Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice
Abstract
:1. Introduction
2. Results
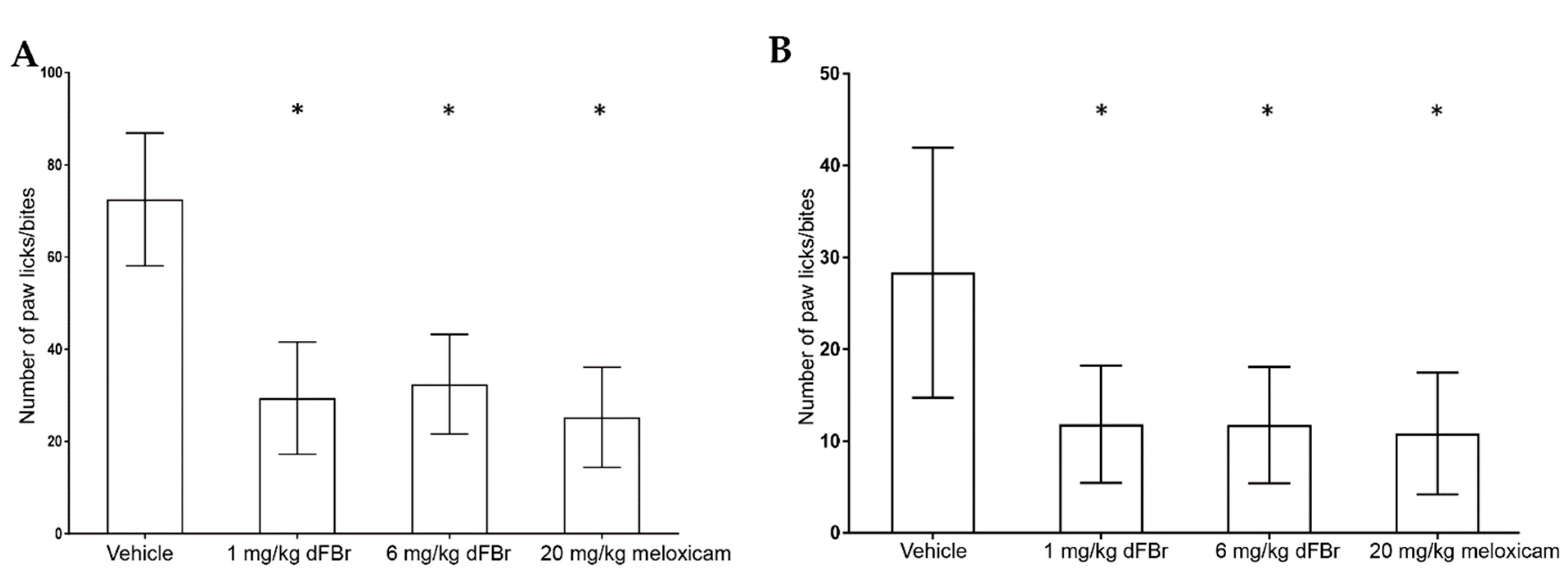
2.1. Formalin-Induced Pain Test
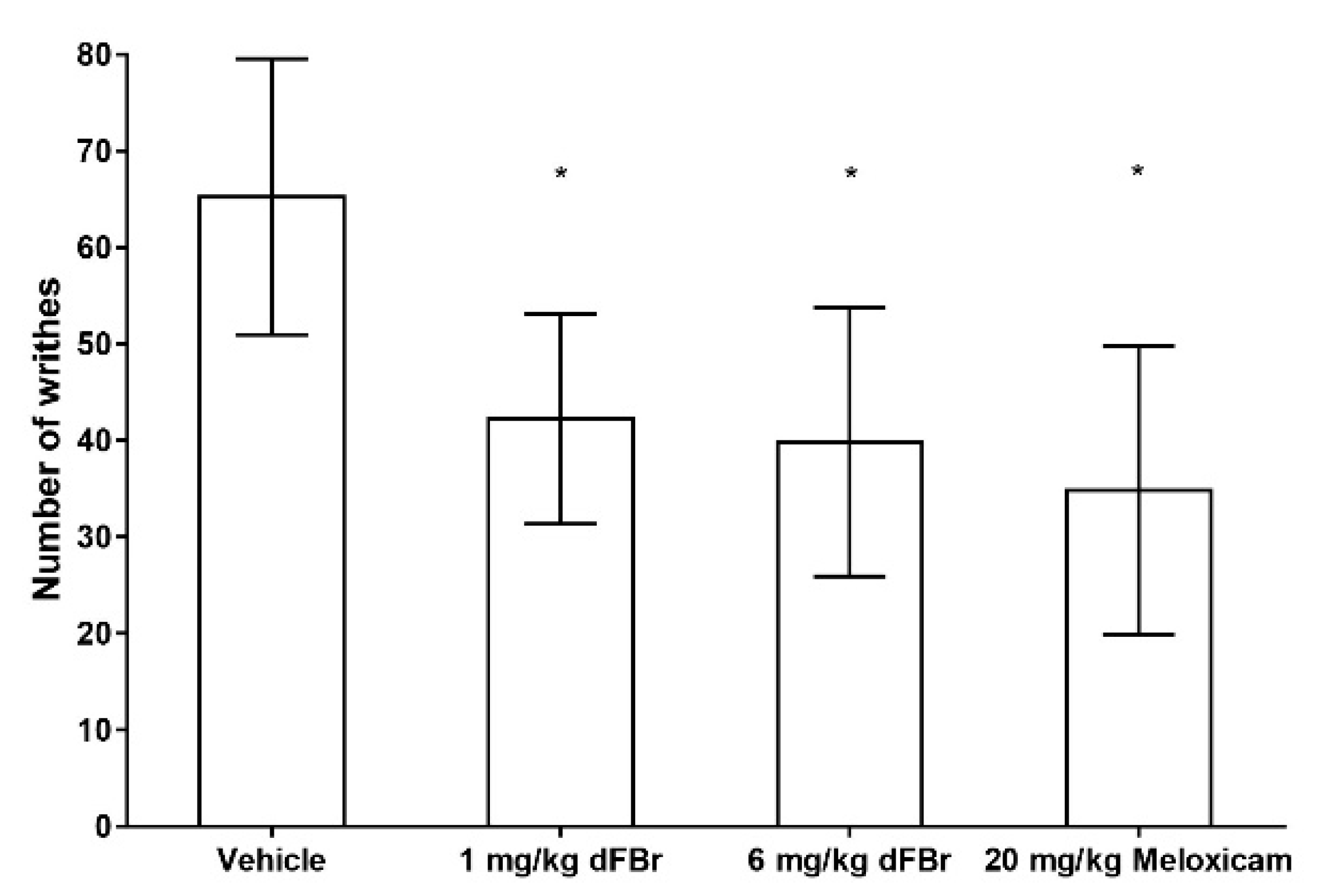
2.2. Acetic Acid-Induced Writhing Response Test
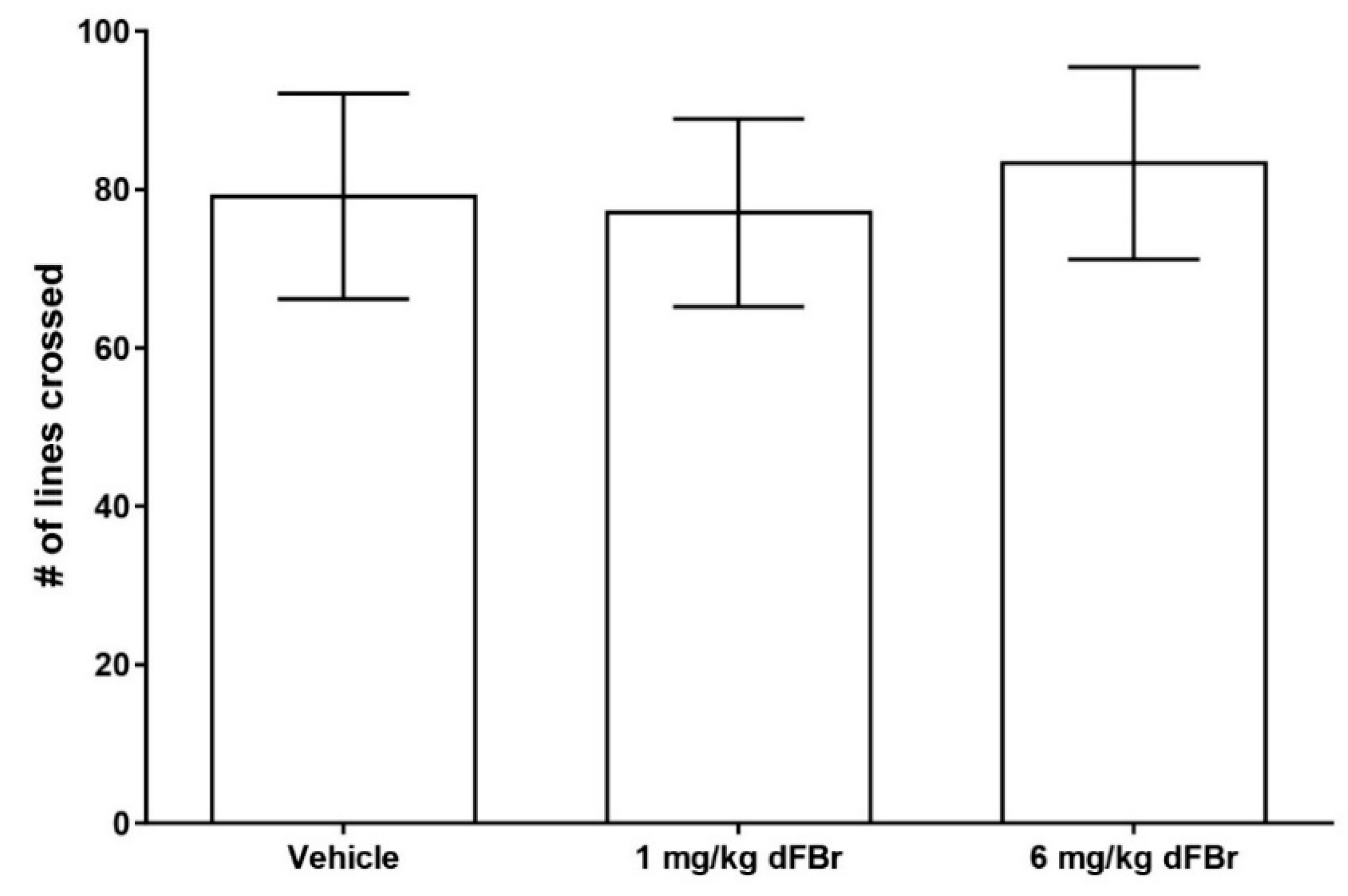
2.3. Open Field Exploration Test
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. Drug Treatment
4.3. Formalin-Induced Pain Test
4.4. Acetic Acid-Induced Writhing Response Test
4.5. Open Field Exploration Test
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Backonja, M.M. Defining neuropathic pain. Anesth. Analg. 2003, 97, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicol, E.; Strassels, S.; Goudas, L.; Lau, J.; Carr, D. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: A systematic review. J. Clin. Oncol. 2004, 22, 1975–1992. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Semble, E.L.; Wu, W.C. NSAID-induced gastric mucosal damage. Am. Fam. Physician 1987, 35, 101–108. [Google Scholar] [PubMed]
- Law, P.Y.; Reggio, P.H.; Loh, H.H. Opioid receptors: Toward separation of analgesic from undesirable effects. Trends Biochem. Sci. 2013, 38, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bannon, A.W.; Decker, M.W.; Holladay, M.W.; Curzon, P.; Donnelly-Roberts, D.; Puttfarcken, P.S.; Bitner, R.S.; Diaz, A.; Dickenson, A.H.; Porsolt, R.D.; et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science 1998, 279, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Umana, I.C.; Daniele, C.A.; McGehee, D.S. Neuronal nicotinic receptors as analgesic targets: it’s a winding road. Biochem. Pharmacol. 2013, 86, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Goura, V.; Abraham, R.; Jayarajan, P. α4β2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur. J. Pharmacol. 2013, 712, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Cucchiaro, G.; Commons, K.G. Alpha 4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse 2003, 49, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Furue, H.; Yoshimura, M.; Ueda, H. Tonic inhibitory role of α4β2 subtype of nicotinic acetylcholine receptors on nociceptive transmission in the spinal cord in mice. Pain 2006, 125, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Webb, J.K.; Shepheard, S.L.; Russell, M.G.; Hill, R.G.; Rupniak, N.M. Analgesic and toxic effects of ABT-594 resemble epibatidine and nicotine in rats. Pain 2000, 85, 443–450. [Google Scholar] [CrossRef]
- Damaj, M.I.; Glassco, W.; Aceto, M.D.; Martin, B.R. Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J. Pharmacol. Exp. Ther. 1999, 291, 390–398. [Google Scholar] [PubMed]
- Lawand, N.B.; Lu, Y.; Westlund, K.N. Nicotinic cholinergic receptors: Potential targets for inflammatory pain relief. Pain 1999, 80, 291–299. [Google Scholar] [CrossRef]
- Kesingland, A.C.; Gentry, C.T.; Panesar, M.S.; Bowes, M.A.; Vernier, J.M.; Cube, R.; Walker, K.; Urban, L. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain 2000, 86, 113–118. [Google Scholar] [CrossRef]
- Cucchiaro, G.; Xiao, Y.; Gonzalez-Sulser, A.; Kellar, K.J. Analgesic effects of Sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology 2008, 109, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.A.; Yakel, J.L. Activation of the α7 nicotinic ACh receptor induces anxiogenic effects in rats which is blocked by a 5-HT1a receptor antagonist. Neuropharmacology 2013, 70, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.A.; Yakel, J.L. Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies. Biochem. Pharmacol. 2013, 86, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.Z.; Chin, C.L.; Rustay, N.R.; Zhong, C.; Mikusa, J.; Chandran, P.; Salyers, A.; Gomez, E.; Simler, G.; Lewis, L.G.; et al. Potentiation of analgesic efficacy but not side effects: Co-administration of an α4β2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem. Pharmacol. 2011, 82, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Rode, F.; Munro, G.; Holst, D.; Nielsen, E.O.; Troelsen, K.B.; Timmermann, D.B.; Ronn, L.C.; Grunnet, M. Positive allosteric modulation of α4β2 nAChR agonist induced behaviour. Brain Res. 2012, 1458, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Yakel, J.L. Allosteric modulator Desformylflustrabromine relieves the inhibition of α2β2 and α4β2 nicotinic acetylcholine receptors by β-amyloid1–42 peptide. J. Mol. Neurosci. 2011, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Yakel, J.L. Allosteric modulators of the α4β2 subtype of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2011, 82, 952–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandya, A.A. Desformylflustrabromine: A Novel Positive Allosteric Modulator for beta2 Subunit Containing Nicotinic Receptor Sub-Types. Curr. Pharm. Des. 2016, 22, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Ergun, D.; Jackson, A.; Toma, W.; Schulte, M.K.; Damaj, M.I. Allosteric modulation of α4β2* nicotinic acetylcholine receptors: Desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. Eur. J. Pain 2018, 22, 84–93. [Google Scholar] [CrossRef] [PubMed]
- King, S.L.; Caldarone, B.J.; Picciotto, M.R. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology 2004, 47 (Suppl. 1), 132–139. [Google Scholar] [CrossRef]
- Panagis, G.; Nisell, M.; Nomikos, G.G.; Chergui, K.; Svensson, T.H. Nicotine injections into the ventral tegmental area increase locomotion and Fos-like immunoreactivity in the nucleus accumbens of the rat. Brain Res. 1996, 730, 133–142. [Google Scholar] [CrossRef]
- Weltzin, M.M.; Schulte, M.K. Desformylflustrabromine Modulates α4β2 Neuronal Nicotinic Acetylcholine Receptor High- and Low-Sensitivity Isoforms at Allosteric Clefts Containing the β2 Subunit. J. Pharmacol. Exp. Ther. 2015, 354, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Mucha, M.; Khatri, S.N.; Glenon, R.; Schulte, M.K.; Bult-Ito, A. Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Front. Behav. Neurosci. 2016, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Zoli, M.; Rimondini, R.; Lena, C.; Marubio, L.M.; Pich, E.M.; Fuxe, K.; Changeux, J.P. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 1998, 391, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Benwell, M.E.; Balfour, D.J. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br. J. Pharmacol. 1992, 105, 849–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, M.F.; Dourish, C.T.; Iversen, S.D. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology 1991, 104, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Govind, A.P.; Vezina, P.; Green, W.N. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009, 78, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonnacott, S.; Sidhpura, N.; Balfour, D.J. Nicotine: From molecular mechanisms to behaviour. Curr. Opin. Pharmacol. 2005, 5, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Gallagher, K.; McGehee, D.S. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J. Neurosci. 2011, 31, 6710–6720. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Positive allosteric modulation of α4β2 nicotinic acetylcholine receptors as a new approach to smoking reduction: Evidence from a rat model of nicotine self-administration. Psychopharmacology 2013, 230, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Jang, I.S. Presynaptic nicotinic acetylcholine receptors enhance GABAergic synaptic transmission in rat periaqueductal gray neurons. Eur. J. Pharmacol. 2010, 640, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Genzen, J.R.; McGehee, D.S. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2005, 1031, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Charlet, A.; Cordero-Erausquin, M.; Tessier, L.H.; Picciotto, M.R.; Schlichter, R.; Poisbeau, P.; Freund-Mercier, M.J.; Barrot, M. Nociceptive thresholds are controlled through spinal β2-subunit-containing nicotinic acetylcholine receptors. Pain 2011, 152, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Eisenach, J.C. Nicotinic acetylcholine receptor regulation of spinal norepinephrine release. Anesthesiology 2002, 96, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Rueter, L.E.; Meyer, M.D.; Decker, M.W. Spinal mechanisms underlying A-85380-induced effects on acute thermal pain. Brain Res. 2000, 872, 93–101. [Google Scholar] [CrossRef]
- Kimura, S.; Kontani, H. Demonstration of antiallodynic effects of the cyclooxygenase-2 inhibitor meloxicam on established diabetic neuropathic pain in mice. J. Pharmacol. Sci. 2009, 110, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I. Synergistic interaction between metformin and sulfonylureas on diclofenac-induced antinociception measured using the formalin test in rats. Pain Res. Manag. 2013, 18, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Since, M.; Freret, T.; Nee, G.; Terme, T.; Vanelle, P.; Boulouard, M. New orally effective 3-(2-nitro)phenylpropanamide analgesic derivatives: Synthesis and antinociceptive evaluation. Eur. J. Med. Chem. 2013, 69, 728–734. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weggel, L.A.; Pandya, A.A. Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice. Molecules 2019, 24, 944. https://doi.org/10.3390/molecules24050944
Weggel LA, Pandya AA. Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice. Molecules. 2019; 24(5):944. https://doi.org/10.3390/molecules24050944
Chicago/Turabian StyleWeggel, Loni A., and Anshul A. Pandya. 2019. "Acute Administration of Desformylflustrabromine Relieves Chemically Induced Pain in CD-1 Mice" Molecules 24, no. 5: 944. https://doi.org/10.3390/molecules24050944



