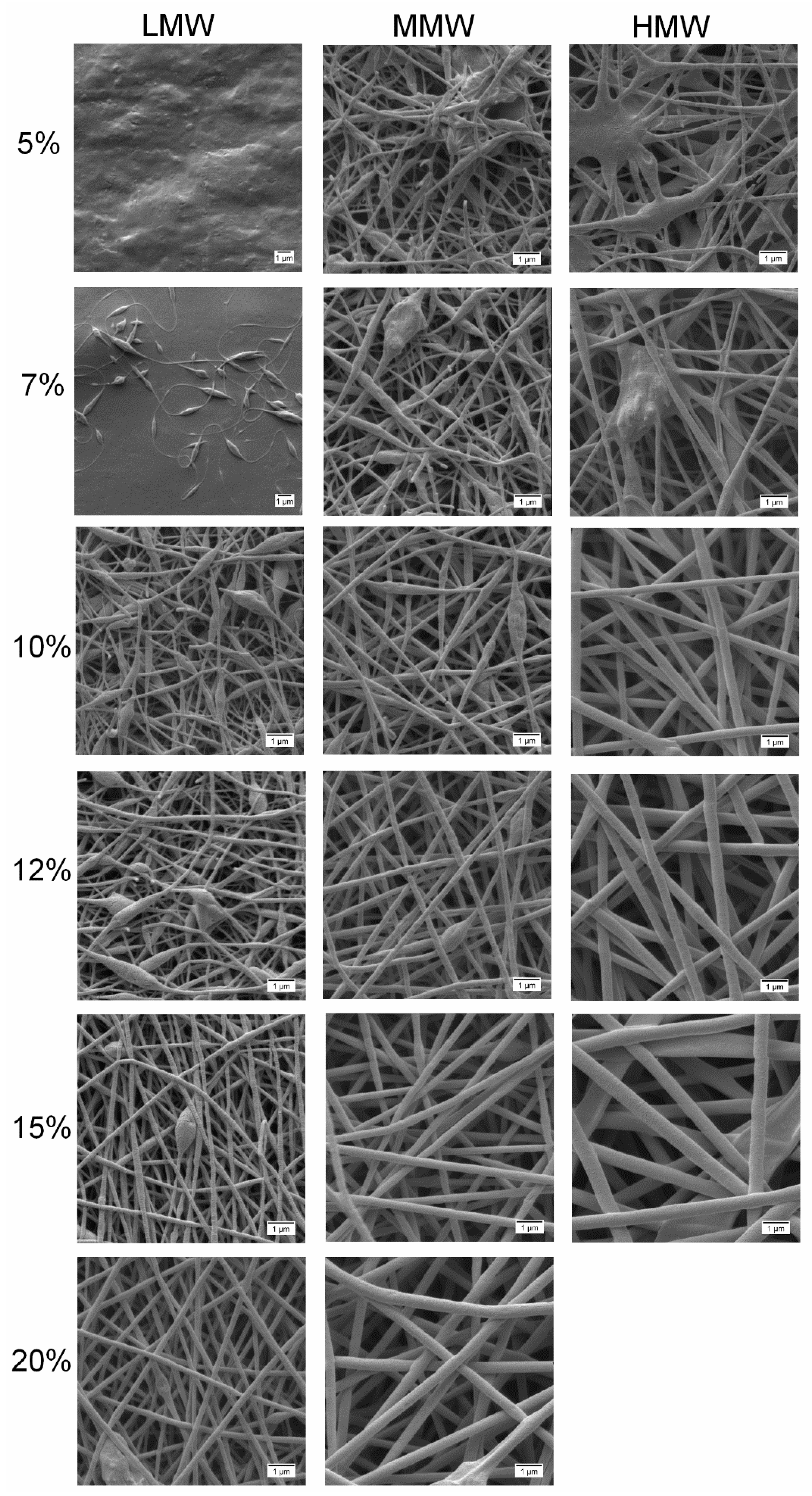
2.6. Fiber Morphology in Different Compositions
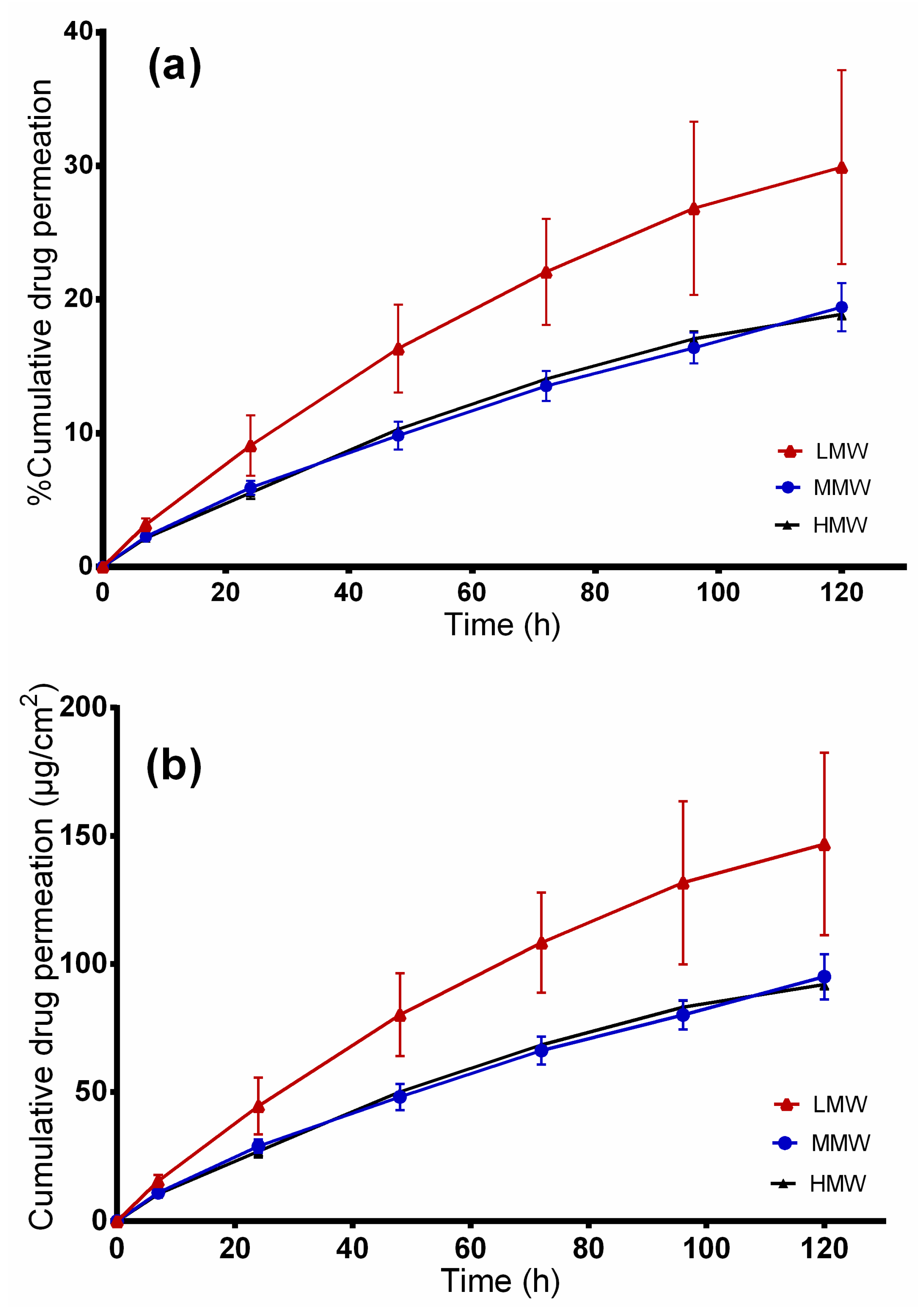
2.7. In Vitro Release Studies
2.8. Ex vivo Permeation through Wounded Skin
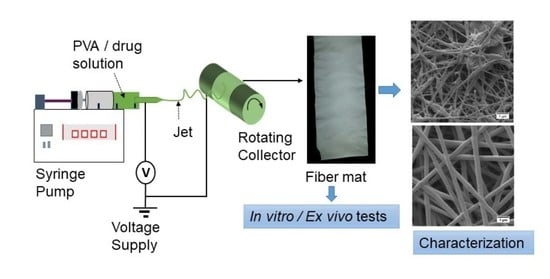
3. Materials and Methods
3.1. Materials
3.2. Preparing the Colloidal Dispersions
3.3. Preparation of Solutions and Electrospinning of Nanofibers
3.4. Electrospinning of Nanofibers
3.5. Rheological Characterization of the Spinning Solutions
3.6. Surface Tension and Conductivity
3.7. Qualitative Analysis of Electrospinning Process and Nanofiber Formation
3.8. Characterization of Nanofibrous Scaffolds
3.9. Ex Vivo Skin Permeation and In Vitro Drug Diffusion Studies
3.10. Betulin Permeation/Release Kinetics Assessment
3.11. HPLC Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehelean, C.A.; Soica, C.M.; Toma, C.-C.; Feflea, S.; Gruia, A.T.; Kasa, P., Jr. Antitumoral activity of betulin, a compound present in birch tree, in formulations with cyclodextrin. Studia Univ. VG Seria St. Vietii 2010, 20, 55–58. [Google Scholar]
- Dehelean, C.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupkó, I.; Gǎluşcan, A.; Pinzaru, S.C.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Central J. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-Y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a Traditional Medicinal Plant to a Rational Drug: Understanding the Clinically Proven Wound Healing Efficacy of Birch Bark Extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef] [Green Version]
- Metelmann, H.-R.; Podmelle, F.; Waite, P.D. Long-Term Cosmetic Benefit of Wound Healing by Betuline. Am. J. Cosmet. Surg. 2012, 29, 19–24. [Google Scholar] [CrossRef]
- Steinbrenner, I.; Houdek, P.; Pollok, S.; Brandner, J.M.; Daniels, R. Influence of the Oil Phase and Topical Formulation on the Wound Healing Ability of a Birch Bark Dry Extract. PLoS ONE 2016, 11, e0155582. [Google Scholar] [CrossRef] [Green Version]
- Färber, A.; Daniels, R. Ex vivo Skin Permeation of Betulin from Water-in-Oil Foams. Ski. Pharmacol. Physiol. 2016, 29, 250–256. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, Chemical and Pharmacological Characterization of a New Oleogel-Forming Triterpene Extract from the Outer Bark of Birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Medica 2019, 85, 524–527. [Google Scholar] [CrossRef] [Green Version]
- Weckesser, S.; Schumann, H.; Laszczyk, M.; Müller, M.; Schempp, C.M. Topical Treatment of Necrotising Herpes Zoster with Betulin from Birch Bark. Forsch. Komplementärmedizin/Res. Complement. Med. 2010, 17, 271–273. [Google Scholar] [CrossRef]
- Schwieger-Briel, A.; Kiritsi, D.; Schempp, C.; Has, C.; Schumann, H. Betulin-Based Oleogel to Improve Wound Healing in Dystrophic Epidermolysis Bullosa: A Prospective Controlled Proof-of-Concept Study. Dermatol. Res. Pr. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- El-Hadi, A.M.; Al-Jabri, F.Y. Influence of Electrospinning Parameters on Fiber Diameter and Mechanical Properties of Poly(3-Hydroxybutyrate) (PHB) and Polyanilines (PANI) Blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, P.; Rezaeian, I.; Siadat, S.O.R.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2009, 21, 77–95. [Google Scholar] [CrossRef]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2014, 45, 659–670. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-loaded electrospun materials in wound-dressing applications and in local cancer treatment. Expert Opin. Drug Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Gajra, B.; Pandya, S.S.; Vidyasagar, G.; Rabari, H.; Dedania, R.R.; Rao, S. Poly vinyl alcohol hydrogel and its pharmaceutical and biomedical applications: A review. Int. J. Pharm. Res. 2012, 4, 20–26. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [Green Version]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Noordin, M.; Idris, A.; Shakir, A.S.A.; Kurniawan, D. Influence of Polyvinyl Alcohol Molecular Weight on the Electrospun Nanofiber Mechanical Properties. Procedia Manuf. 2015, 2, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Hasan, A.; Nath, V.K.Y.; Thomas, J.; Augustine, A.; Kalarikkal, N.; Al Moustafa, A.-E.; Thomas, S. Electrospun polyvinyl alcohol membranes incorporated with green synthesized silver nanoparticles for wound dressing applications. J. Mater. Sci. Mater. Med. 2018, 29, 163. [Google Scholar] [CrossRef]
- Saeed, S.M.; Mirzadeh, H.; Zandi, M.; Barzin, J. Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing. Prog. Biomater. 2017, 6, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Aruan, N.M.; Sriyanti, I.; Edikresnha, D.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Polyvinyl Alcohol/Soursop Leaves Extract Composite Nanofibers Synthesized Using Electrospinning Technique and their Potential as Antibacterial Wound Dressing. Procedia Eng. 2017, 170, 31–35. [Google Scholar] [CrossRef]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract. Pharmaceutics 2020, 12, 770. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Daniels, R. Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer. Pharmaceutics 2020, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-W.; Yang, R.-J.; He, J.-Y.; Yang, L. Rheological behaviors of PVA/H2O solutions of high-polymer concentration. J. Appl. Polym. Sci. 2009, 116, 1459–1466. [Google Scholar] [CrossRef]
- Gao, H.; He, J.; Yang, R.; Yang, L. Characteristic rheological features of high concentration PVA solutions in water with different degrees of polymerization. J. Appl. Polym. Sci. 2010, 116, 2734–2741. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly(vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Hay, W.T.; Byars, J.A.; Fanta, G.F.; Selling, G.W. Rheological characterization of solutions and thin films made from amylose-hexadecylammonium chloride inclusion complexes and polyvinyl alcohol. Carbohydr. Polym. 2017, 161, 140–148. [Google Scholar] [CrossRef]
- Sousa, A.M.; Souza, H.K.; Uknalis, J.; Liu, S.-C.; Gonçalves, M.; Liu, L. Electrospinning of agar/PVA aqueous solutions and its relation with rheological properties. Carbohydr. Polym. 2015, 115, 348–355. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Huang, C.-C. Electrospinning PVA solution-rheology and morphology analyses. Fibers Polym. 2012, 13, 44–50. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ray, P. Studies on surface tension of poly(vinyl alcohol): Effect of concentration, temperature, and addition of chaotropic agents. J. Appl. Polym. Sci. 2004, 93, 122–130. [Google Scholar] [CrossRef]
- Le Corre Bordes, D.; Hofman, K.; Bordes, N.; Tucker, N.; Huber, T.; Staiger, M.P. Electrospinning window: Solution properties for uniform fibres from electrospinnable biopolymers. In Proceedings of the International Conference on Processing and Fabrication of Advanced Materials, Auckland, New Zealand, 22–25 January 2017; pp. 22–25. [Google Scholar]
- Shin, J.; Kim, Y.; Lim, Y.M.; Nho, Y.C. Removal of sodium acetate in poly(vinyl alcohol) and its quantification by1H NMR spectroscopy. J. Appl. Polym. Sci. 2007, 107, 3179–3183. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the electrospinning of poly(vinyl alcohol) nanofiber mats: A revisit. J. Appl. Polym. Sci. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Toh Tuck Link, Singapore, 2005. [Google Scholar]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y.; Chen, X. Fabrication and characterization of electrospun feather keratin/poly(vinyl alcohol) composite nanofibers. RSC Adv. 2017, 7, 9854–9861. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical properties and biodegradability of electrospun soy protein Isolate/PVA hybrid nanofibers. Polym. Degrad. Stab. 2012, 97, 747–754. [Google Scholar] [CrossRef]
- Imani, R.; Yousefzadeh, M.; Nour, S. Functional Nanofiber for Drug Delivery Applications. In Handbook of Nanofibers; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 1–55. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of Lutein-Loaded PVA/Sodium Alginate Nanofibers and Investigation of Its Release Behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [Green Version]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lunter, D.J. Confocal Raman microspectroscopy as an alternative method to investigate the extraction of lipids from stratum corneum by emulsifiers and formulations. Eur. J. Pharm. Biopharm. 2018, 127, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D.J.; Rottke, M.; Daniels, R. Oil-in-Oil-Emulsions with Enhanced Substantivity for the Treatment of Chronic Skin Diseases. J. Pharm. Sci. 2014, 103, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D.; Daniels, R. In vitro Skin Permeation and Penetration of Nonivamide from Novel Film-Forming Emulsions. Ski. Pharmacol. Physiol. 2013, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Suvakanta, D.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Armbruster, M.; Mönckedieck, M.; Scherließ, R.; Daniels, R.; Wahl, M.A. Birch Bark Dry Extract by Supercritical Fluid Technology: Extract Characterisation and Use for Stabilisation of Semisolid Systems. Appl. Sci. 2017, 7, 292. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



| TE Composition | Specific Surface Area | Particle Size D50% |
|---|---|---|
| Betulin 81.60%, lupeol 2.08%, betulinic acid 3.84%, erythrodiol 1.05%, oleanolic acid 0.97%, Betulinic acid methyl ester 0.52%, undisclosed substances 9.94% | 42 ± 0.4 m2/g | 5.8 µm |
| Molecular Weight | PVA (wt.%) | Average Diameter (nm) ± SD | Minimum Diameter (nm) | Maximum Diameter (nm) |
|---|---|---|---|---|
| LMW | 5% | - | - | - |
| 7% | - | - | - | |
| 10% | 147 ± 24 | 91 | 202 | |
| 12% | 174 ± 25 | 113 | 228 | |
| 15% | 224 ± 22 | 178 | 271 | |
| 20% | 390 ± 36 | 304 | 466 | |
| MMW | 5% | 183 ± 46 | 108 | 307 |
| 7% | 224 ± 35 | 162 | 276 | |
| 10% | 372 ± 26 | 288 | 418 | |
| 12% | 543 ± 70 | 437 | 670 | |
| 15% | 655 ± 46 | 589 | 759 | |
| 20% | 1357± 450 | 742 | 2333 | |
| HMW | 5% | 269 ± 24 | 226 | 311 |
| 7% | 358 ± 38 | 305 | 443 | |
| 10% | 688 ± 94 | 482 | 863 | |
| 12% | 975 ± 170 | 768 | 1364 | |
| 15% | 1135 ± 405 | 762 | 2337 | |
| 20% | 2204 ± 151 | 1500 | 2397 |
| Molecular Weight | PVA (wt.%) | Average Diameter (nm) ± SD | Minimum Diameter (nm) | Maximum Diameter (nm) |
|---|---|---|---|---|
| LMW | 12% | 143 ± 29 | 88 | 225 |
| 15% | 187 ± 31 | 152 | 272 | |
| 20% | 241 ± 28 | 191 | 298 | |
| MMW | 10% | 174 ± 22 | 129 | 225 |
| 12% | 241 ± 32 | 178 | 317 | |
| 15% | 308 ± 29 | 263 | 373 | |
| 20% | 424 ± 33 | 355 | 497 | |
| HMW | 10% | 319 ± 56 | 267 | 503 |
| 12% | 392 ± 41 | 341 | 499 | |
| 15% | 605 ± 55 | 509 | 689 |
| Sample | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| LMW | 0.8674 | 0.8955 | 0.9518 | 0.9727 | 0.41 |
| MMW | 0.9062 | 0.9233 | 0.9705 | 0.9875 | 0.46 |
| HMW | 0.9504 | 0.9670 | 0.9947 | 0.9967 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwiiri, F.K.; Daniels, R. Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing. Molecules 2020, 25, 4799. https://doi.org/10.3390/molecules25204799
Mwiiri FK, Daniels R. Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing. Molecules. 2020; 25(20):4799. https://doi.org/10.3390/molecules25204799
Chicago/Turabian StyleMwiiri, Francis Kamau, and Rolf Daniels. 2020. "Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing" Molecules 25, no. 20: 4799. https://doi.org/10.3390/molecules25204799