Characterization and Structure–Property Relationships of Organic–Inorganic Hybrid Composites Based on Aluminum–Magnesium Hydroxycarbonate and Azo Chromophore
Abstract
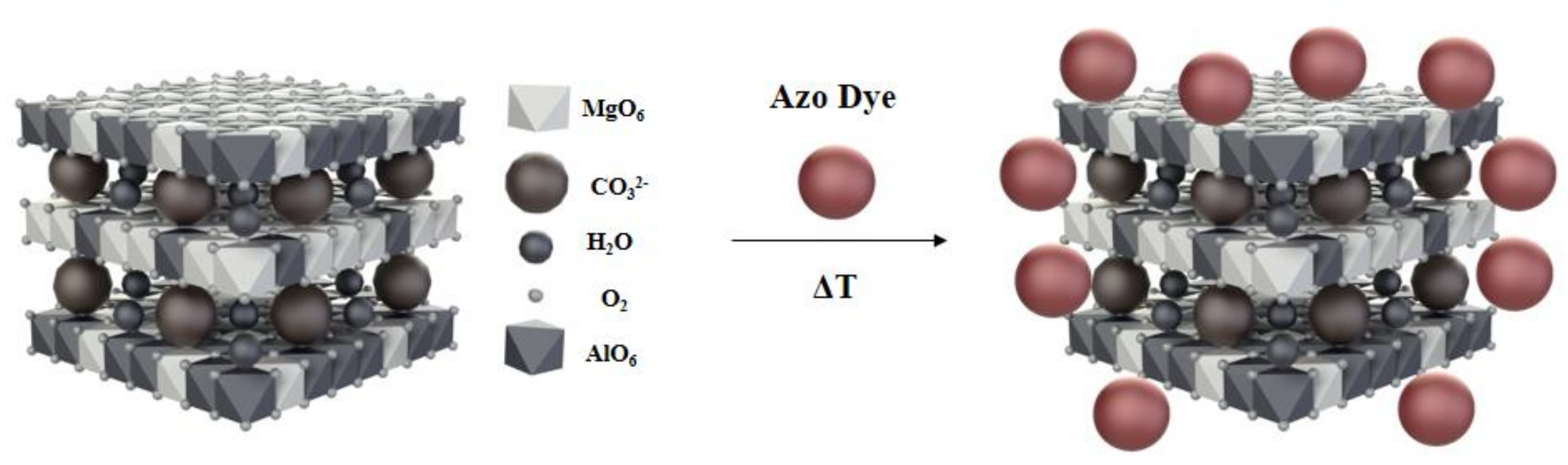
:1. Introduction
2. Results and Discussion
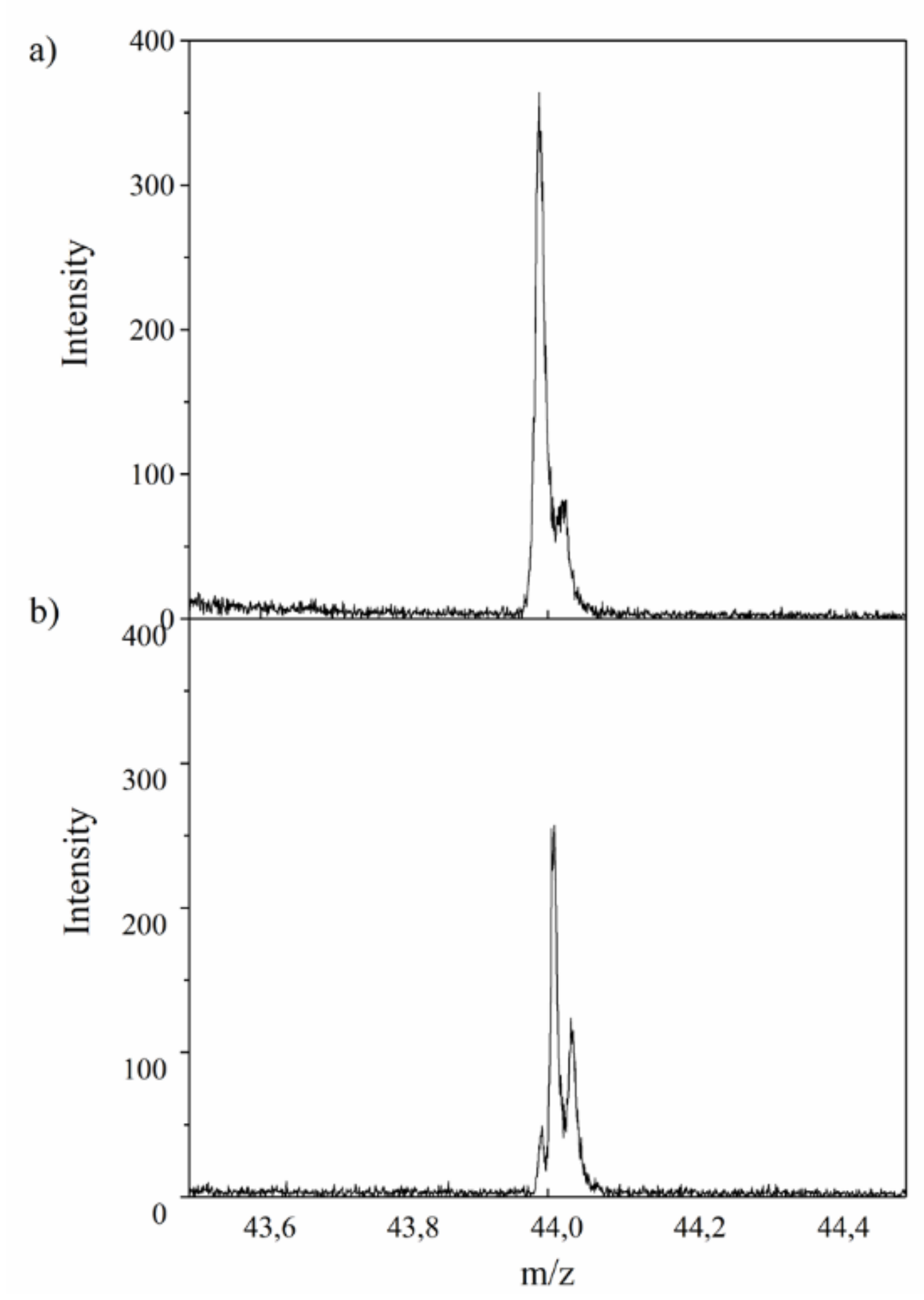
2.1. Secondary Ion Mass Spectrometry (TOF-SIMS)
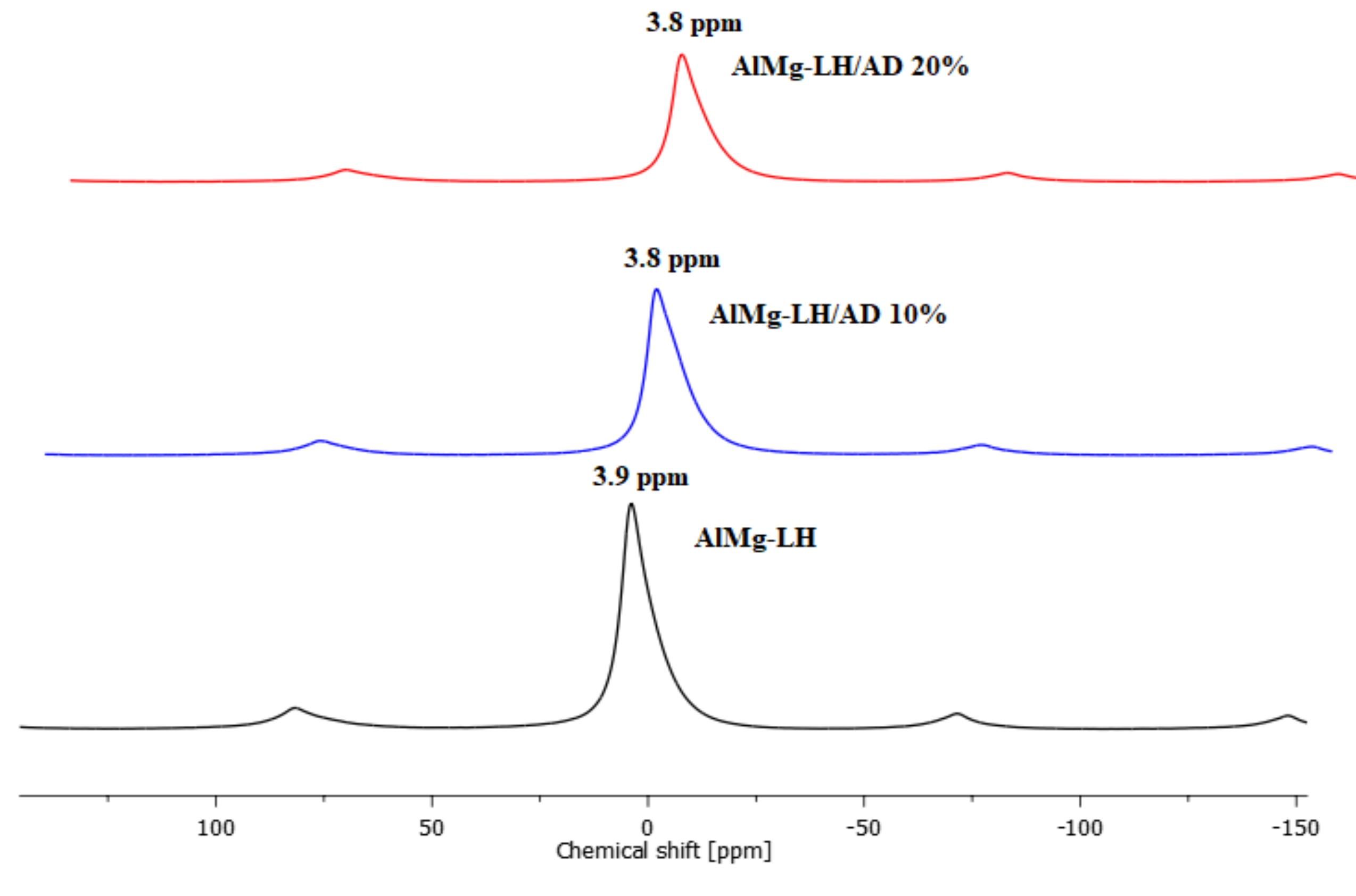
2.2. Solid State Nuclear Magnetic Resonance of 27Al (MAS NMR)
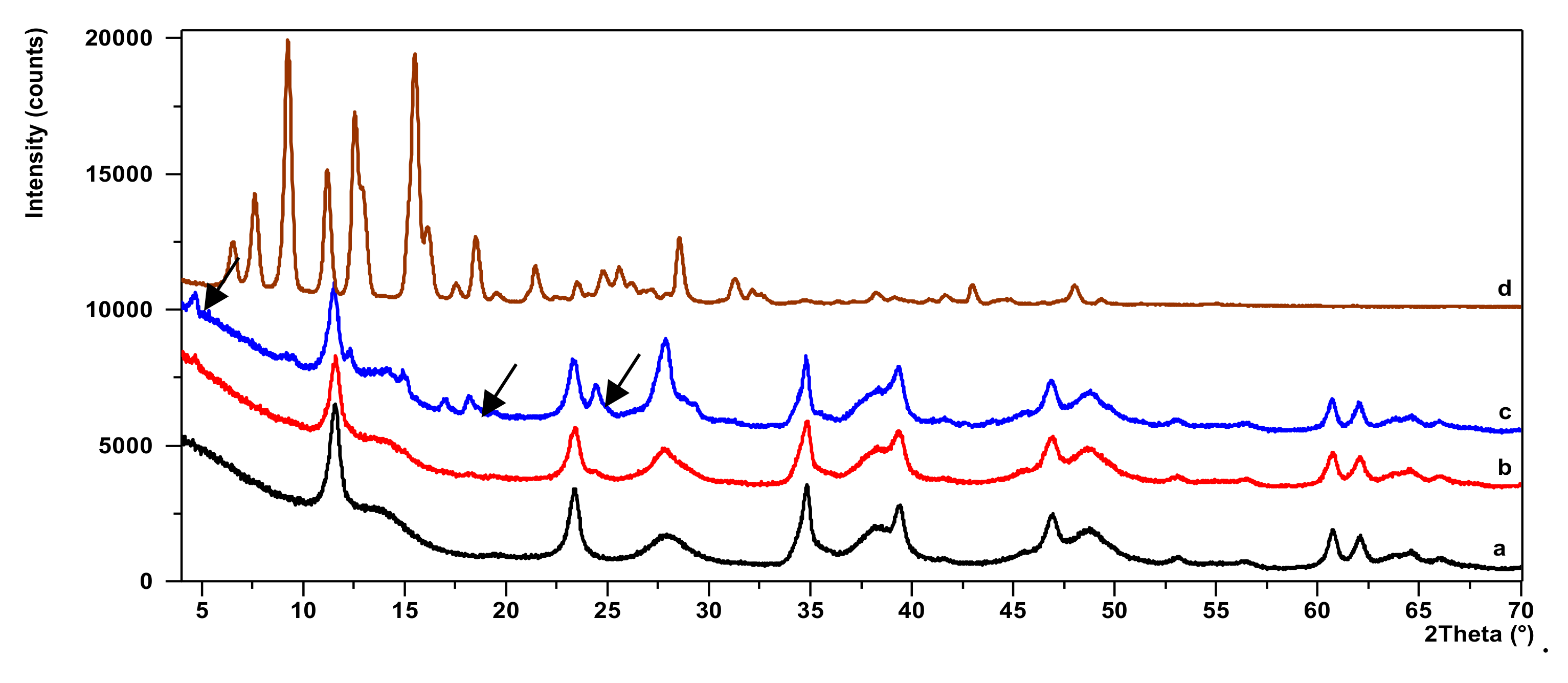
2.3. X-ray Diffraction Study (XRD)
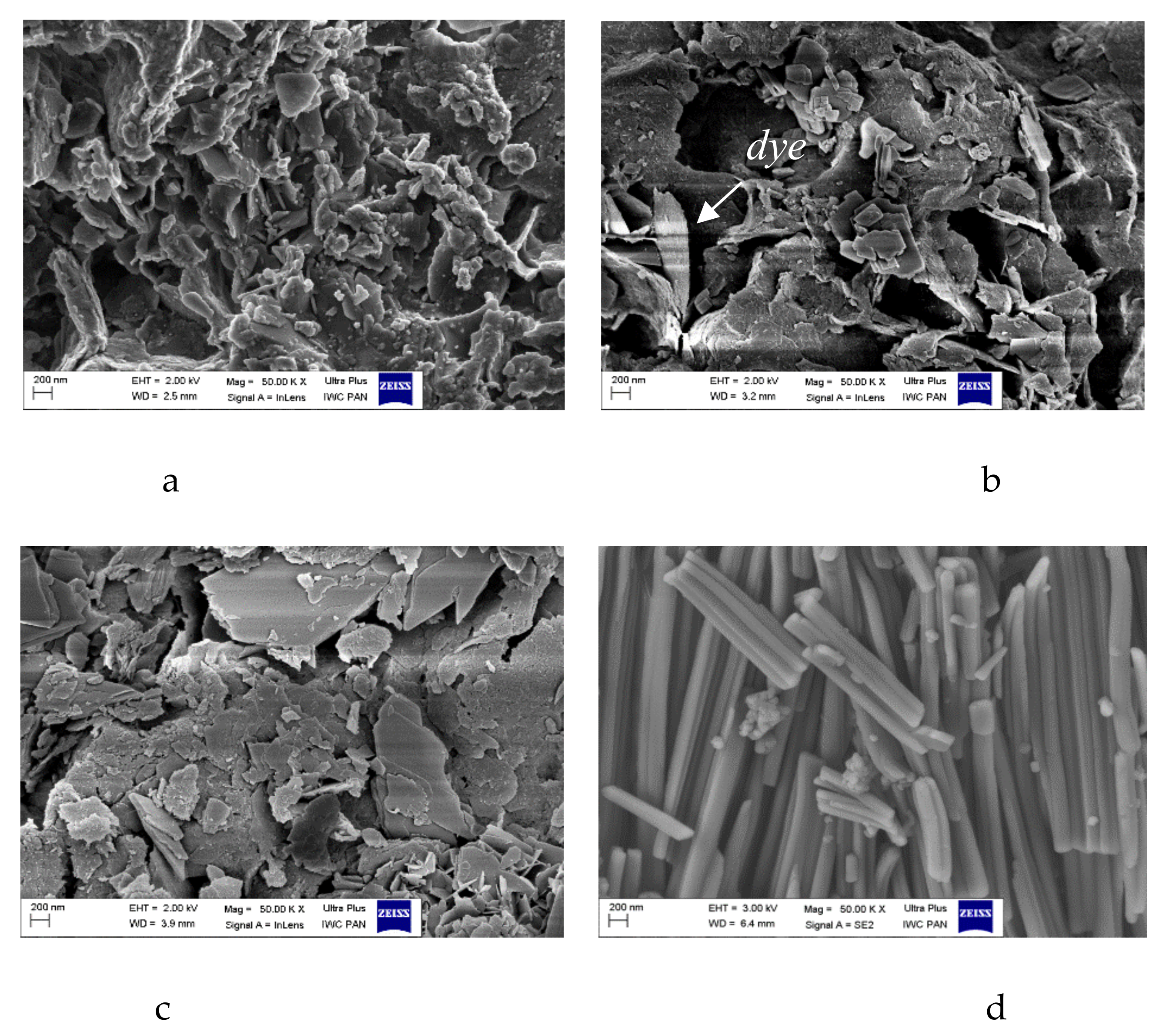
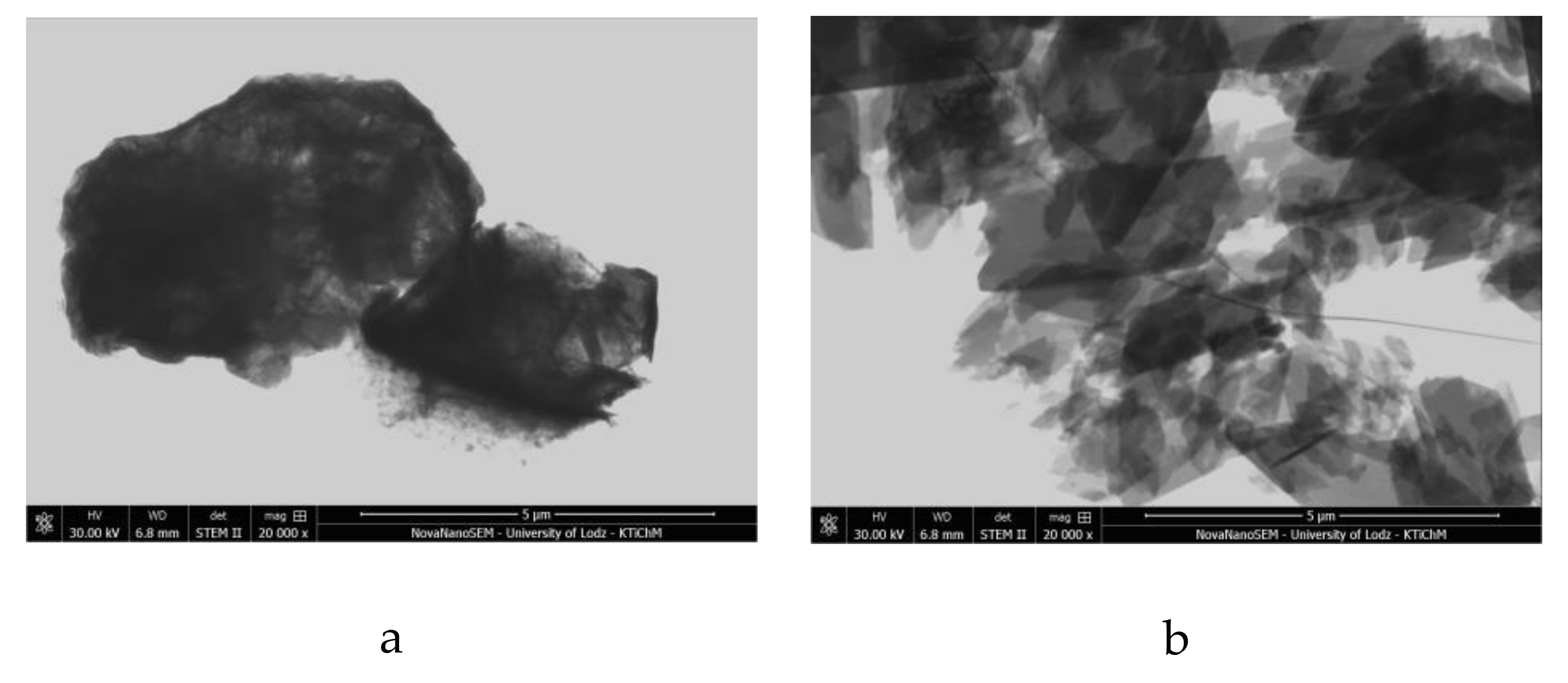
2.4. Morphology of Hybrid Pigment (SEM/STEM)
2.5. Solvent Resistance
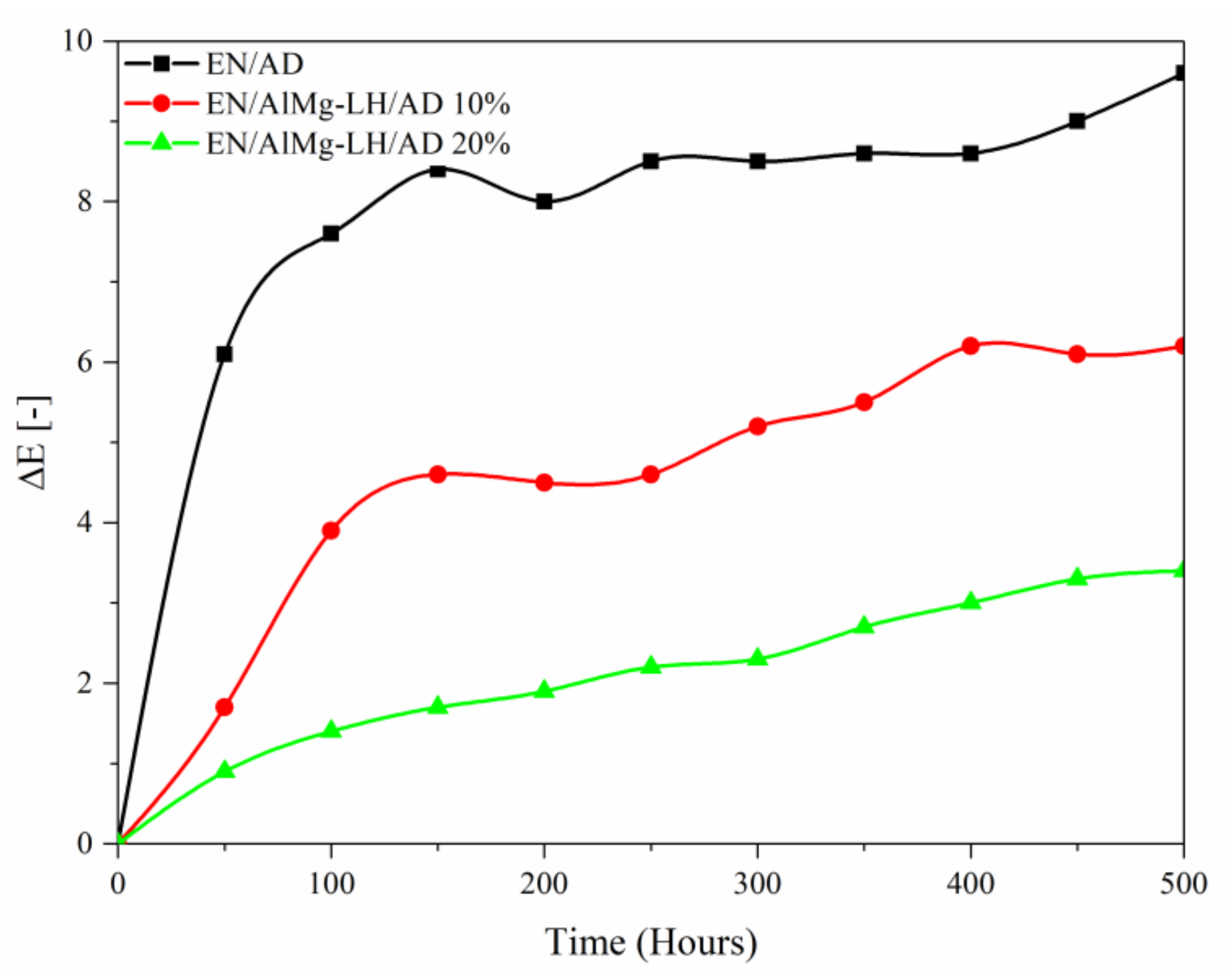
2.6. Thermal Analysis (TGA) and Photostability of Hybrid Pigments
3. Experimental
3.1. Materials
3.1.1. Azo Dye Synthesis
3.1.2. Hybrid Pigment Preparation
3.1.3. Preparation of Ethylene-Norbornene (EN) Composites
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tang, P.; Feng, Y.; Li, D. Improved thermal and photostability of an anthraquinone dye by intercalation in a zinc-aluminum layered double hydroxides host. Dyes Pigm. 2012, 94, 437–442. [Google Scholar] [CrossRef]
- Liu, P.; Liu, P.; Zhao, K.; Li, L. Photostability enhancement of azoic dyes adsorber and intercalated into Mg-Al-layered double hydroxide. Opt. Laser. Technol. 2015, 74, 23–28. [Google Scholar] [CrossRef]
- Pardo, R.; Zayat, M.; Levy, D. Photochromic organic–inorganic hybrid materials. Chem. Soc. Rev. 2011, 40, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Feng, Y.; Li, D. Facile synthesis of multicolor organic–inorganic hybrid pigments based on layered double hydroxides. Dyes Pigm. 2014, 104, 131–136. [Google Scholar] [CrossRef]
- Shi, W.; He, S.; Wei, M.; Evans, D.; Duan, X. Optical pH sensor with rapid response based on a fluorescein-intercalated layered double hydroxide. Adv. Funct. Mater. 2010, 20, 3856–3863. [Google Scholar] [CrossRef]
- Latterini, L.; Nocchetti, M.; Aloisi, G.G.; Costantino, U.; Elisei, F. Organized chromophores in layered inorganic matrices. Inorg. Chim. Acta. 2007, 360, 728–740. [Google Scholar] [CrossRef]
- Martinez-Zapata, O.; Mendez-Vivar, J.; Bosch, P.; Lara, VH. Synthesis and characterization of amorphous aluminosilicates prepared by sol-gel to encapsulate organic dyes. J. Non-Cryst Solids 2011, 357, 3480–3485. [Google Scholar] [CrossRef]
- Perez, E.; Lima, E.; Guzman, A. Natural betalains supported on γ-alumina: A wide family of stable pigments. Dyes Pigm. 2015, 120, 161–168. [Google Scholar] [CrossRef]
- Jesionowski, T. Characterisation of pigments obtained by adsorption of CI Basic Blue 9 and CI Acid Orange 52 dyes onto silica particles precipitated via the emulsion route. Dyes Pigm. 2005, 67, 81–92. [Google Scholar] [CrossRef]
- Loera, S.; Ibarra, I.A.; Laguna, H.; Lima, E.; Bosch, P.; Lara, V.; Haro-Poniatowski, E. Colored sodalite and A zeolites. Ind. Eng. Chem. Res. 2006, 45, 9195–9200. [Google Scholar] [CrossRef]
- Maas, H.; Khatyr, A.; Calzaferri, G. Phenoxazine dyes in zeolite L, synthesis and properties. Microporous Mesoporous Mater. 2003, 65, 233–242. [Google Scholar] [CrossRef]
- Hwang, S.H.; Jung, S.C.; Yoon, S.M.; Kim, D.K. Preparation and characterization of dye-intercalated ZnAl-layered double hydroxide and its surface modification by silica coating. J. Solid State Chem. 2008, 69, 1061–1065. [Google Scholar] [CrossRef]
- Kohno, Y.; Totsuka, K.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Photostability enhancement of anionic natural dye by intercalation into hydrotalcite. J. Colloid Interface Sci. 2009, 337, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, S.A.; Tavares, S.R.; Leitao, A.A.; Wypych, F. Intercalation of indigo carmine anions into zinc hydroxide salt: a novel alternative blue pigment. Dyes Pigm. 2016, 128, 158–164. [Google Scholar] [CrossRef]
- Bourhis, K.; Blanc, S.; Mathe, C.; Dupin, JC.; Vieillescazes, C. Spectroscopic and chromatographic analysis of yellow flavonoidic lakes: quercitin chromophore. Appl. Clay. Sci. 2011, 53, 598–607. [Google Scholar] [CrossRef]
- Doskocz, M.; Kubas, K.; Frackowiak, A.; Gancarz, R. NMR and ab initio studies of Mg2+, Ca2+, Zn2+, Cu2+ alizarin complexes. Polyhedron 2009, 28, 2201–2205. [Google Scholar] [CrossRef]
- Deveoglu, O.; Cakmakc, E.; Taskopru, T.; Torgan, E.; Karadag, R. Identification by RP-HPLC-DAD, FTIR, TGA and FESEM-EDAX of natural pigments prepared from Datisca cannabinaL. Dyes Pigm. 2012, 94, 437–442. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Yahaya, A.H.; Ping, L.M. Dye-interleaved nanocomposite: Evan’s Blue in the lamella of Mg-Al-layered double hydroxide. Dyes Pigm. 2004, 63, 135–140. [Google Scholar] [CrossRef]
- Guillermin, D.; Debroise, T.; Trigueiro, P.; de Viguerie, L.; Rigaud, B.; Morlet-Savary, F.; Balme, S.; Janot, J.M.; Tielens, F.; Michot, L.; et al. New pigments based on carminic acid and smectites: A molecular investigation. Dyes Pigm. 2019, 160, 971–982. [Google Scholar] [CrossRef]
- Perez, E.; Ibarra, I.A.; Guzman, A.; Lima, E. Hybrid pigments resulting from several guest dyes onto γ-alumina host: A spectroscopic analysis. Spectrochim. Acta A. 2017, 172, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Girdthep, S.; Sirirak, J.; Daranarong, D.; Daengngern, R.; Chayabutra, S. Physico-chemical characterization of natural lake pigments obtained from Caesalpinia Sappan Linn. and their composite films for poly(lactic acid)-based packaging material. Dyes Pigm. 2018, 157, 27–39. [Google Scholar] [CrossRef]
- Marangoni, R.; Ramos, L.P.; Wypych, F. New multifunctional materials obtained by the intercalation of anionic dyes into layered zinc hydroxide nitrate followed by dispersion into poly(vinyl alcohol)(PVA). J. Colloid. Interface Sci. 2009, 330, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Xu, X.; Lin, Y.; Li, D. Enhancement of the thermo- and photo-stability of an anionic dye by intercalation in a zinc-aluminum layered double hydroxide host. End. Eng. Chem. Res. 2008, 47, 2478–2483. [Google Scholar] [CrossRef]
- Chakraborty, C.; Dana, K.; Malik, S. Intercalation of perylenediimde dye into LDH clays: enhancement of photostability. J. Phys. Chem. C. 2010, 115, 1996–2004. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirb, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Evans, D.G.; Duan, X. Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine. Chem. Commun. 2006, 5, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prevot, V.; Taviot-Gueho, C. Handbook of Clay Science. 2006, 745–782. [Google Scholar]
- Sun, Z.; Jin, L.; Shi, W.; Wei, M.; Duan, X. Preparation of an anion dye intercalated into layered double hydroxides and its controllable luminescence properties. Chem. Eng. J. 2010, 161, 293–300. [Google Scholar] [CrossRef]
- Bauer, J.; Behrens, P.; Speckbacher, M.; Langhals, H. Composites of perylene chromophores and layered double hydroxides: direct synthesis, characterization, and photo- and chemical stability. Adv. Funct. Mater. 2003, 13, 241–248. [Google Scholar] [CrossRef]
- Kutlu, B.; Leuteritz, A.; Häußler, L.; Oertel, U.; Heinrich, G. Stabilization of polypropylene using dye modified layered double hydroxides. Polym. Degrad. Stab. 2014, 102, 9–14. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Stewart, H.; Eremin, K.; Stenger, J. Lithol red: A systematic structural study on salts of a sulfonated azo pigment. Chem. Eur. J. 2012, 18, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.M.; Mackay, J.L. Metal salt azo pigments. Color. Technol. 2008, 124, 133–134. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Moszyński, M.; Kozanecki, M.; Zaborski, M. Characterization and properties of new color-tunable hybrid pigments based on layered double hydroxides (LDH) and 1,2-dihydroxyanthraquinone dye. J. Ind. Eng. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Fournier, F.; Viguerie, L.; Balme, S.; Janot, J.M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay. Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Park, T.J.; Choi, S.S.; Kim, Y. 27Al solid-state NMR structural studies of hydrotalcite compounds calcined at at different temperatures. Bull. Korean Chem. Soc. 2009, 30, 149–152. [Google Scholar]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kushwaha, P.K.; Srivastava, V.K.; Bhatt, S.D.; Jasra, R.V. Effect of hydrothermal conditions on structural and textural properties of synthetic hydrotalcites of varying Mg/Al ratio. Ind. Eng. Chem. Res. 2007, 46, 4856–4865. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg-Al layered double hydroxide by anionic surfactants: preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Tewari, A.K.; Mishra, A. Synthesis and antiviral activities of N-substituted-2-substituted benzimidazole derivatives. Indian J. Chem. Sec. B. 2006, 45, 489–493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Hybrid Pigment | Solvent Resistance | ||||
|---|---|---|---|---|---|
| Water | Acetone | Toluene | Ethanol | Butyl Acetate | |
| AlMg−LH/AD 10% | 5 | 5 | 5 | 5 | 5 |
| AlMg−LH/AD 20% | 5 | 5 | 5 | 5 | 5 |
| Sample | T051 (°C) | T101 (°C) | T201 (°C) | T301 (°C) |
|---|---|---|---|---|
| AlMg−LH | 119 | 205 | 330 | 441 |
| AlMg−LH/AD 10% | 131 | 214 | 353 | 466 |
| AlMg−LH/AD 20% | 145 | 217 | 370 | 488 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Zaborski, M. Characterization and Structure–Property Relationships of Organic–Inorganic Hybrid Composites Based on Aluminum–Magnesium Hydroxycarbonate and Azo Chromophore. Molecules 2019, 24, 880. https://doi.org/10.3390/molecules24050880
Marzec A, Szadkowski B, Rogowski J, Maniukiewicz W, Zaborski M. Characterization and Structure–Property Relationships of Organic–Inorganic Hybrid Composites Based on Aluminum–Magnesium Hydroxycarbonate and Azo Chromophore. Molecules. 2019; 24(5):880. https://doi.org/10.3390/molecules24050880
Chicago/Turabian StyleMarzec, Anna, Bolesław Szadkowski, Jacek Rogowski, Waldemar Maniukiewicz, and Marian Zaborski. 2019. "Characterization and Structure–Property Relationships of Organic–Inorganic Hybrid Composites Based on Aluminum–Magnesium Hydroxycarbonate and Azo Chromophore" Molecules 24, no. 5: 880. https://doi.org/10.3390/molecules24050880
APA StyleMarzec, A., Szadkowski, B., Rogowski, J., Maniukiewicz, W., & Zaborski, M. (2019). Characterization and Structure–Property Relationships of Organic–Inorganic Hybrid Composites Based on Aluminum–Magnesium Hydroxycarbonate and Azo Chromophore. Molecules, 24(5), 880. https://doi.org/10.3390/molecules24050880