Chromatographic Analysis and Anti-Oxidative Property of Naoxinqing Tablet, a Proprietary Preparation of Diospyros Kaki Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stock Solutions and Working Standards
2.3. Extraction of the Naoxinqing (NXQ)
2.4. Ultra Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (UPLC-ESI-MS/MS) Instrumentation
2.5. UPLC-ESI-MS/MS Conditions
2.6. Method Validation
2.7. 2,2-Di(4-Tert-Octylphenyl)-1-Picrylhydrazyl (DPPH)Scavenging Activity
2.8. 2,2′-Azino-Bis(3-Ethylbenzthiazoline-6-Sulphonic Acid(ABTS)Scavenging Activity
2.9. EA.hy 926 Cell Culture
2.10. Measurement of Intracellular Reactive Oxygen Species (ROS) Level
2.11. Statistical Analysis
3. Results
3.1. UPLC-ESI-MS/MS
3.2. Method Validation Results
3.3. Comparison of Concentrations of the Analytes in Different Batches of NXQ
3.4. DPPH and ABTS Scavenging Activity
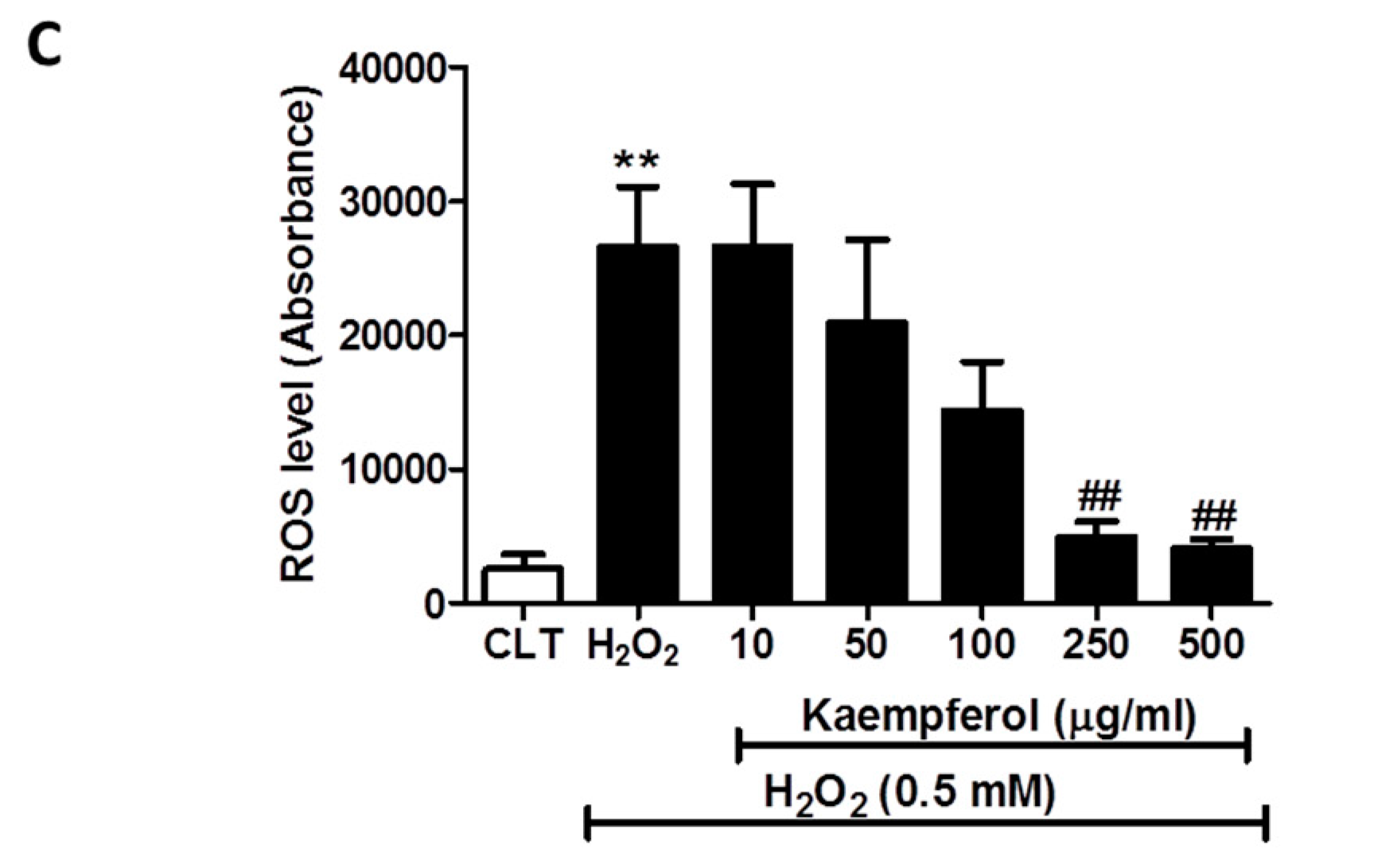
3.5. Effect of the NXQ Total Extract and the Two Major Components (Quercetin and Kaempferol) of NXQ on the Intracellular ROS Generation in H2O2-Treated EA.hy926 Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Manhas, N.; Raghubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.-W.; Chang, D.; Jenkins, A.; Bensoussan, A.; Kiat, H. Angiogenesis in Ischemic Stroke and Angiogenic Effects of Chinese Herbal Medicine. J. Clin. Med. 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Dyken, M.L. What lessons have we learned in the past 40 years? Stroke 2010, 41, 1073–1075. [Google Scholar] [CrossRef]
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. In Baylor University Medical Center Proceedings; Taylor & Francis: Milton Park, UK, 2011; Volume 24, pp. 257–259. [Google Scholar]
- Sandercock, P. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [PubMed]
- Gong, X.; Sucher, N.J. Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. Trends Pharmacol. Sci. 1999, 20, 191–196. [Google Scholar] [CrossRef]
- Arab, L.; Liu, W.; Elashoff, D. Green and black tea consumption and risk of stroke: A meta-analysis. Stroke 2009, 40, 1786–1792. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Vita, J.A. Grapes and cardiovascular disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wu, J.S.; Yang, S.T.; Huang, C.Y.; Chang, C.; Sun, G.Y.; Lin, T.N. Stroke, angiogenesis and phytochemicals. Front. Biosci. (Schol. Ed.) 2012, 4, 599–610. [Google Scholar]
- Bei, W.; Peng, W.; Ma, Y.; Xu, A. NaoXinQing, an anti-stroke herbal medicine, reduces hydrogen peroxide-induced injury in NG108-15 cells. Neurosci. Lett. 2004, 363, 262–265. [Google Scholar] [CrossRef]
- Bei, W.; Peng, W.; Ma, Y.; Xu, A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005, 76, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Wei, M. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, S.X.; Wang, H.Z.; Zhang, R.Q. Kakispyrol, a new biphenyl derivative from the leaves of Diospyros kaki. J. Asian Nat. Prod. Res. 2005, 7, 265–268. [Google Scholar] [CrossRef]
- Li, W.; Yi, S.; Wang, Z.; Chen, S.; Xin, S.; Xie, J.; Zhao, C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int. J. Pharm. 2011, 420, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Huang, S.W.; Wang, W.; Song, S.J. Triterpene saponins with neuroprotective effects from the leaves of Diospyros kaki Thunb. Fitoterapia 2018, 129, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.Y.; Ma, Y.J.; Zhang, L.; Wang, L.J.; Wu, X.F.; Liu, X.P. Flavonoids extracted from leaves of Diospyros kaki regulates RhoA activity to rescue synapse loss and reverse memory impairment in APP/PS1 mice. Neuroreport 2018, 29, 564–569. [Google Scholar] [CrossRef]
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.K. Effect of pre-treatment and extraction conditions on the antioxidant properties of persimmon (Diospyros kaki) leaves. Biosci. Biotechnol. Biochem. 2017, 81, 2079–2085. [Google Scholar] [CrossRef]
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Xu, S.; Kodama, H. Effect of five triterpenoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation in human polymorphonuclear leukocytes. Clin. Chim. Acta. 2002, 320, 11–16. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Luo, J.; Bei, W.-J.; Xu, T.; Huang, W.-H.; Li, M.M. Studies on quality specification of Naoxinqing tablets. Chin. New Drugs J. 2004, 13, 625–627. [Google Scholar]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.; Chang, D.; Ko, W.; Zhou, X.; Kiat, H.; Bensoussan, A.; Liu, J. Sailuotong Prevents Hydrogen Peroxide (H2O2)-Induced Injury in EA. hy926. Cells. Int. J. Mol. Sci. 2017, 18, 95. [Google Scholar] [CrossRef]
- Sabernavaei, M.; Kobarfard, F.; Hadjiakhoondi, A.; Aghaahmadi, M.; Amin, M.; Yassa, N. Biological Evaluation of the Isolated Compounds from Methanol Fraction of Leutea avicennia Mozaff. Iran. J. Pharm. Res. 2018, 17, 1386–1391. [Google Scholar]
- Yi, J.; Wu, J.G.; Wu, Y.B.; Peng, W. Antioxidant and Anti-proliferative Activities of Flavonoids from Bidens pilosa L. var radiata Sch Bip. Trop. J. Pharm. Res. 2016, 15, 341–348. [Google Scholar] [CrossRef]
- Park, S.N.; Kim, S.Y.; Lim, G.N.; Jo, N.R.; Lee, M.H. In vitro skin permeation and cellular protective effects of flavonoids isolated from Suaeda asparagoides extracts. J. Ind. Eng. Chem. 2012, 18, 680–683. [Google Scholar] [CrossRef]
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, H.-Z.; Liang, Y.-Z.; Liang, X.; Pang, J.-M.; Liao, J.-M. HPLC Determination of Protocatochuic Acid in Diangui’ainaxiang Pian. Guangzhou Chem. Ind. 2012, 19. [Google Scholar]
- Liang, J.Y.; Luo, J.; Yin, R.J.; Li, Y.; Wu, R.; Wang, D.Q. Simultaneous Determination of Quercetin and Kaempferol in Naoxinqing Tablets by HPLC. Pharm. Today 2012, 8. [Google Scholar]
- Wen, Y.; Dongxu, S.; Lishuang, D. Determination of Kaempferol in Naoxinqing Granules by HPLC. Chin. Pharm. Aff. 2011, 5, 25. [Google Scholar]
- Yin, R.; Wang, D.; Luo, J.; Li, Y.; Liang, J.; Li, C. Study on HPLC-DAD Fingerprint of Naoxinqing Tablets. Tradit. Chin. Drug Res. Clin. Pharm. 2013, 1, 21. [Google Scholar]
- Yin, R.J.; Li, C.Y.; Liang, J.Y.; Luo, J.; Li, Y.; Wu, R. Determination of Furoic Acid in Naoxinqing Tablets. Pharm. Today 2012, 3, 7. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agri. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Rezaei-Sadabady, R.; Eidi, A.; Zarghami, N.; Barzegar, A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 128–134. [Google Scholar] [CrossRef]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Libová, L.; Lukáč, N. Protective Effects of Quercetin on Selected Oxidative Biomarkers in Bovine Spermatozoa Subjected to Ferrous Ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Zholobenko, A.; Mouithys-Mickalad, A.; Modriansky, M.; Serteyn, D.; Franck, T. Polyphenols from Silybum marianum inhibit in vitro the oxidant response of equine neutrophils and myeloperoxidase activity. J. Vet. Pharmacol. Ther. 2016, 39, 592–601. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, G.B.; Sun, B.; Wu, Y.; He, L.; Wang, X.; Sun, X.B. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology 2012, 292, 53–62. [Google Scholar] [CrossRef]
- Miles, S.L.; McFarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: Need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arun, K.B.; Syama, H.P.; Nisha, P.; Sundaresan, A.; Kumar, T.S.; Jayamurthy, P. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014, 158, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
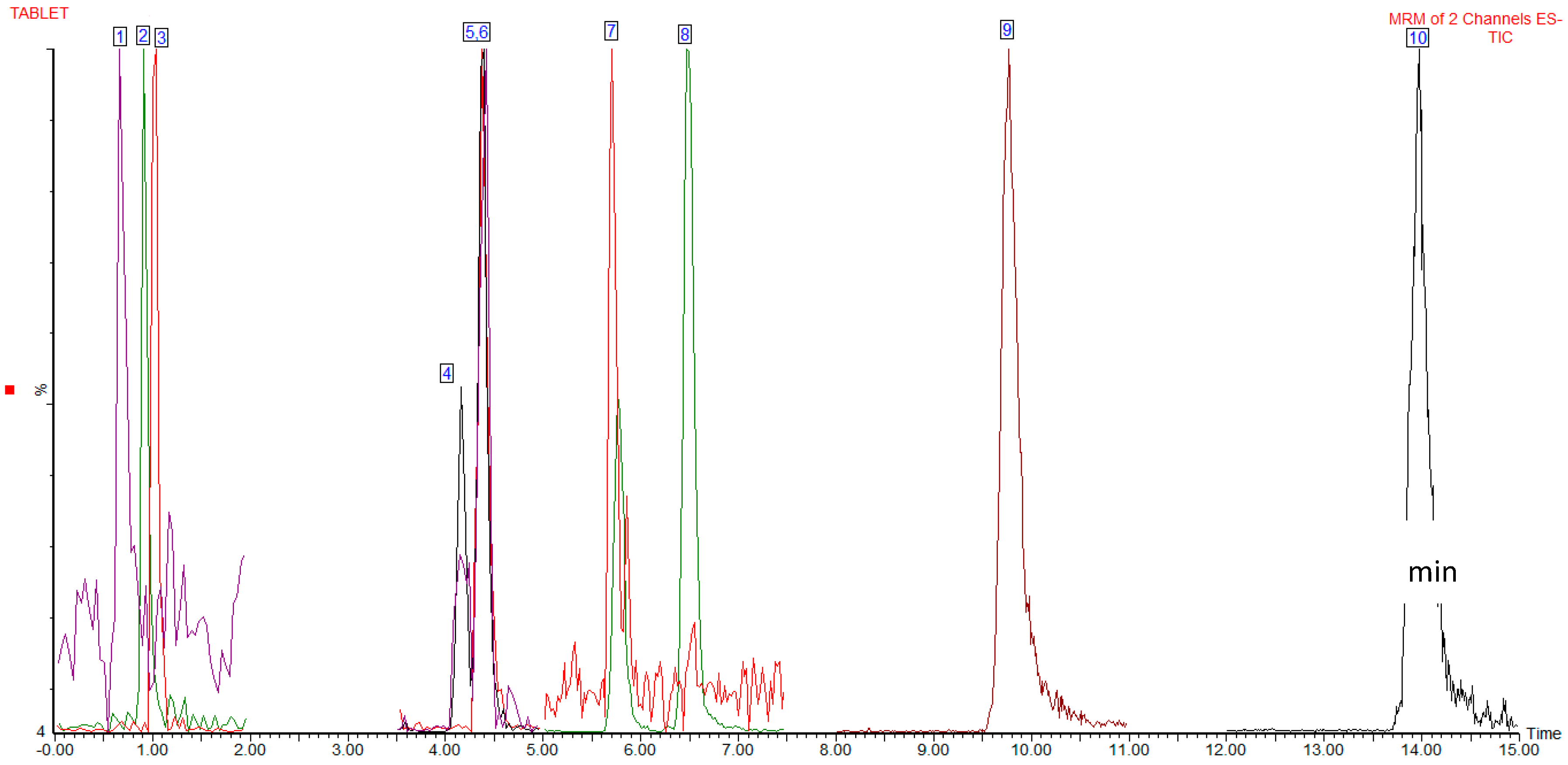

| Analytes | Energy for the Fragmentation (V) | Retention Time Window (min) |
|---|---|---|
| Kaempferol-3-O-glucoside | 30 | 5–7.5 |
| Quercetin-3-O-galactoside | 30 | 3.5–5 |
| Quercetin-3-O-glucoside | 35 | 3.5–5 |
| Kaempferol | 40 | 12–15 |
| 3,4-Dihydroxybenzoic acid | 15 | 0–2 |
| Furan-2-carboxylic acid | 10 | 0–2 |
| Quercetin | 20 | 8–11 |
| Analytes | m/z Ions | Relative Intensity (%) | |||
|---|---|---|---|---|---|
| Precursor [M−] | Product | Standard Peak 1 | Sample Peak 1 | Relative Difference 2 | |
| Kaempferol-3-O-glucoside | 447.75 | 285.05 | 100 | 100 | 0.00 |
| 255.93 | 27.3 | 26.94 | 1.32 | ||
| Quercetin-3-O-galactoside | 463.51 | 300.95 | 100 | 100 | 0.00 |
| 271.95 | 4.59 | 4.39 | 4.36 | ||
| Quercetin-3-O-glucoside | 463.7 | 301.07 | 100 | 100 | 0.00 |
| 271.88 | 30.16 | 30.91 | 2.49 | ||
| Kaempferol | 285.22 | 159.04 | 64.49 | 80.71 | 2.85 |
| 116.98 | 100 | 100 | 0.00 | ||
| 3,4-Dihydroxybenzoic acid | 153.89 | 110.03 | 100 | 100 | 0.00 |
| 109.02 | 24.37 | 12.37 | 4.92 | ||
| Furan-2-carboxylic acid | 111.89 | 67.88 | 100 | 100 | 0.00 |
| 66.81 | 18.32 | 17 | 7.21 | ||
| Quercetin | 301.42 | 178.82 | 42.93 | 39.9 | 7.06 |
| 150.88 | 100 | 100 | 0.00 | ||
| Quercetin-3-O-rutinoside3 | 609.08 | 299.86 | 100 | 100 | 0 |
| 271.02 | 0.91 | 4.93 | 45.05 | ||
| Succinic acid4 | 118 | 99.83 | 10.04 | 7.55 | 24.80 |
| 73.95 | 100 | 100 | 0 | ||
| Myricetin3 | 317.66 | 178.95 | 74.97 | 70.22 | 6.34 |
| 150.94 | 100 | 100 | 0 | ||
| Analytes | R2 1 | Concentration (mg g−1) ± SD2 | LOD/LOQ (mg g−1) 3 | Recovery (%) (±RSD) 5 | |||
|---|---|---|---|---|---|---|---|
| 50 % Spike | 100 % Spike | 200 % Spike | Average % | ||||
| Kaempferol-3-O-glucoside | 0.9998 | 3.12 ± 0.21 | 0.6/2.1 | 92.8 ± 0.8 | 111.0 ± 1.6 | 124.3 ± 0.5 | 109.4 ± 1.0 |
| Quercetin-3-O-galactoside | 0.9990 | 1.68 ± 0.14 | 0.4/1.4 | 84.3 ± 0.5 | 118.0 ± 1.6 | 94.7 ± 1.9 | 99.0 ± 1.3 |
| Quercetin-3-O-glucoside | 0.9998 | 1.65 ± 0.18 | 0.6/1.8 | 88.6 ± 1.4 | 99.9 ± 1.4 | 117.1 ± 0.7 | 101.9 ± 1.2 |
| Kaempferol | 0.9996 | 2.73 ± 0.15 | 0.5/1.5 | 90.4± 0.8 | 104.2 ± 2.6 | 119.7 ± 0.9 | 104.8 ± 1.4 |
| 3,4-Dihydroxybenzoic acid | 0.9963 | 0.52 ± 0.13 | 0.4/1.3 | 107.4 ± 1.7 | 121.8 ± 2.4 | 95.4 ± 1.1 | 108.2 ± 1.7 |
| Furan-2-carboxylic acid | 0.9987 | 5.19 ± 0.10 | 0.3/1.0 | 101.2 ± 5.3 | 106.7 ± 3.8 | 114.9 ± 4.2 | 107.6 ± 4.4 |
| Quercetin | 0.9999 | 6.43 ± 0.35 | 1.1/3.5 | 95.6 ± 1.3 | 96.5 ± 1.9 | 104.5 ± 1.2 | 98.8 ± 1.5 |
| Batch | Kaempferol-3-O-glucoside | Quercetin-3-O-galactoside | Quercetin-3-O-glucoside | Kaempferol | 3,4-Dihydroxybenzoic acid | Furan-2-carboxylic acid | Quercetin | DOM 2 |
|---|---|---|---|---|---|---|---|---|
| A-1 | 3.99 | 0.83 | 2.54 | 2.71 | 0.63 | 4.76 | 3.35 | 5/1/2011 |
| A-2 4 | 3.12 | 1.68 | 1.65 | 2.73 | 0.52 | 5.19 | 6.43 | 8/1/2011 |
| A-3 | 1.85 | 3.80 | 0.50 | 3.61 | 0.47 | 2.78 | 2.28 | 11/1/2011 |
| A-4 | 3.05 | 0.95 | 1.79 | 2.49 | 1.01 | 4.80 | 4.18 | 7/5/2011 |
| A-5 | 1.14 | 0.23 | 0.48 | 1.05 | <LOD | 5.29 | 4.39 | 5/6/2011 |
| A-6 | 1.02 | 0.10 | 0.43 | 1.00 | <LOQ | 5.57 | 6.55 | 24/6/2011 |
| A-7 | 1.05 | 0.27 | 0.34 | 0.96 | <LOQ | 1.46 | 3.54 | 2/8/2011 |
| A-8 | 0.82 | 0.20 | 0.24 | 0.89 | <LOQ | 3.92 | 4.03 | 17/8/2011 |
| B-1 | 1.27 | 0.39 | 0.69 | 1.15 | <LOQ | 2.44 | 3.13 | 11/1/2012 |
| B-2 | 1.65 | 0.30 | 0.87 | 1.47 | <LOQ | 3.57 | 5.28 | 19/5/2012 |
| B-3 | 1.86 | 0.28 | 0.99 | 1.54 | <LOQ | 3.68 | 5.48 | 20/5/2012 |
| B-4 | 1.56 | 0.31 | 0.79 | 1.29 | <LOQ | 3.64 | 4.30 | 21/5/2012 |
| B-5 | 1.68 | 0.49 | 0.92 | 1.35 | <LOQ | 3.41 | 4.51 | 26/5/2012 |
| B-6 | 1.47 | 0.13 | 0.79 | 1.32 | <LOQ | 3.14 | 5.87 | 28/5/2012 |
| B-7 | 1.45 | 0.45 | 0.82 | 1.32 | <LOQ | 3.53 | 4.10 | 29/5/2012 |
| Fold variation 3 | 4.87 | 36.61 | 10.58 | 4.08 | 33.67 5 | 3.82 | 2.87 |
| Rank | Analyte | DPPH Activity (EC50 µM ± SD) | ABTS Activity (EC50 µM ± SD) | Average Concentration (mg g−1) | Ranking Score 1 |
|---|---|---|---|---|---|
| 1 | Quercetin | 140 ± 2 | 81 ± 2 | 4.49 | 100 |
| 2 | Kaempferol | 119 ± 6 | 80 ± 3 | 1.66 | 37 |
| 3 | Quercetin-3-O-glucoside | 148 ± 2 | 68 ± 3 | 0.92 | 21 |
| 4 | Kaempferol-3-O-glucoside | >1000 | 164 ± 5 | 1.80 | 18 |
| 5 | Quercetin-3-O-galactoside | 175 ± 5 | 90 ± 1 | 0.69 | 15 |
| 6 | 3,4-Dihydroxybenzoic acid | 181 ± 10 | 350 ± 13 | 0.20 | 4 |
| 7 | Furan-2-carboxylic acid | >1000 | >1000 | 3.81 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazzem, M.; Sun, Y.-T.; Low, M.; Seto, S.W.; Chang, D.; Lee, S.; Suresh, H.; Khoo, C.S.; Bensoussan, A.; Kiat, H. Chromatographic Analysis and Anti-Oxidative Property of Naoxinqing Tablet, a Proprietary Preparation of Diospyros Kaki Leaves. Molecules 2019, 24, 1101. https://doi.org/10.3390/molecules24061101
Kazzem M, Sun Y-T, Low M, Seto SW, Chang D, Lee S, Suresh H, Khoo CS, Bensoussan A, Kiat H. Chromatographic Analysis and Anti-Oxidative Property of Naoxinqing Tablet, a Proprietary Preparation of Diospyros Kaki Leaves. Molecules. 2019; 24(6):1101. https://doi.org/10.3390/molecules24061101
Chicago/Turabian StyleKazzem, Magdy, Yu-Ting Sun, Mitchell Low, Sai Wang Seto, Dennis Chang, Samiuela Lee, Harsha Suresh, Cheang S. Khoo, Alan Bensoussan, and Hosen Kiat. 2019. "Chromatographic Analysis and Anti-Oxidative Property of Naoxinqing Tablet, a Proprietary Preparation of Diospyros Kaki Leaves" Molecules 24, no. 6: 1101. https://doi.org/10.3390/molecules24061101






