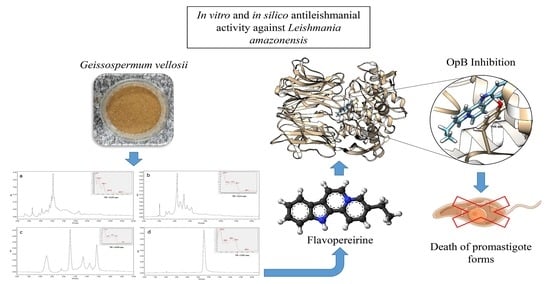
Flavopereirine—An Alkaloid Derived from Geissospermum vellosii—Presents Leishmanicidal Activity In Vitro
Abstract
:1. Introduction
2. Results
2.1. In Vitro Results
2.1.1. G. vellosii Prospection and Phytochemical Profile Show the Presence of an Alkaloid
2.1.2. The Alkaloid Derived from Flavopereirine Presents High Antipromastigote Activity
2.1.3. Cytotoxicity and Selectivity Index of Flavopereirine Improved with Exposure Time in Comparison to Amphotericin B
2.2. In Silico Results
2.2.1. Flavopereirine Presented Better Theoretical Properties than Antipain
2.2.2. Flavopereirine Appears to Inhibit OpB by Directly Binding to Tyr-499 in Silico
2.2.3. Flavopereirine Shows High Affinity to OpB in the Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Theoretical Molecular Properties
4.2. Molecular Docking
4.3. Molecular Dynamics Simulation
4.4. Plant Material
4.5. Biological Material
4.6. Phytochemical Studies
4.7. Antipromastigote Assay
4.8. Cell Viability Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vásquez, S.P.F. Conhecimento, uso e conservação da diversidade vegetal em quatro comunidades ribeirinhas no município Manacapuru, Amazonas. Instituto Nacional de Pesquisas da Amazônia 2014, xiv, 87f. [Google Scholar]
- Ferreira, I.C.P.; Lonardoni, M.V.C.; Machado, G.M.C.; Leon, L.L.; Filho, L.G.; Pinto, L.H.B.; De Oliveira, A.J.B. Anti-leishmanial activity of alkaloidal extract from Aspidosperma ramiflorum. Mem. Inst. Oswaldo Cruz 2004, 99, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.C.A.; da Silva, C.C.; Ferreira, I.C.P.; Machado, G.M.C.; Leon, L.L.; de Oliveira, A.J.B. Antileishmanial activity of indole alkaloids from Aspidosperma ramiflorum. Phytomedicine 2007, 14, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Reina, M.; Ruiz-Mesia, W.; López-Rodríguez, M.; Ruiz-Mesia, L.; González-Coloma, A.; Martínez-Díaz, R. Indole alkaloids from Geissospermum reticulatum. J. Nat. Prod. 2012, 75, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Mbeunkui, F.; Grace, M.H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. In vitro antiplasmodial activity of indole alkaloids from the stem bark of Geissospermum vellosii. J. Ethnopharmacol. 2012, 139, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.P.; Veitch, N.C.; Kite, G.C.; Simmonds, M.S.J.; Warhurst, D.C. Indole and β-carboline alkaloids from Geissospermum sericeum. J. Nat. Prod. 2002, 65, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Marion, L. Chapter XIII The Indole alkaloids. In Alkaloids: Chemistry and Physiology; Manske, R.H.F., Holmes, H.L., Eds.; Academic Press: New York, NY, USA, 1952; Volume 2, pp. 369–498. [Google Scholar]
- Rapoport, H.; Onak, T.P.; Hughes, N.A.; Reinecke, M.G. Alkaloids of Geissospermum vellosii. J. Am. Chem. Soc. 1958, 80, 1601–1604. [Google Scholar] [CrossRef]
- Hughes, N.; Rapoport, H. Flavopereirine, an alkaloids from Geissospermum vellosii. J. Am. Chem. 1958, 80, 1604–1609. [Google Scholar] [CrossRef]
- Rapoport, H.; Moore, R.E. Alkaloids of Geissospermum vellosii. Isolation and structure determinations of Vellosimine, Vellosiminol, and Geissolosimine. J. Org. Chem. 1962, 27, 2981–2985. [Google Scholar] [CrossRef]
- Moore, R.E.; Rapoport, H. Geissovelline, a new alkaloid from Geissospermum vellossi. J. Org. Chem. 1973, 38, 215–230. [Google Scholar] [CrossRef]
- Ishiyama, H.; Matsumoto, M.; Sekiguchi, M.; Shigemori, H.; Ohsaki, A.; Kobayashi, J. Two new indole alkaloids from Aspidosperma subincanum and Geissospermum vellosii. Heterocycles 2005, 66, 651–658. [Google Scholar] [CrossRef]
- Werner, J.A.T.; Oliveira, S.M.; Martins, D.F.; Mazzardo, L.; de Fátima Gaspari Dias, J.; Lordello, A.L.L.; Miguel, O.G.; Royes, L.F.; Ferreira, J.; Santos, A.R.S. Evidence for a role of 5-HT1A receptor on antinociceptive action from Geissospermum vellosii. J. Ethnopharmacol. 2009, 125, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.D.F.G. Fitoquímica e ensaios biológicos do extrato bruto etanólico, frações e substâncias isoladas provenientes das cascas de Geissospermum Vellosii ALLEMÃO (Apocynaceae), Universidade Federal do Paraná. 2012. Available online: https://acervodigital.ufpr.br/handle/1884/28287?show=full (accessed on 26 September 2018).
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Freitas, C.S.; Marcon, R.; Schwanke, R.C.; Siqueira, J.M.; Calixto, J.B. Non-clinical studies required for new drug development—Part I: Early in silico and in vitro studies, new target discovery and validation, proof of principles and robustness of animal studies. Br. J. Med. Biol. Res. 2016, 49, e5644. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Rawlings, N.D. Families and clans of serine peptidases. Arch. Biochem. Biophys. 1995, 318, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J. MEROPS: The peptidase database. Nucleic Acids Res. 1999, 27, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Morty, R.E.; Lonsdale-Eccles, J.D.; Mentele, R.; Auerswald, E.A.; Coetzer, T.H.T. Trypanosome-derived oligopeptidase B is released into the plasma of infected rodents, where it persists and retains full catalytic activity. Infect. Immun. 2001, 69, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- Swenerton, R.K.; Zhang, S.; Sajid, M.; Medzihradszky, K.F.; Craik, C.S.; Kelly, B.L.; McKerrow, J.H. The oligopeptidase B of Leishmania regulates parasite enolase and immune evasion. J. Biol. Chem. 2011, 286, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Hrabinováb, M.; Kulhánkováa, A.; Benešováa, N.; Chlebeka, J.; Jun, D.; Novák, Z.; Macáková, K.; Kuneš, J.; Kuča, K.; et al. Alkaloids from Chlidanthus fragrans and their Acetylcholinesterase, Butyrylcholinesterase and Prolyl Oligopeptidase Activities. Nat. Prod. Commun. 2013, 8, 1541–1544. [Google Scholar] [PubMed]
- Cahlíková, L.; Hulová, L.; Hrabinová, M.; Chlebek, J.; Hošťálková, A.; Adamcová, M.; Šafratová, M.; Jun, D.; Opletal, L.; Ločárek, M.; et al. Isoquinoline alkaloids as prolyl oligopeptidase inhibitors. Fitoterapia 2015, 103, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Šafratová, M.; Hostalková, A.; Hulcová, D.; Breiterová, K.; Hrabcová, V.; Machado, M.; Fontinha, D.; Prudêncio, M.; Kuneš, J.; Chlebek, J.; et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch. Pharm. Res. 2018, 41, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 3 October 2018).
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Maaß, P.C.; Rarey, M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Hazell, C.; Andrews, N.W.; Morty, R.E.; Fülöp, V. Expression, purification and preliminary crystallographic analysis of oligopeptidase B from Trypanosoma brucei. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, T.H.T.; Goldring, J.P.D.; Huson, L.E.J. Oligopeptidase B: A processing peptidase involved in pathogenesis. Biochimie 2008, 90, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Siatka, T.; Adamcová, M.; Opletal, L.; Cahlíková, L.; Jun, D.; Hrabinová, M.; Kuneš, J.; Chlebek, J. Cholinesterase and prolyl oligopeptidase inhibitory activities of alkaloids from argemone platyceras (Papaveraceae). Molecules 2017, 22, 1181. [Google Scholar] [CrossRef] [PubMed]
- Oligopeptidase B—An overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/oligopeptidase-b (accessed on 5 November 2018).
- McLuskey, K.; Paterson, N.G.; Bland, N.D.; Isaacs, N.W.; Mottram, J.C. Crystal structure of Leishmania major oligopeptidase B gives insight into the enzymatic properties of a trypanosomatid virulence factor. J. Biol. Chem. 2010, 285, 39249–39259. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; McLuskey, K.; Brown, E.; Coombs, G.H.; Mottram, J.C. Oligopeptidase B deficient mutants of Leishmania major. Mol. Biochem. Parasitol. 2011, 175, 49–57. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 64, 3–25. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Accoounts Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free energy calculations by the molecular mechanics poisson-boltzmann surface area method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Su, P.C.; Tsai, C.C.; Mehboob, S.; Hevener, K.E.; Johnson, M.E. Comparison of radii sets, entropy, QM methods, and sampling on MM-PBSA, MM-GBSA, and QM/MM-GBSA ligand binding energies of F. tularensis enoyl-ACP reductase (FabI). J. Comput. Chem. 2015, 36, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Database CID=65171. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/65171 (accessed on 1 April 2018).
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham III, T.E.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Walker, R.C.; Zhang, W.; Merz, K.M.; et al. Amber 12; University of California: San Francisco, CA, USA, 2012. [Google Scholar]
- Joung, I.S.; Cheatham, T.E. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 2008, 112, 9020–9041. [Google Scholar] [CrossRef] [PubMed]
- Courant, R. Variational methods for the solution of problems of equilibrium and vibrations. Bull. Am. Math. Soc. 1943, 49, 1–24. [Google Scholar] [CrossRef]
- Fletcher, R. Function minimization by conjugate gradients. Comput. J. 1964, 7, 149–154. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Perez, A.; MacCallum, J.L.; Brini, E.; Simmerling, C.; Dill, K.A. Grid-based backbone correction to the ff12SB protein force field for implicit-solvent simulations. J. Chem. Theory Comput. 2015, 11, 4770–4779. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Vale, V.V.; Vilhena, T.C.; Trindade, R.C.S.; Ferreira, M.R.C.; Percário, S.; Soares, L.F.; Pereira, W.L.A.; Brandão, G.C.; Oliveira, A.B.; Dolabela, M.F.; et al. Anti-malarial activity and toxicity assessment of Himatanthus articulatus, a plant used to treat malaria in the Brazilian Amazon. Malar. J. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Mota, E.F.; Rosario, D.M.; Silva Veiga, A.S.; Brasil, D.D.S.B.; Silveira, F.T.; Dolabela, M.F. Biological activities of Croton palanostigma Klotzsch. Pharmacogn. Mag. 2016, 12, 96. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ngure, P.K.; Tonui, W.K.; Ingonga, J.; Mutai, C.; Ng, Z.; Rukunga, G.; Kimutai, A. In vitro antileishmanial activity of extracts of Warburgia ugandensis (Canellaceae), a Kenyan medicinal plant. J. Med. Plants Res. 2009, 3, 61–66. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound flavopereirine are available from the authors upon requesting. |





| Samples | Yield (%) | TLC | |
|---|---|---|---|
| UV Light | Dragendorff | ||
| EE | 2.0 | + | + |
| NF | 42.8 | + | + |
| AF | 27.5 | + | + |
| FrHex | 1.8 | + | + |
| FrDcm | 5.8 | + | + |
| FrAcOET | 2.2 | + | + |
| FrMeOH | 85.2 | + | + |
| Samples | IC50 (µg/mL) + SD | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| EE | 50.25 ± 0.36 | 29.57 ± 0.83 | 14.71 ± 0.70 |
| FrHex | 84.31 ± 0.50 | >200 | >200 |
| FrDcm | 5.56 ± 0.70 | 20.85 ± 0.25 | 10.06 ± 0.80 |
| FrAcOET | 12.32 ± 0.69 | 82.06 ± 0.26 | 88.61 ± 0.20 |
| FrMeOH | 1.71 ± 0.15 | 3.75 ± 0.52 | 5.95 ± 0.66 |
| NF | 34.59 ± 0.83 | 21.33 ± 0.19 | 18.25 ± 0.91 |
| AF | 6.22 ± 0.25 | 10.19 ± 0.18 | 1.07 ± 0.24 |
| F6AF | 1.56 ± 0.16 * | 31.50 ± 0.76 * | 1.24 ± 0.15 * |
| Flavopereirine | 0.23 ± 0.10 * (0.93 µM) | 2.34 ± 0.50 * 9.3 µM | 0.15 ± 0.06 * 0.61 µM |
| Amphotericin | 0.42 ± 0.09 (0.45 µM) | 1.79 ± 0.06 (1.94 µM) | 0.35 ± 0.01 (0.30 µM) |
| Samples | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|
| CC50 * | SI | CC50 * | SI | CC50 * | SI | |
| EE | 178.0 ± 0.36 | 13.1 | 581.1 ± 0.60 | 19.7 | 829.4 ± 1.09 | 56.4 |
| NF | 115.9 ± 0.35 | 3.4 | 514.4 ± 0.61 | 23.4 | 744.3 ± 0.23 | 32.4 |
| AF | 121.4 ± 0.29 | 19.5 | 535.4 ± 0.43 | 52.5 | 774.3 ± 0.47 | 723.7 |
| F6AF | 443 ± 0.45 | 147.8 | 625.7 ± 0.34 | 19.9 | 629.4 ± 0.91 | 508.0 |
| Flavopereirine | 225.5 ± 0.9 (910 µM) | 976.2 | 533.3 ± 0.15 (2156 µM) | 228.2 | 734.0 ± 0.86 (2968 µM) | 4993.2 |
| Amphotericin | 272.7 ± 0.09 (295.1 µM) | 655.5 | 584.6 ± 0.46 (632.6 µM) | 326.6 | 637.7 ± 0.72 (690 µM) | 1811.7 |
| Properties | Antipain | Flavopereirine | |
|---|---|---|---|
| Molecules | |||
| tLogP | −3.01 | 0.90 | |
| TPSA | 282.51 | 19.89 | |
| Molecular Weight | 604.71 | 247.32 | |
| nON | 16 | 2 | |
| nOHNH | 13 | 1 | |
| nViolations | 3 | 0 | |
| nRotB | 19 | 1 | |
| Volume | 563.14 | 233.44 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva e Silva, J.V.; Cordovil Brigido, H.P.; Oliveira de Albuquerque, K.C.; Miranda Carvalho, J.; Ferreira Reis, J.; Vinhal Faria, L.; Coelho-Ferreira, M.R.; Silveira, F.T.; da Silva Carneiro, A.; Percário, S.; et al. Flavopereirine—An Alkaloid Derived from Geissospermum vellosii—Presents Leishmanicidal Activity In Vitro. Molecules 2019, 24, 785. https://doi.org/10.3390/molecules24040785
da Silva e Silva JV, Cordovil Brigido HP, Oliveira de Albuquerque KC, Miranda Carvalho J, Ferreira Reis J, Vinhal Faria L, Coelho-Ferreira MR, Silveira FT, da Silva Carneiro A, Percário S, et al. Flavopereirine—An Alkaloid Derived from Geissospermum vellosii—Presents Leishmanicidal Activity In Vitro. Molecules. 2019; 24(4):785. https://doi.org/10.3390/molecules24040785
Chicago/Turabian Styleda Silva e Silva, João Victor, Helliton Patrick Cordovil Brigido, Kelly Cristina Oliveira de Albuquerque, Josiwander Miranda Carvalho, Jordano Ferreira Reis, Lara Vinhal Faria, Márlia Regina Coelho-Ferreira, Fernando Tobias Silveira, Agnaldo da Silva Carneiro, Sandro Percário, and et al. 2019. "Flavopereirine—An Alkaloid Derived from Geissospermum vellosii—Presents Leishmanicidal Activity In Vitro" Molecules 24, no. 4: 785. https://doi.org/10.3390/molecules24040785