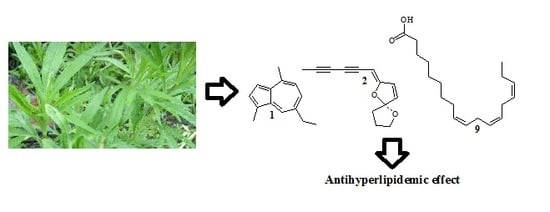
Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia integrifolia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–10 from the Crude Lipophilic Components
2.2. Acute Toxicity
2.3. Effects of Crude Lipophilic Components and Compounds 1,2,9 on Triton WR-1339-Induced Hyperlipidemia in Rats
3. Experimental Section
3.1. General Information
3.2. Plant Material and the Crude Lipophilic Components from A. integrifolia
3.3. Isolation of the Crude Lipophilic Components
3.4. Characterization Data of Known Compounds
3.5. Experimental Animals
3.6. Acute Toxicity
3.7. Triton WR-1339-Induced Hyperlipidemic in Rats
3.8. Biochemical Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frishman, W.H. Biologic markers as predictors of cardiovascular disease. Am. J. Med. 1998, 104, 18S–27S. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Hazzard, W.R.; Schrott, H.G.; Bierman, E.L.; Motulsky, A.G. Hyperlipidemia in coronary heart disease I. Lipid levels in 500 survivors of myocardial infarction. J. Clin. Investig. 1973, 52, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Hulley, S.B.; Browner, W.S.; Kuller, L.H.; Wentworth, D. Serum cholesterol, blood pressure, and mortality: Implications from a cohort of 361,662 men. Lancet 1986, 2, 933–936. [Google Scholar] [CrossRef]
- West, K.M.; Ahuja, M.S.; Bennet, P.H. The role of circulating glucose and triglyceride concentrations and their interactions with other “risk factors” as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 1983, 6, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, F.; Ray, A.B.; Chansouria, J.P.N. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J. Nat. Prod. 1993, 56, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Poornima, K.; Chella, P.P.; Gopalakrishnan, V.K. Protective effect of ethanolic extract of Tabernaemontana divaricata (L.) R. Br. against DEN and Fe NTA Induced Liver Necrosis in Wistar Albino Rats. Biomed. Res. Int. 2014, 2014, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.J.; Xiang, B.P.; He, J.G. Modulation effects of Artemisia integrifolia on immue function of mice. Food Sci. 2008, 29, 405–408. [Google Scholar]
- Tu, Y.; Liu, X.H. The Daur people eating-Artemisia integrifolia. Chin. Folk. Ther. 2007, 15, 10–11. [Google Scholar]
- Jin, Y.R.; Du, Y.F.; Shi, X.W.; Liu, P.W. Simultaneous quantification of 19 diterpenoids in Isodon amethystoides by high performance liquid chromatography electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. 2010, 53, 403–411. [Google Scholar] [CrossRef]
- Zhao, R.G.T.; Wu, S.Y.; Han, N.R.C.G.T.; Wang, Q.H. Isolation and identification of fatty compounds of the petroleum ether extracts from Artemisia integrifolia L. J. Inn. Mong. Univ. Natl. (Nat. Sci.) 2016, 31, 247–249. [Google Scholar]
- Liu, R.; Wamg, X.H.; Li, J.M. Isolation and identification of polysaccharides from Artemisia integrifolia. Food Sci. 2009, 30, 81–83. [Google Scholar]
- Wang, Q.H.; Hao, J.S.; Xu, Y.H.; Bao, Y.P. Structure elucidation of a new iridoid from Artemisia integrifolia. Rec. Nat. Prod. 2018, 12, 544–548. [Google Scholar] [CrossRef]
- Wang, M.X.; Huang, F.H.; Liu, C.S.; Wang, J.W.; Huang, Q.J. Study on antioxidative effects of natural antioxidants to α-ethyl linolenate. Chin. J. Oil Crop Sci. 2007, 29, 466–469. [Google Scholar]
- Maierdan, M.H.M.T.; Liu, Y.; Nijiati, R.H.M. The protective effect of extracts from Lithospermum erythrorhizon on acute liver injury induced by D- galactosamine in mice. Chin. J. Chin. Mat. Med. 2006, 31, 1646–1648. [Google Scholar]
- Zhang, W.J.; You, C.X.; Yang, K.; Wang, Y.; Su, Y.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W.; Wang, Y.Y. Boiactivity and chemical constituents of the essential oil from Dendranthema indicum (L.) Des Moul. Against two stored insects. J. Oleo Sci. 2015, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Jiwajinda, A.S.; Ohigashi, H. Isolation and identification of acetylenic spiroketal enol ethers from Artemisia lactiflora as inhibitors of superoxide generation induced by a tumor promoter in pifferentiated HL-60 cells. J. Agric. Food Chem. 1998, 46, 5031–5036. [Google Scholar] [CrossRef]
- Zan, L.F.; Tuliguer, B.; Bao, H.Y. Chemical composition in fruiting body of Cortinarius rufo-ofivaceus. Mycosystema 2008, 27, 284–288. [Google Scholar]
- Deng, X.H.; Zheng, C.J.; Wu, Y. Chemical constituents of whole plant of Veronicastrum axillare (Sieb. et Zucc.) Yamazaki. Chin. Pharm. J. 2013, 48, 777–781. [Google Scholar]
- Yang, Q.; Wang, S.W.; Wang, J.B. Study on extraction and identification from Semen zanthoxyli bungeani. Chin. J. New Drugs 2008, 17, 318–320. [Google Scholar]
- Lago, J.H.G.; Brochini, C.B.; Roque, N.F. Terpenoids from Guarea guidonia. Phytochemistry 2002, 60, 333–338. [Google Scholar] [CrossRef]
- Ghatak, A.; Asthana, O.P. Recent trends in hyperlipoproteinemias and its pharmacotherapy. Indian J. Pharmacol. 1995, 27, 14–19. [Google Scholar]
- Han, X.; Zhang, M.Q.; Chang, B.; Wang, X.; Hao, L.; Liu, Y.; Shi, C.; Guo, Q.H.; Zhang, Y.X. Effects of Rhizoma Alismatis decoction on lipid metabolism and PPARα and ACO expression in rats with hyperlipemia. Chin. Pharmacol. Bull. 2017, 33, 1761–1765. [Google Scholar]
- Saravanan, M.; Pandikumar, P.; Prakash Babu, N.; Ignacimuthu, S. Antihyperlipidemic activity of Ichnocarpus frutescens in triton WR-1339-induced and high-fat diet animals. Pharm. Biol. 2011, 49, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, S.P.; Mei, X.L. Progress on Fructus Choerospondiatis, a Mongolian folk medicine. Straits Pharm. J. 2017, 29, 1–5. [Google Scholar]
- Yan, Y.; Wuliji, O.; Zhao, X.J.; Ye, X.C.; Zhang, C.; Hao, J.J.; He, J.J.; Zhu, X.; Xu, H.B.; Yang, X.L. Effect ofessentialoilof Syringa pinnatifolia Hemsl. var. alashanensis on ischemia of myocardium, hypoxia and platelet taggregation. J. Ethnopharmacol. 2010, 131, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Qin, H.L.; He, H.B.; Luo, T.; Liu, H.J.; Liu, X.; Zhou, J.G.; Ye, P.; Xu, Q. Cardioprotective effects of Salvia miltiorrhiza on heart failure rats and SIRT1 and SDF-1, CXCR4 relationship. Pharmacol. Clin. Chin. Mater. Med. 2014, 30, 96–101. [Google Scholar]
- Harnafi, H.; Caid, H.S.; Bouanani, N.H.; Aziz, M.; Amrani, S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008, 108, 205–212. [Google Scholar] [CrossRef]
- Xie, W.; Wang, W.; Su, H.; Xing, D.; Cai, G.; Du, L. Hypolipidemic mechanisms of Ananas comosus L. leaves in mice: Different from fibrates but similar to statins. J. Pharmacol. Sci. 2007, 103, 267–274. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; Trindade de Paula, M.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef]
- Otway, S.; Robinson, D.S. The effect of the nonionic detergent (Triton) on the removal of triglyceride fatty acids from the blood of the rats. J. Physiol. 1967, 19, 309–319. [Google Scholar] [CrossRef]
- Malloy, M.J.; Kan, J.P. Medical management of hyperlipidemic states. Adv. Intern. Med. 1994, 39, 603–631. [Google Scholar]
- Anila, L.; Vijayalakshmi, N.R. Flavonoids from Emblica officinalis and Mangifera indica—Effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef]
- Roux, S.; Sable, E.; Porsolt, R.D. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effect on behavior and physiological function. Curr. Protoc. Pharmacol. 2004, 10, 1–23. [Google Scholar]
- Silva, G.N.; Martins, F.R.; Matheus, M.E. Investigation of anti-inflammatory and antinociceptive activities of Lantana trifolia. J. Ethnopharmacol. 2005, 100, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Schurr, P.E.; Shultz, J.R. Parkinson TM. Triton-induced hypelipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972, 1, 68–74. [Google Scholar] [CrossRef]
- Yusuf, A.H.; Ghassan, S.; Tariq, A.Q.; Waseem, E.H.; Ghassan, A.S.; Suhair, H. Antihyperlipidemic Properties of Novel N-(Benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules 2011, 16, 8292–8304. [Google Scholar]
Sample Availability: Samples of the compounds 1–10 are available from the authors. |

| Position | δH (ppm), J (Hz) | δC (ppm) | HMBC |
|---|---|---|---|
| 1 | — | 126.9 | |
| 2 | 7.39 (brs, 1H) | 125.2 | C-4, C-6, C-7, C-1′ |
| 3 | — | 129.0 | |
| 4 | — | 162.1 | |
| 5 | 6.77 (d, 1H, J = 8.5 Hz) | 109.5 | C-1, C-3 |
| 6 | 7.43 (brd, 1H, J = 8.5 Hz) | 130.4 | C-2, C-4, C-7 |
| 7 | 7.57 (d, 1H, J = 15.5 Hz) | 145.3 | C-2, C-6, C-8, C-9 |
| 8 | 6.42 (d, 1H, J = 15.5 Hz) | 114.5 | C-1, C-9 |
| 9 | — | 167.5 | |
| 1′ | 3.17 (dd, 1H, J = 16.0, 9.0 Hz) 2.98 (dd, 1H, J = 16.0, 8.5 Hz) | 31.6 | C-2, C-4, C-2′, C-3′ |
| 2′ | 4.77 (dd, 1H, J = 9.0, 4.5 Hz | 85.7 | C-3, C-4′, C-5′ |
| 3′ | 1.95 (m, 1H) | 40.7 | |
| 4′ | 3.51 (dd, 1H, J = 10.5, 5.0 Hz) 3.05 (dd, 1H, J = 10.5, 6.5 Hz) | 62.8 | C-2, C-4, C-2′, C-3′ |
| 5′ | 0.86 (d, 3H, J = 7.0 Hz) | 12.3 | C-2′, C-3′, C-4′ |
| -OCH3 | 3.69 (3H, s) | 51.7 | C-9 |
| Groups | Average Body Weight (g) | |
|---|---|---|
| Day 1 | Day 15 | |
| I | 195.73 ± 9.77 | 250.15 ± 9.96 |
| II | 195.12 ± 11.73 | 249.63 ± 12.86 |
| III | 195.91 ± 11.07 | 233.75 ± 6.59 * |
| IV | 198.06 ± 10.77 | 240.85 ± 4.55 |
| V | 196.7 ± 11.29 | 231.28 ± 9.89 * |
| VI | 198.41 ± 10.86 | 242.67 ± 10.83 |
| VII | 199.35 ± 11.92 | 247.55 ± 10.50 |
| VIII | 198.93 ± 11.96 | 232.08 ± 11.71 * |
| Parameters | TGs (mmol/L) | TC (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|
| CG | 0.68 ± 0.15 | 1.53 ± 0.18 | 1.17 ± 0.19 | 0.27 ± 0.07 |
| HG | 11.8 ± 1.32 ### | 7.14 ± 1.73 ### | 0.81 ± 0.10 ### | 0.95 ± 0.21 ### |
| PCG (simvastatin, 4 mg/kg) | 1.53 ± 1.04 ** | 2.19 ± 0.64 ** | 0.97 ± 0.25 | 0.65 ± 0.15 ** |
| TG IV (crude lipophilic components, 100 mg/kg) | 1.19 ± 0.56 *** | 4.65 ± 1.40 * | 1.15 ± 0.15 * | 0.70 ± 0.23 * |
| TG V (crude lipophilic components, 200 mg/kg) | 0.69 ± 0.16 *** | 2.13 ± 0.50 ** | 1.26 ± 0.26 ** | 0.55 ± 0.19 ** |
| TG VI (compound 1, 4 mg/kg) | 6.13 ± 1.52 ** | 4.80 ± 1.67 * | 1.24 ± 0.34 ** | 0.72 ± 0.20 * |
| TG VII (compound 2, 4 mg/kg) | 7.93 ± 1.41 ** | 4.77 ± 1.48 * | 1.39 ± 0.52 ** | 0.67 ± 0.17 ** |
| TG VIII (compound 9, 4 mg/kg) | 0.75 ± 0.22 *** | 2.54 ± 0.75 *** | 1.06 ± 0.12 * | 0.58 ± 0.14 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Q.; Bao, W.; Pa, B. Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia integrifolia. Molecules 2019, 24, 725. https://doi.org/10.3390/molecules24040725
Xu Y, Wang Q, Bao W, Pa B. Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia integrifolia. Molecules. 2019; 24(4):725. https://doi.org/10.3390/molecules24040725
Chicago/Turabian StyleXu, Yanhua, Qinghu Wang, Wenqiang Bao, and Biligetu Pa. 2019. "Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia integrifolia" Molecules 24, no. 4: 725. https://doi.org/10.3390/molecules24040725