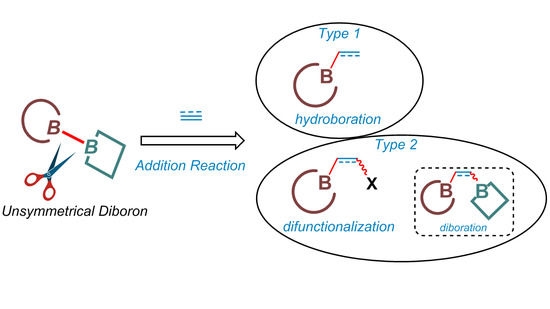
Unsymmetrical Diboron Reagents: Application in Borylation Reactions of Unsaturated Bonds
Abstract
:1. Introduction
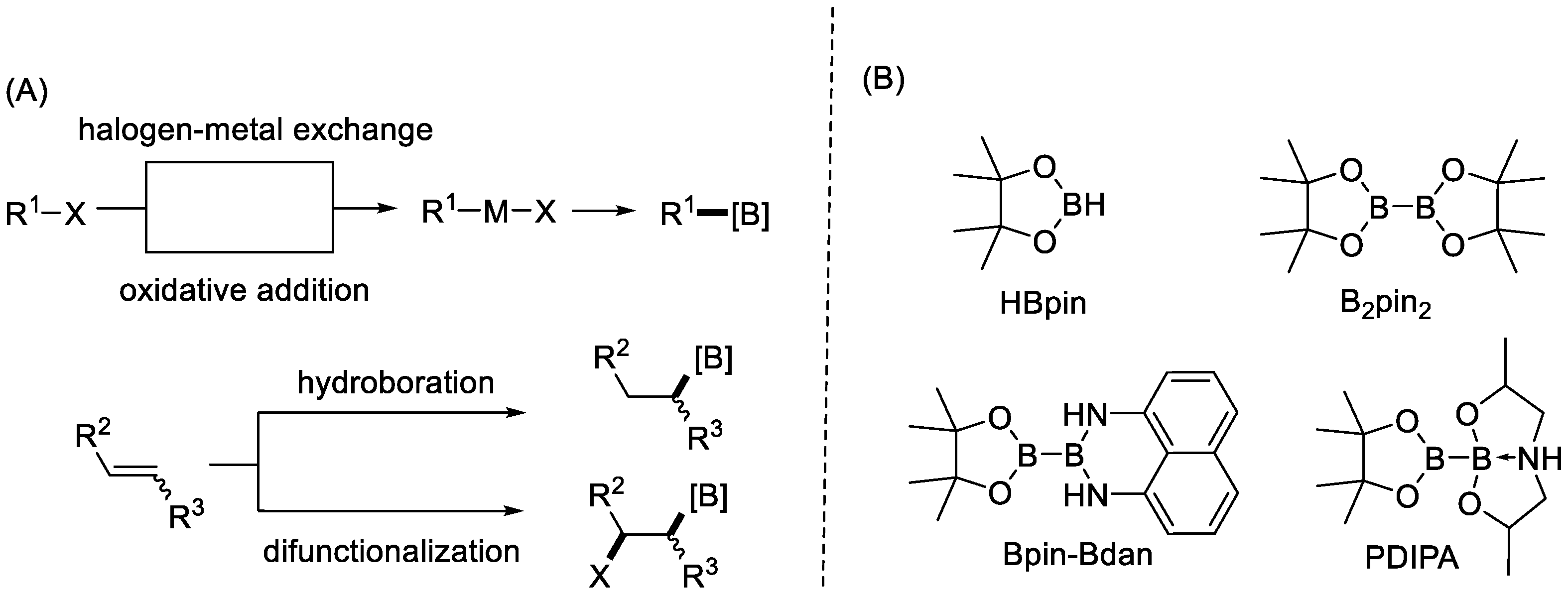
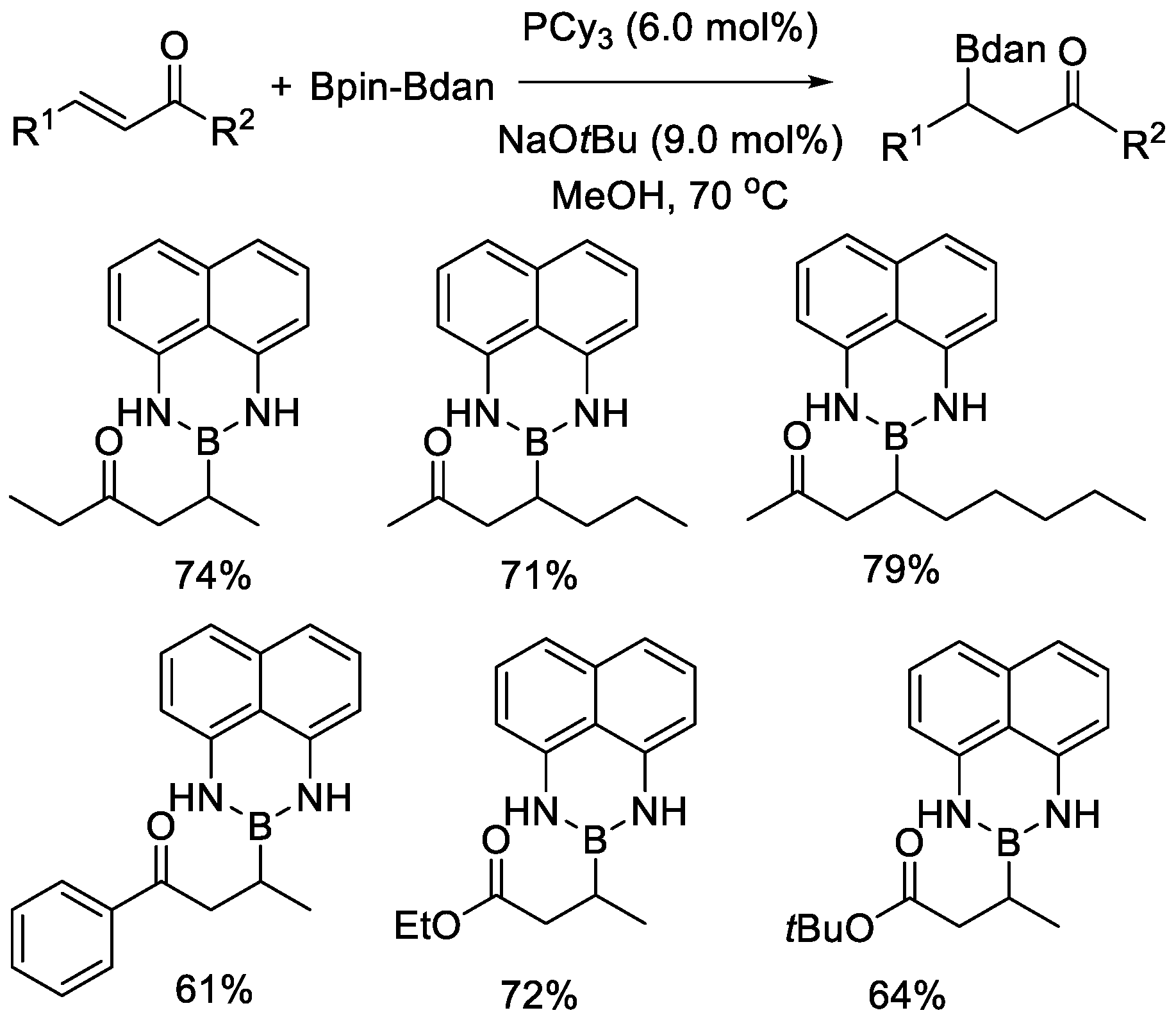
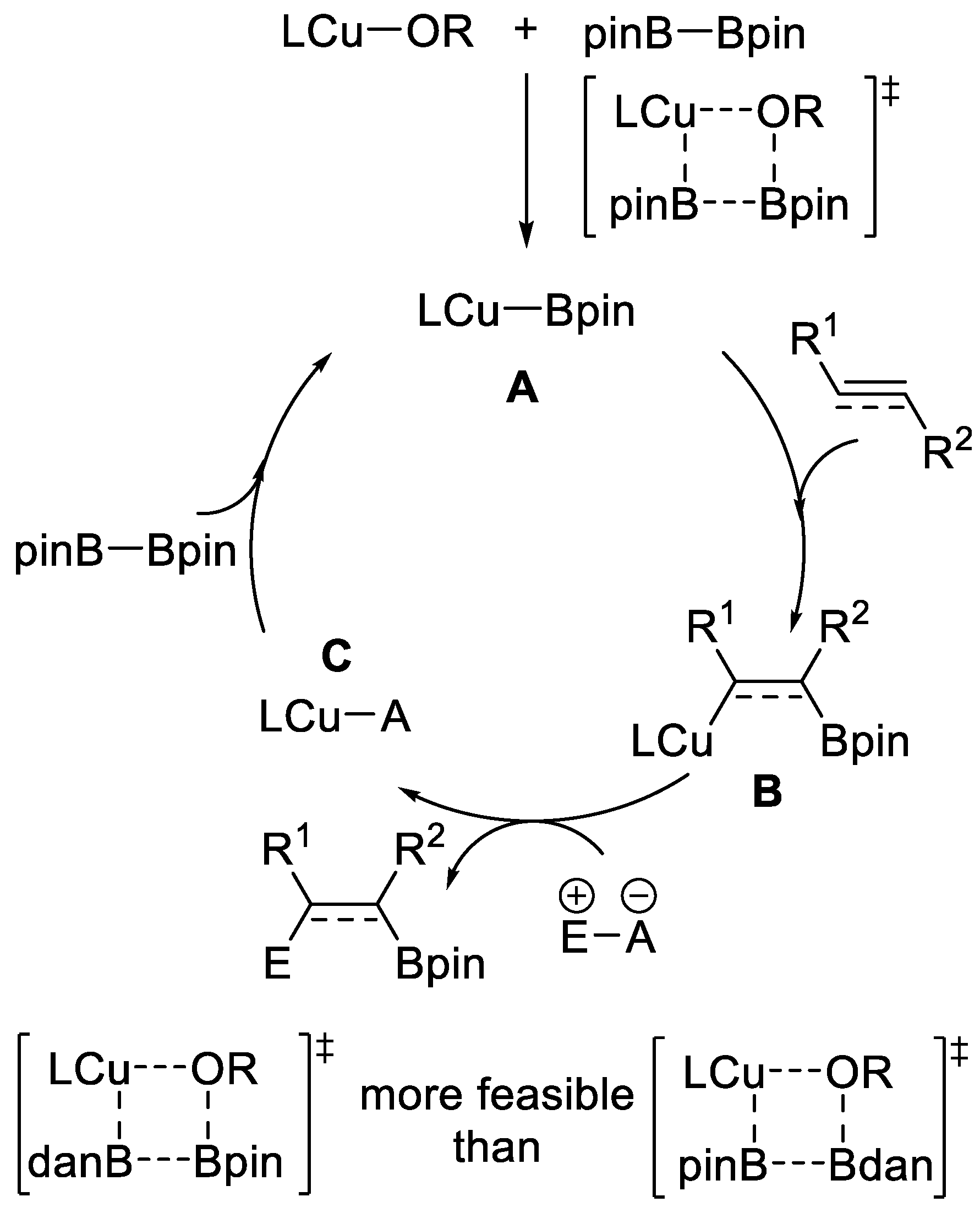
2. sp2-sp3 Diboron Reagents
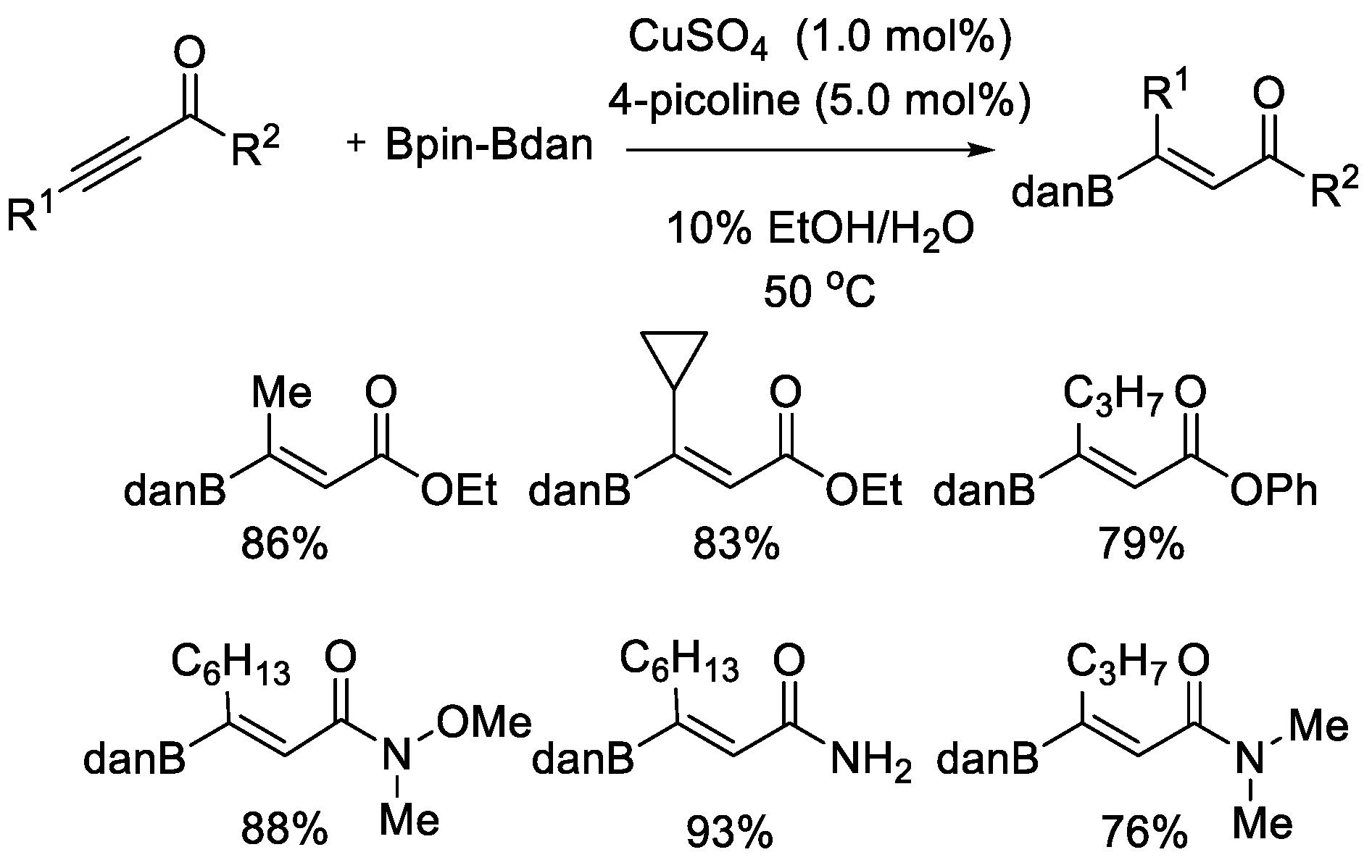
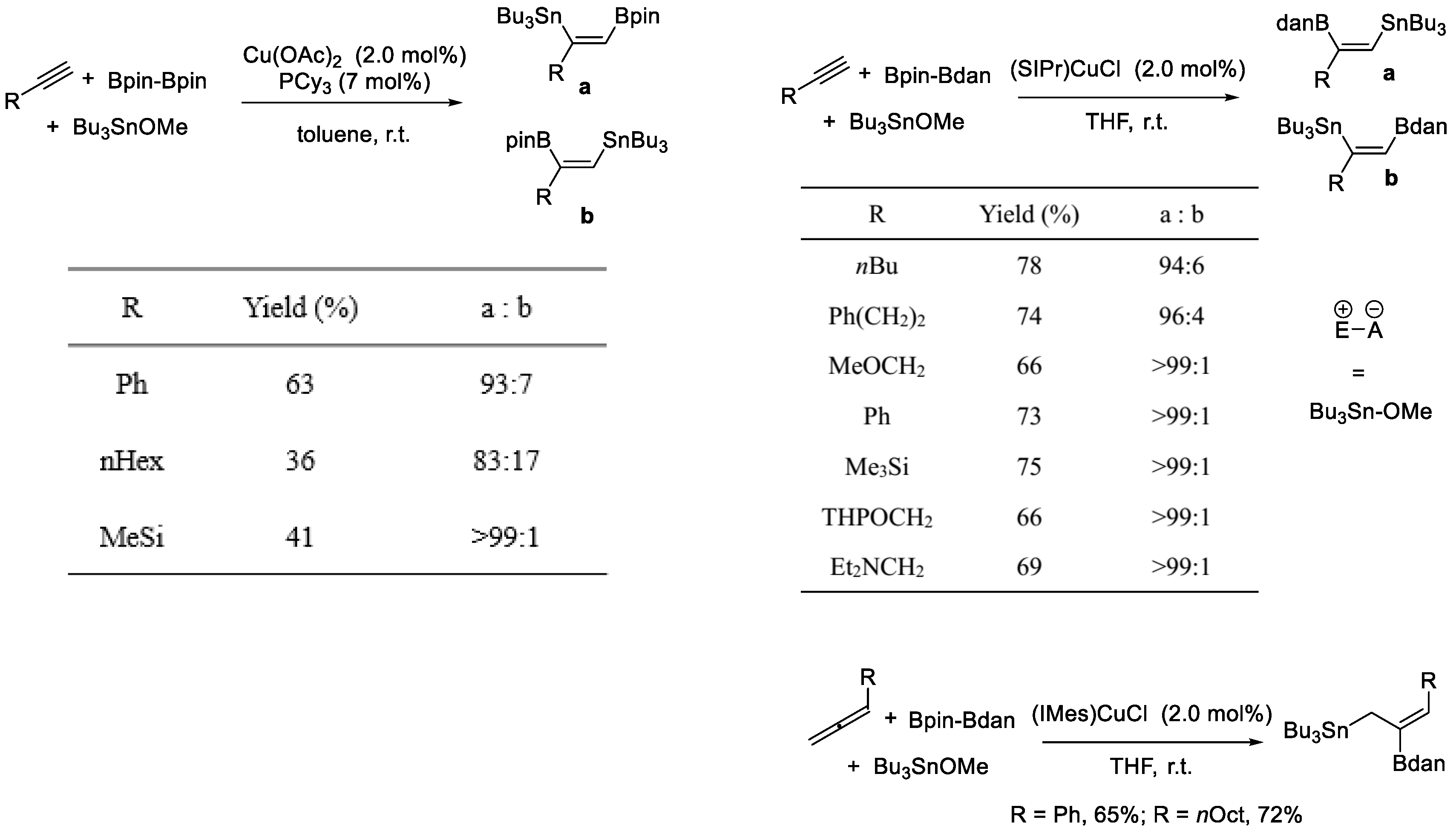
3. sp2-sp2 Diboron Reagents
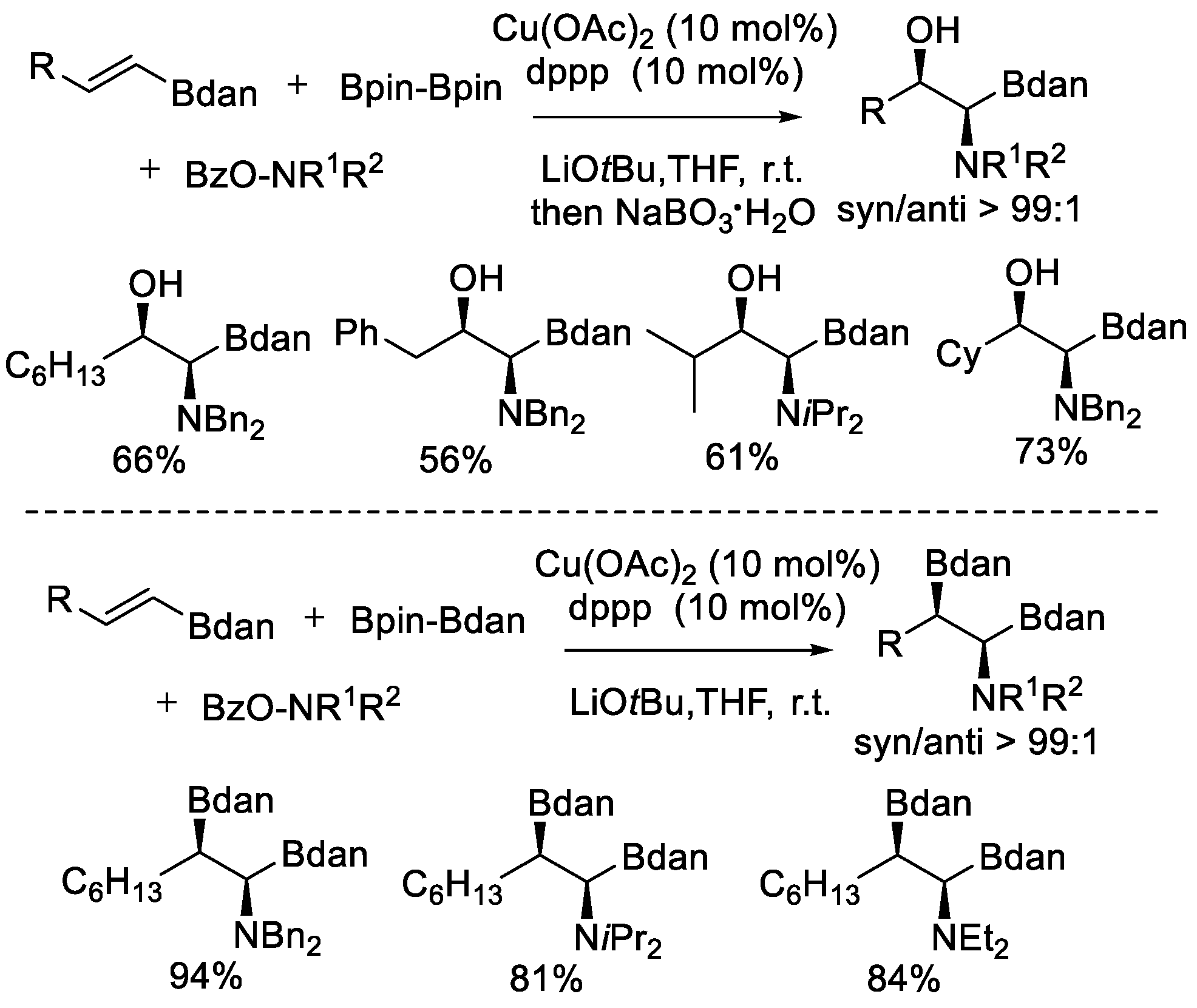
3.1. Addition Reactions: Diboration
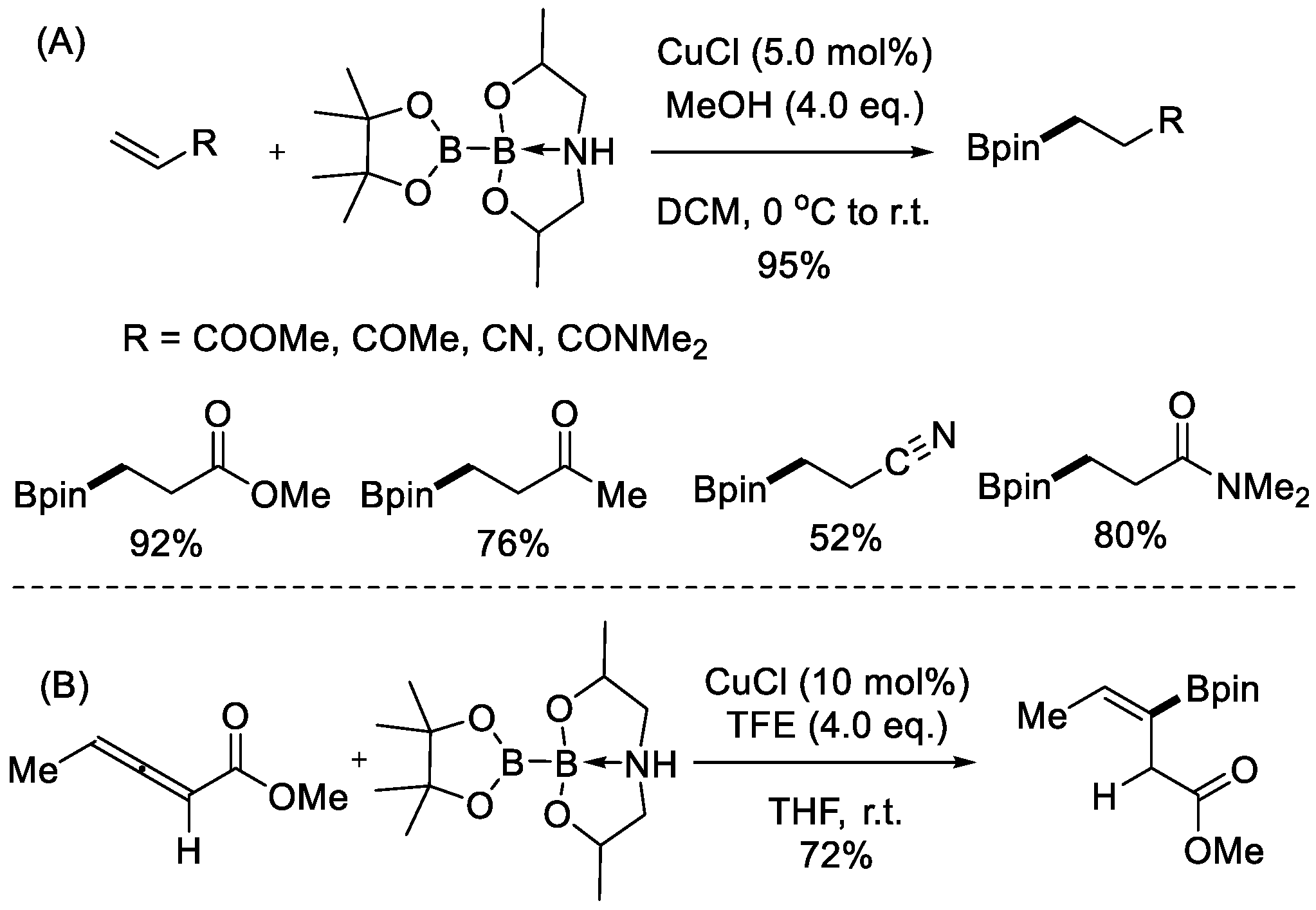
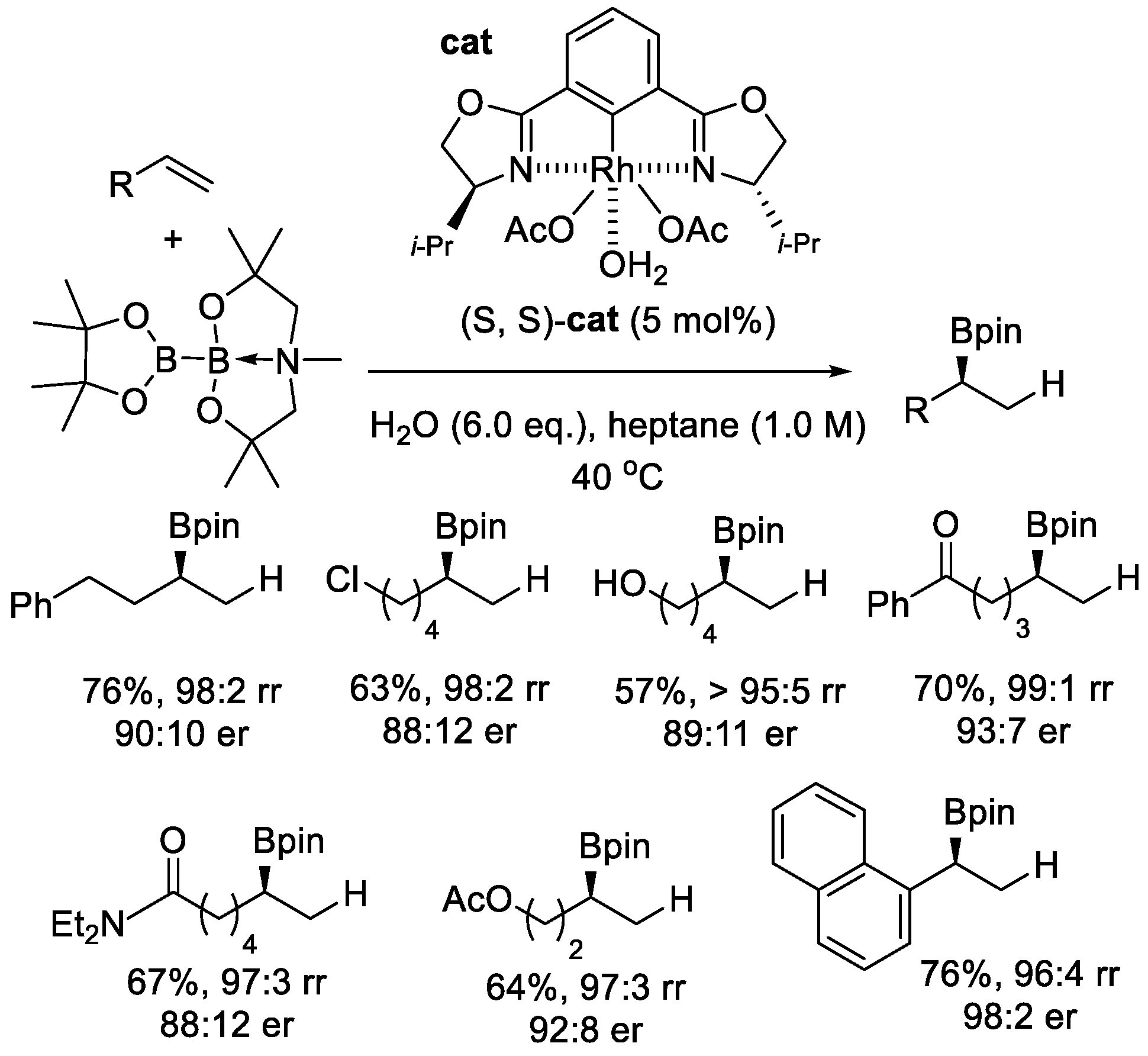
3.2. Addition Reactions: Hydroboration
3.3. Other Types of Addition Reactions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fyfe, J.W.B.; Watson, A.J.B. Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem 2017, 3, 31–55. [Google Scholar] [CrossRef]
- Lennox, A.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Lam, P. Copper-Promoted Carbon-Heteroatom Bond Cross-Coupling with Boronic Acids and Derivatives. Synthesis 2010, 2011, 829–856. [Google Scholar] [CrossRef]
- Sanjeeva Rao, K.; Wu, T.-S. Chan–Lam coupling reactions: Synthesis of heterocycles. Tetrahedron 2012, 68, 7735–7754. [Google Scholar] [CrossRef]
- Ros, A.; Fernández, R.; Lassaletta, J.M. Functional group directed C–H borylation. Chem. Soc. Rev. 2014, 43, 3229–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeve, E.C.; Geier, S.J.; Mkhalid, I.A.I.; Westcott, S.A.; Marder, T.B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161. [Google Scholar] [CrossRef]
- Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Recent advances in catalytic C–H borylation reactions. Tetrahedron 2017, 73, 7123–7157. [Google Scholar] [CrossRef]
- Yan, G.; Huang, D.; Wu, X. Recent Advances in C–B Bond Formation through a Free Radical Pathway. Adv. Synth. Catal. 2017, 360, 1040–1053. [Google Scholar] [CrossRef]
- Chen, K.; Wang, L.; Meng, G.; Li, P. Recent Advances in Transition-Metal-Free Aryl C–B Bond Formation. Synthesis 2017, 49, 4719–4730. [Google Scholar]
- Cuenca, A.B.; Shishido, R.; Ito, H.; Fernández, E. Transition-metal-free B–B and B–interelement reactions with organic molecules. Chem. Soc. Rev. 2017, 46, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Jia, X.; Li, P.; Zhao, J.; Zhou, Y.; Wang, J.; Liu, H. Catalytic and catalyst-free diboration of alkynes. Org. Chem. Front. 2017, 4, 2235–2255. [Google Scholar] [CrossRef]
- Hemming, D.; Fritzemeier, R.; Westcott, S.A.; Santos, W.L.; Steel, P.G. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 2018, 47, 7477–7494. [Google Scholar]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Brown, H.C. Hydroboration—A powerful synthetic tool. Tetrahedron 1961, 12, 117–138. [Google Scholar] [CrossRef]
- Brown, H.C.; Gupta, S.K. 1,3,2-Benzodioxaborole, a convenient monofunctional hydroborating agent. Simple new synthesis of alkaneboronic esters and acids from olefins via hydroboration. J. Am. Chem. Soc. 1971, 93, 1816–1818. [Google Scholar] [CrossRef]
- Männig, D.; Nöth, H. Catalytic Hydroboration with Rhodium Complexes. Angew. Chem. Int. Ed. Engl. 1985, 24, 878–879. [Google Scholar] [CrossRef]
- Partyka, D.V. Transmetalation of Unsaturated Carbon Nucleophiles from Boron-Containing Species to the Mid to Late d-Block Metals of Relevance to Catalytic C–X Coupling Reactions (X = C, F, N, O, Pb, S, Se, Te). Chem. Rev. 2011, 111, 1529–1595. [Google Scholar] [CrossRef]
- Dang, L.; Lin, Z.; Marder, T.B. Boryl ligands and their roles in metal-catalysed borylation reactions. Chem. Commun. 2009, 27, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, R.D.; Neeve, E.C.; Braunschweig, H.; Marder, T.B. sp2-sp3 diboranes: Astounding structural variability and mild sources of nucleophilic boron for organic synthesis. Chem. Commun. 2015, 51, 9594–9607. [Google Scholar] [CrossRef] [PubMed]
- Borner, C.; Wlecha, M.T.; Kleeberg, C. Unsymmetrical Diborane (4) Derivatives: A Comparative Study. Eur. J. Inorg. Chem. 2017, 38, 4485–4492. [Google Scholar] [CrossRef]
- Oschmann, W.; Borner, C.; Kleeberg, C. Unsymmetrical diborane (4) derivatives by copper mediated B-B coupling. Dalton Trans. 2018, 47, 5318–5327. [Google Scholar] [CrossRef]
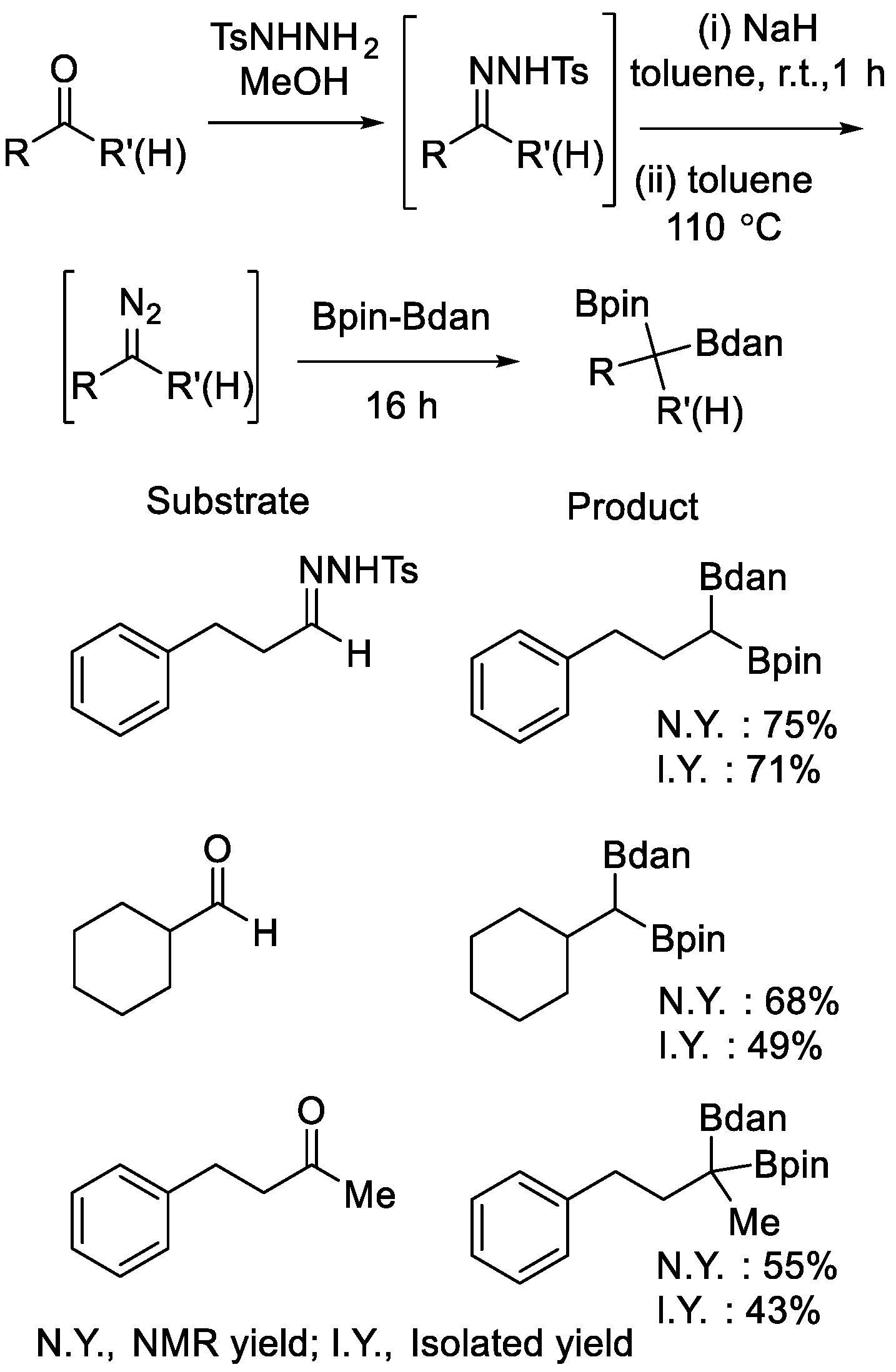
- Gao, M.; Thorpe, S.B.; Santos, W.L. sp2-sp3 Hybridized Mixed Diboron: Synthesis, Characterization, and Copper-Catalyzed β-Boration of α,β-Unsaturated Conjugated Compounds. Org. Lett. 2009, 11, 3478–3481. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Thorpe, S.B.; Kleeberg, C.; Slebodnick, C.; Marder, T.B.; Santos, W.L. Structure and Reactivity of a Preactivated sp2-sp3 Diboron Reagent: Catalytic Regioselective Boration of α,β-Unsaturated Conjugated Compounds. J. Org. Chem. 2011, 76, 3997–4007. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.B.; Guo, X.; Santos, W.L. Regio- and stereoselective copper-catalyzed [small beta]-borylation of allenoates by a preactivated diboron. Chem. Commun. 2011, 47, 424–426. [Google Scholar] [CrossRef]
- Smith, J.R.; Collins, B.S.L.; Hesse, M.J.; Graham, M.A.; Myers, E.L.; Aggarwal, V.K. Enantioselective Rhodium(III)-Catalyzed Markovnikov Hydroboration of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2017, 139, 9148–9151. [Google Scholar] [CrossRef]
- Iwadate, N.; Suginome, M. Differentially Protected Diboron for Regioselective Diboration of Alkynes: Internal-Selective Cross-Coupling of 1-Alkene-1,2-diboronic Acid Derivatives. J. Am. Chem. Soc. 2010, 132, 2548–2549. [Google Scholar] [CrossRef]
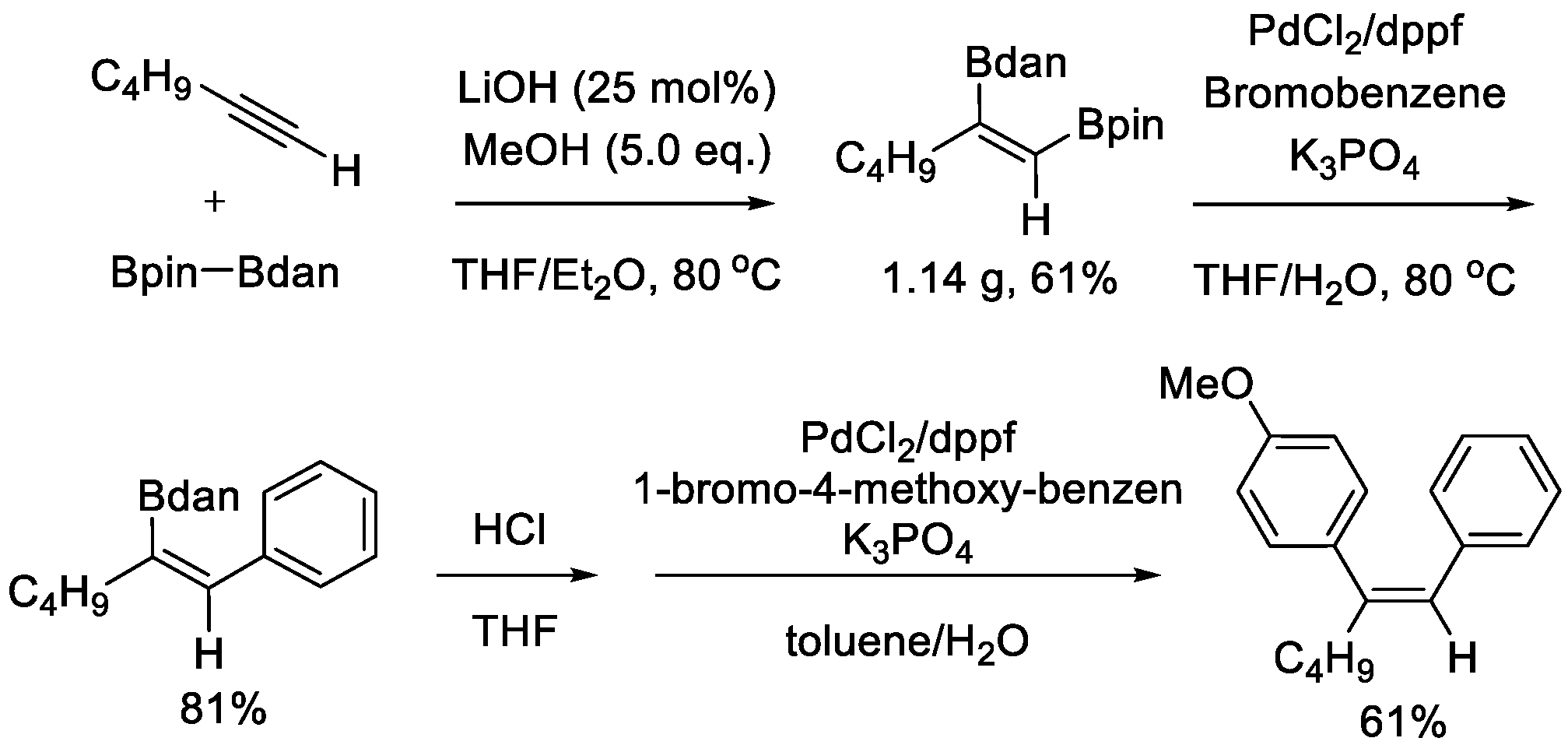
- Peng, S.; Liu, G.; Huang, Z. Mixed Diboration of Alkynes Catalyzed by LiOH: Regio- and Stereoselective Synthesis of cis-1,2-Diborylalkenes. Org. Lett. 2018, 20, 7363–7366. [Google Scholar] [CrossRef]
- Nagashima, Y.; Hirano, K.; Takita, R.; Uchiyama, M. Trans-Diborylation of Alkynes: Pseudo-Intramolecular Strategy Utilizing a Propargylic Alcohol Unit. J. Am. Chem. Soc. 2014, 136, 8532–8535. [Google Scholar] [CrossRef] [PubMed]
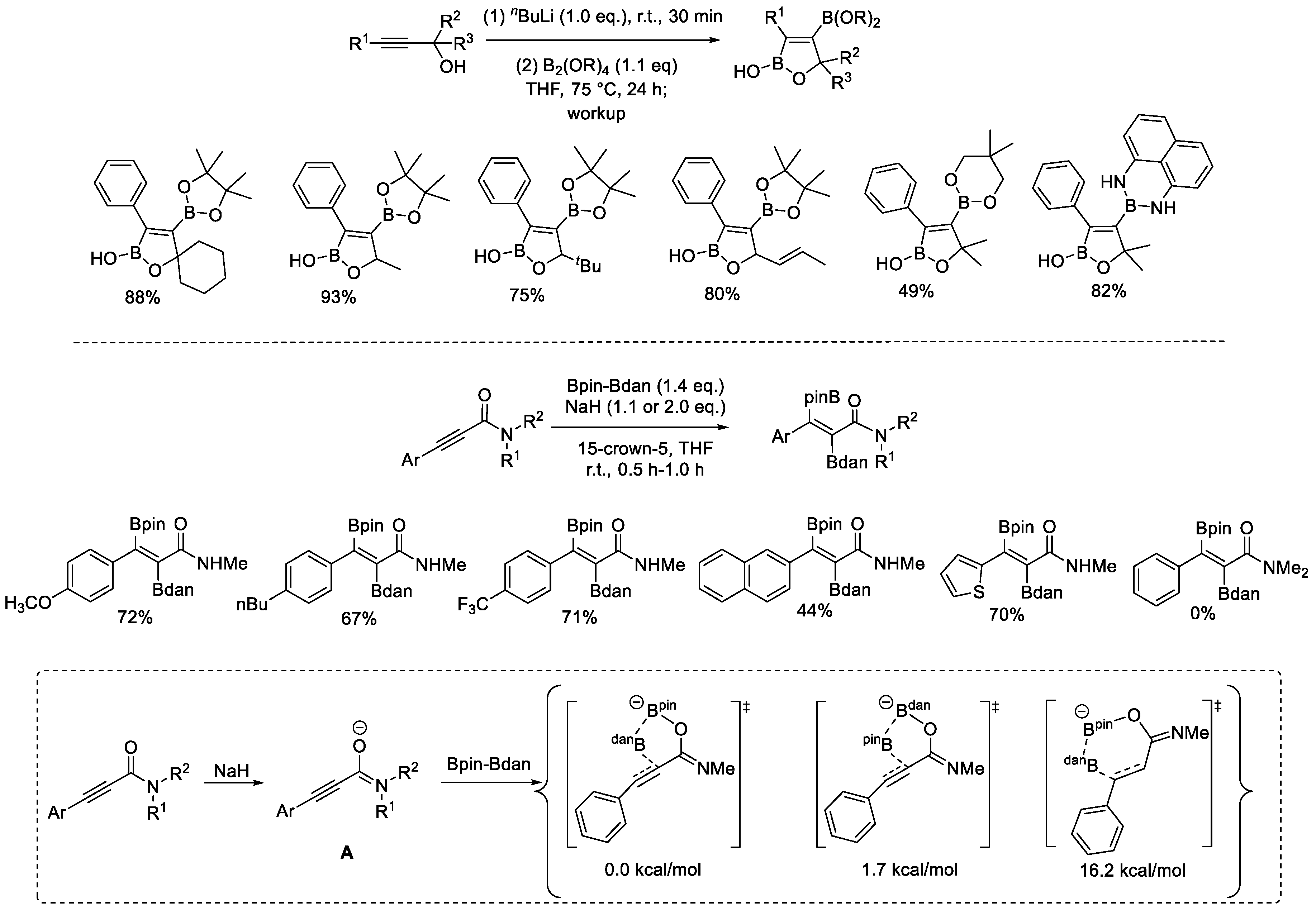
- Verma, A.; Snead, R.F.; Dai, Y.; Slebodnick, C.; Yang, Y.; Yu, H.; Yao, F.; Santos, W.L. Substrate-Assisted, Transition-Metal-Free Diboration of Alkynamides with Mixed Diboron: Regio- and Stereoselective Access to trans-1,2-Vinyldiboronates. Angew. Chem. Int. Ed. 2017, 56, 5193–5197. [Google Scholar] [CrossRef]
- Kojima, C.; Lee, K.-H.; Lin, Z.; Yamashita, M. Direct and Base-Catalyzed Diboration of Alkynes Using the Unsymmetrical Diborane(4), pinB-BMes2. J. Am. Chem. Soc. 2016, 138, 6662–6669. [Google Scholar] [CrossRef]
- Guo, X.; Nelson, A.K.; Slebodnick, C.; Santos, W.L. Regio- and Chemoselective Diboration of Allenes with Unsymmetrical Diboron: Formation of Vinyl and Allyl Boronic Acid Derivatives. ACS Catal. 2015, 5, 2172–2176. [Google Scholar] [CrossRef]
- Miralles, N.; Cid, J.; Cuenca, A.B.; Carbo, J.J.; Fernandez, E. Mixed diboration of alkenes in a metal-free context. Chem. Commun. 2015, 51, 1693–1696. [Google Scholar] [CrossRef] [Green Version]
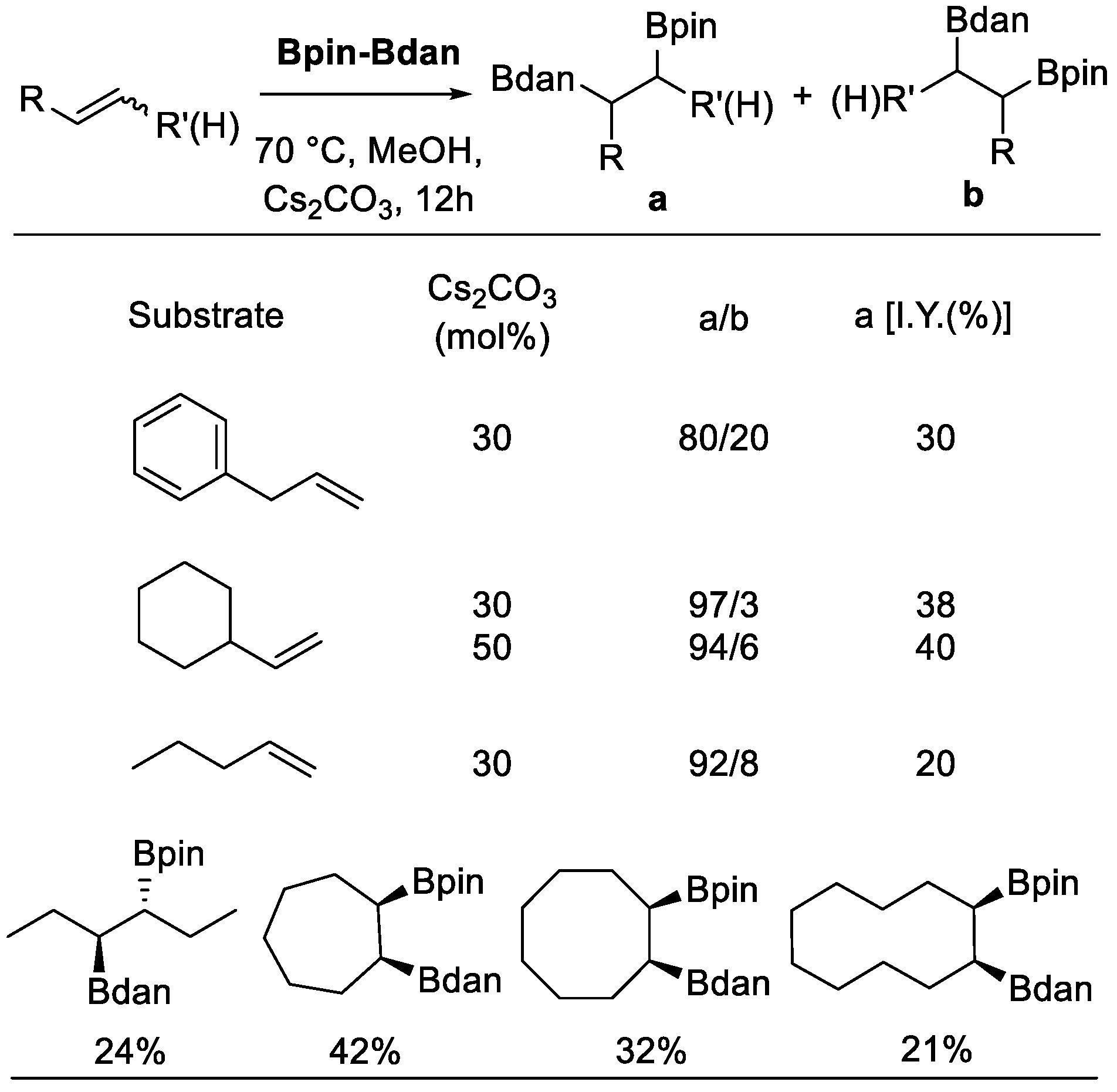
- Cuenca, A.B.; Cid, J.; García-López, D.; Carbó, J.J.; Fernández, E. Unsymmetrical 1,1-diborated multisubstituted sp3-carbons formed via a metal-free concerted-asynchronous mechanism. Org. Biomol. Chem. 2015, 13, 9659–9664. [Google Scholar] [CrossRef] [PubMed]
- Krautwald, S.; Bezdek, M.J.; Chirik, P.J. Cobalt-Catalyzed 1,1-Diboration of Terminal Alkynes: Scope, Mechanism, and Synthetic Applications. J. Am. Chem. Soc. 2017, 139, 3868–3875. [Google Scholar] [CrossRef] [Green Version]
- Cid, J.; Carbó, J.J.; Fernández, E. A Clear-Cut Example of Selective BpinBdan Activation and Precise Bdan Transfer on Boron Conjugate Addition. Chem. A Eur. J. 2014, 20, 3616–3620. [Google Scholar] [CrossRef]
- Cascia, E.L.; Sanz, X.; Bo, C.; Whiting, A.; Fernandez, E. Asymmetric metal free β-boration of α,β-unsaturated imines assisted by (S)-MeBoPhoz. Org. Biomol. Chem. 2015, 13, 1328–1332. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Takemoto, Y.; Takaki, K. A masked diboron in Cu-catalysed borylation reaction: Highly regioselective formal hydroboration of alkynes for synthesis of branched alkenylborons. Chem. Commun. 2014, 50, 8299–8302. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.K.; Peck, C.L.; Rafferty, S.M.; Santos, W.L. Chemo-, Regio-, and Stereoselective Copper(II)-Catalyzed Boron Addition to Acetylenic Esters and Amides in Aqueous Media. J. Org. Chem. 2016, 81, 4269–4279. [Google Scholar] [CrossRef]
- Yoshida, H.; Takemoto, Y.; Takaki, K. Borylstannylation of alkynes with inverse regioselectivity: Copper-catalyzed three-component coupling using a masked diboron. Chem. Commun. 2015, 51, 6297–6300. [Google Scholar] [CrossRef]
- Takemoto, Y.; Yoshida, H.; Takaki, K. Copper-Catalyzed Three-Component Borylstannylation of Alkynes. Chem. A Eur. J. 2012, 18, 14841–14844. [Google Scholar] [CrossRef]
- Sakae, R.; Hirano, K.; Satoh, T.; Miura, M. Copper-Catalyzed Stereoselective Aminoboration of Bicyclic Alkenes. Angew. Chem. Int. Ed. 2014, 54, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Sakae, R.; Hirano, K.; Miura, M. Ligand-Controlled Regiodivergent Cu-Catalyzed Aminoboration of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2015, 137, 6460–6463. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, D.; Hirano, K.; Miura, M. Copper-Catalyzed Regio- and Stereoselective Aminoboration of Alkenylboronates. Org. Lett. 2016, 18, 4856–4859. [Google Scholar] [CrossRef] [PubMed]
- Kageyuki, I.; Osaka, I.; Takaki, K.; Yoshida, H. Copper-Catalyzed B(dan)-Installing Carboboration of Alkenes. Org. Lett. 2017, 19, 830–833. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Xu, L.; Miao, Z. Unsymmetrical Diboron Reagents: Application in Borylation Reactions of Unsaturated Bonds. Molecules 2019, 24, 1325. https://doi.org/10.3390/molecules24071325
Ding S, Xu L, Miao Z. Unsymmetrical Diboron Reagents: Application in Borylation Reactions of Unsaturated Bonds. Molecules. 2019; 24(7):1325. https://doi.org/10.3390/molecules24071325
Chicago/Turabian StyleDing, Siyi, Liang Xu, and Zongcheng Miao. 2019. "Unsymmetrical Diboron Reagents: Application in Borylation Reactions of Unsaturated Bonds" Molecules 24, no. 7: 1325. https://doi.org/10.3390/molecules24071325