Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium
Abstract
:1. Introduction
2. Results and Discussion
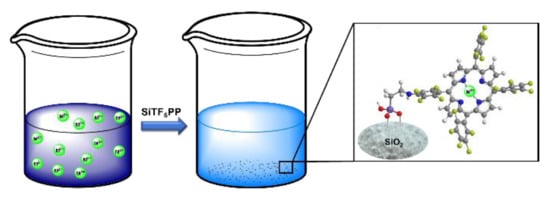
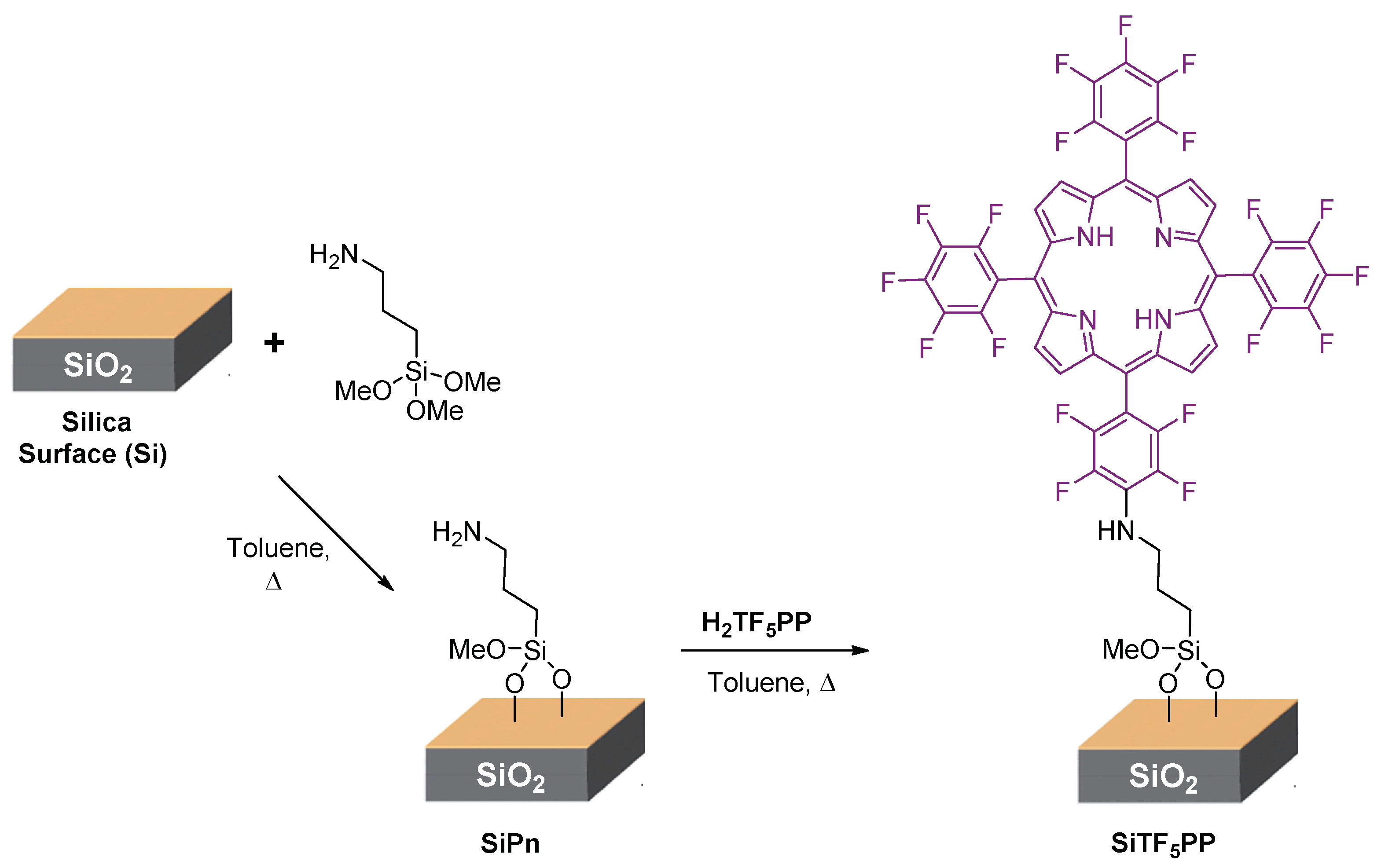
2.1. Synthesis and Characterization of the Organic-Inorganic Hybrid Material SiTF5PP
2.2. Metal Cations Adsorption
2.2.1. Effect of pH
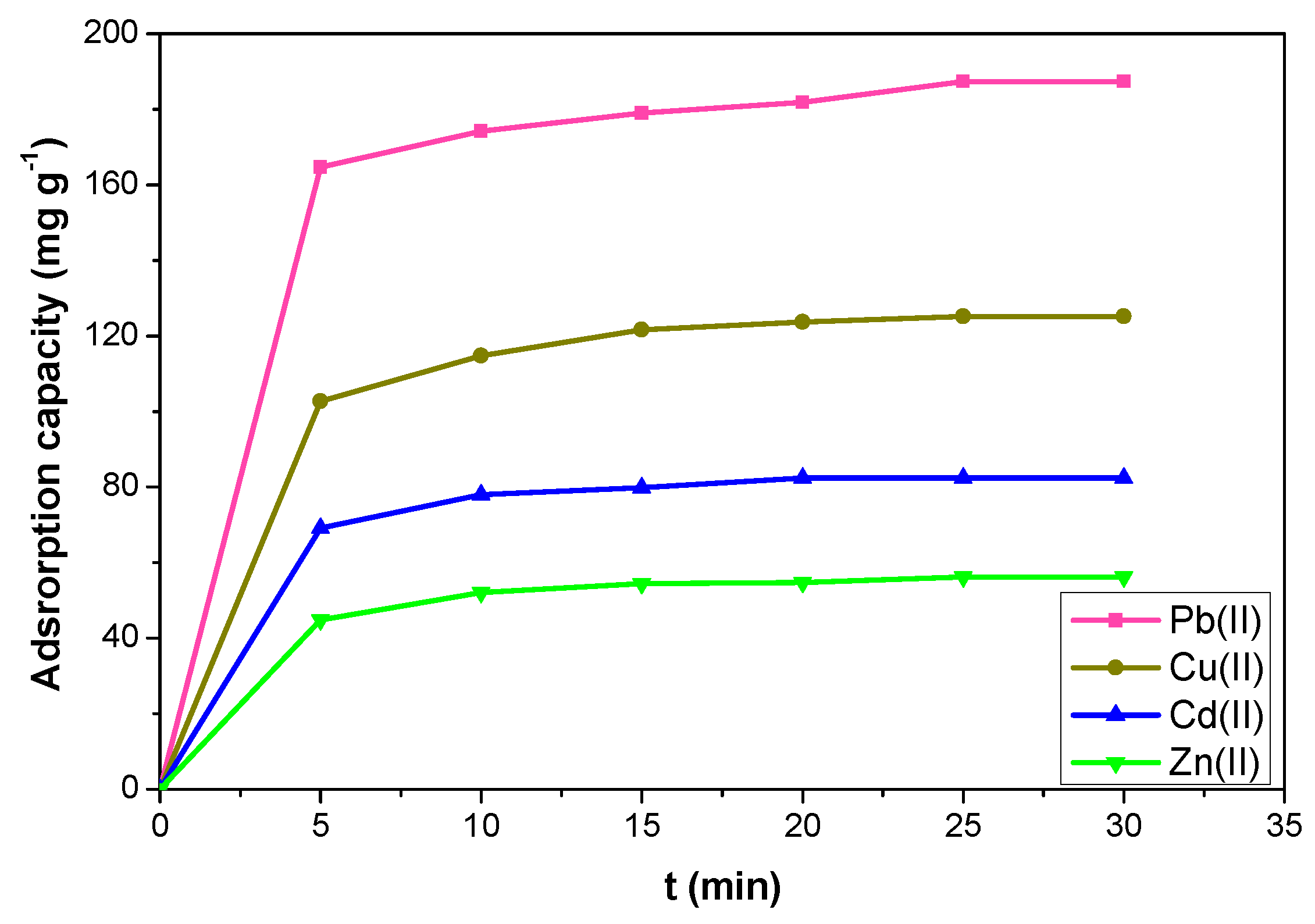
2.2.2. Effect of Contact Time and Adsorption Kinetics
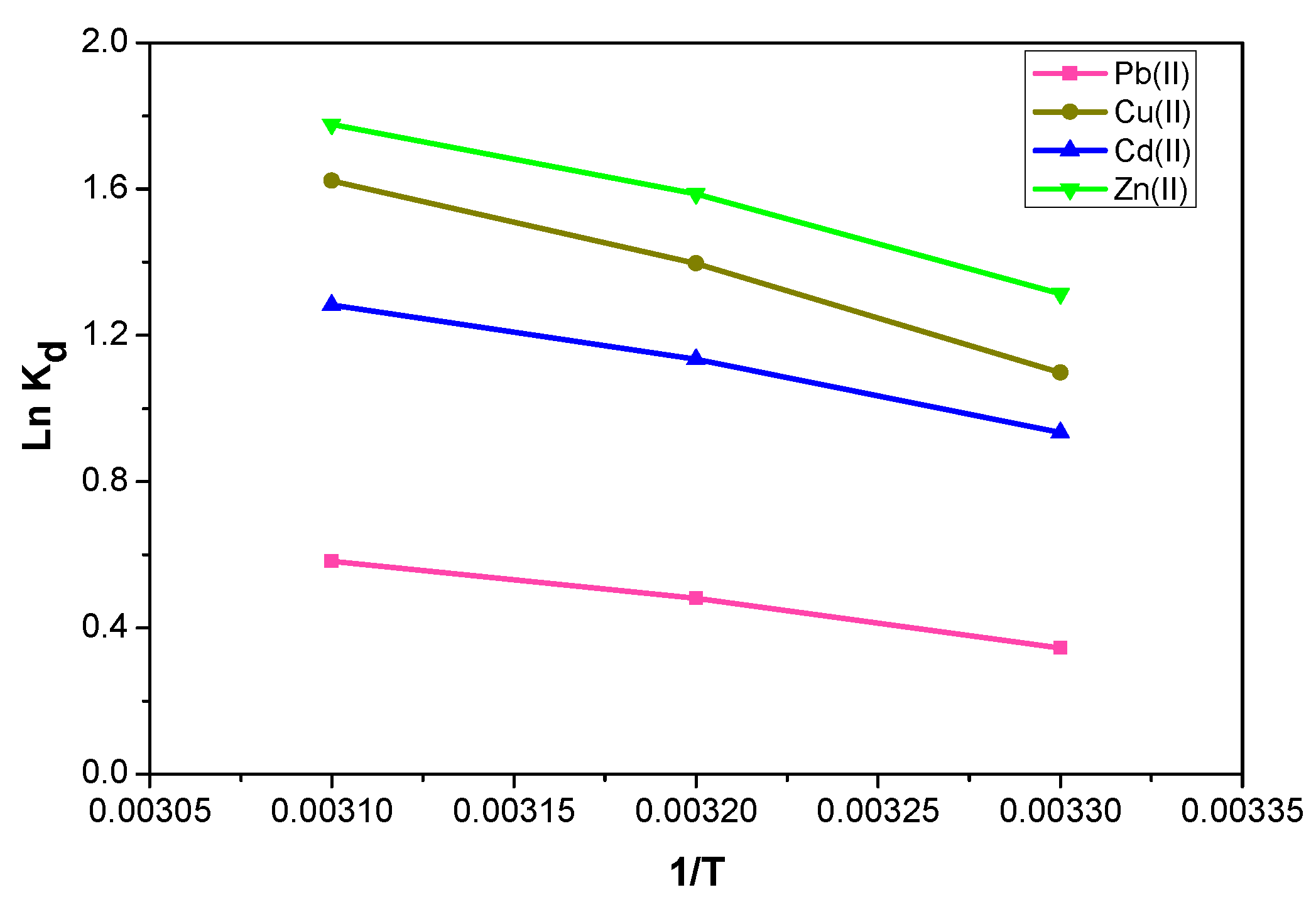
2.2.3. Thermodynamic Studies
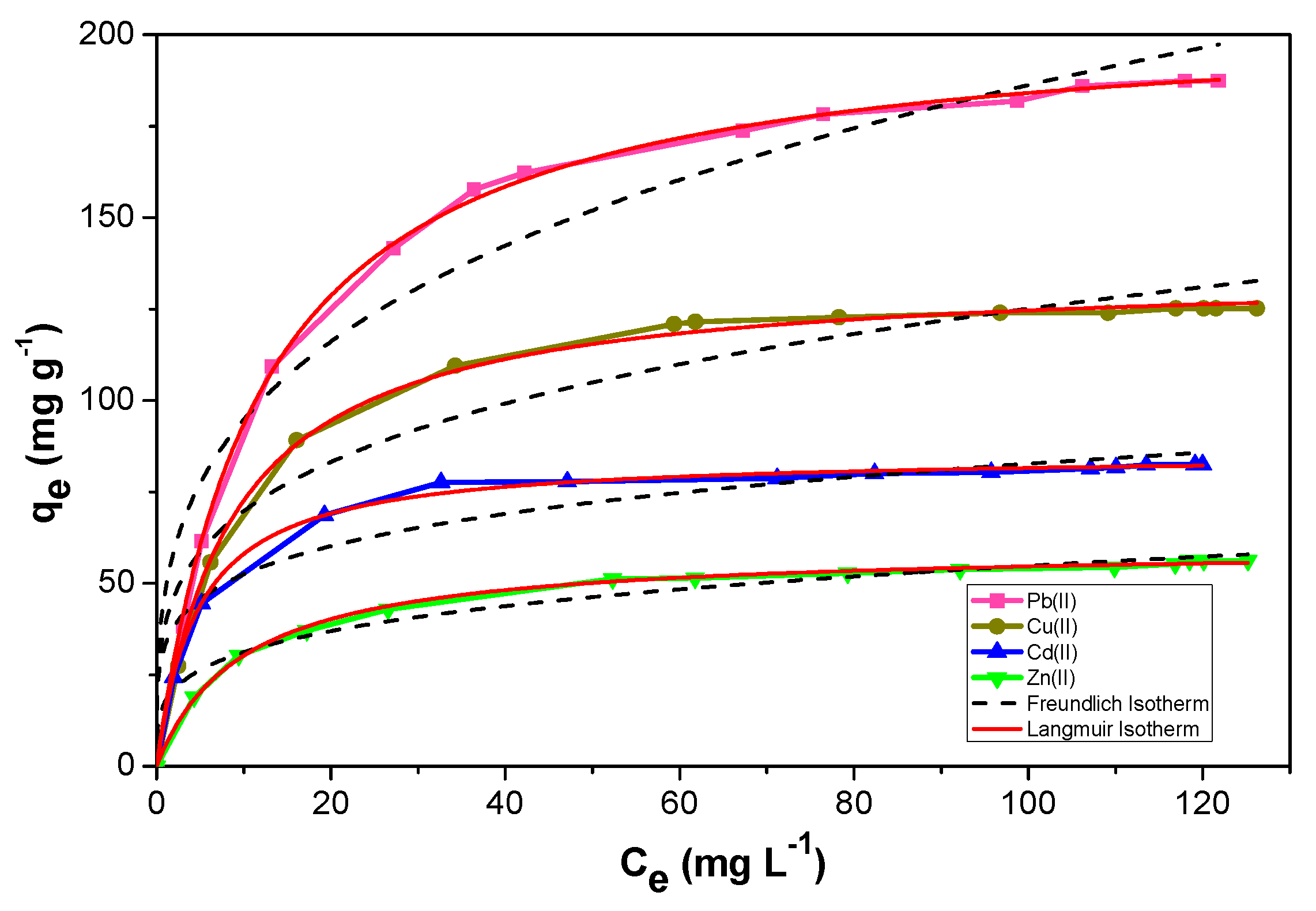
2.2.4. Adsorption Isotherms
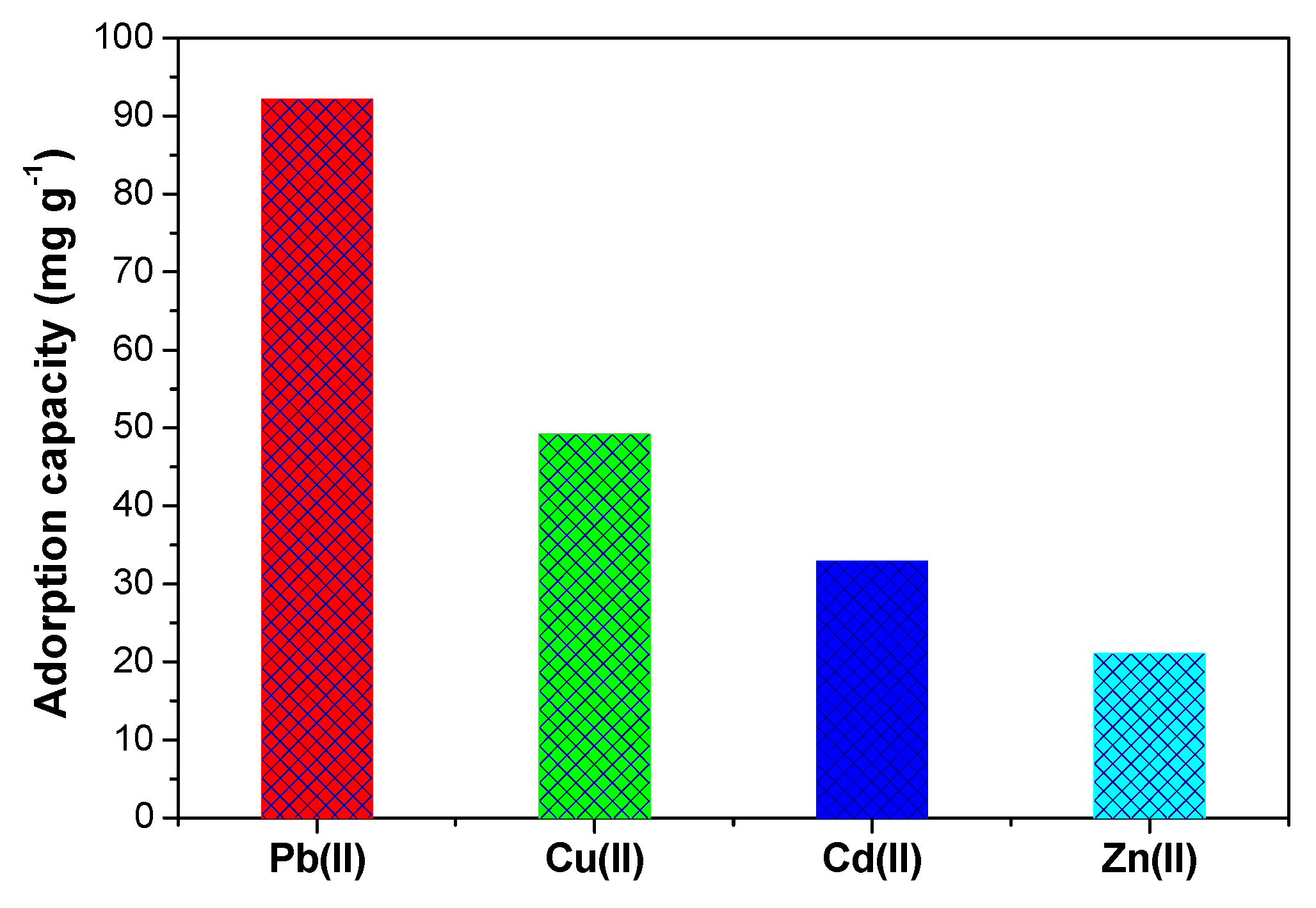
2.2.5. Selectivity of SiTF5PP
2.2.6. Applicability of SiTF5PP for the Removal Potentially Toxic Transition Metals from Real Samples
2.2.7. Regeneration Ability of SiTF5PP
2.2.8. Comparison with Alternative Adsorbents
3. Materials and Methods
3.1. General Remarks
3.2. Preparation of 3-Aminopropylsilica (SiPn)
3.3. Synthesis of Porphyrin-Substituted Silica (SiTF5PP)
3.4. Metal Cation Adsorption Experiments
3.5. Batch Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bernard, A. Cadmium and its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557. [Google Scholar] [PubMed]
- Hajdu, I.; Bodnár, M.; Csikós, Z.; Wei, S.; Daróczi, L.; Kovács, B.; Győri, Z.; Tamás, J.; Borbély, J. Combined nano-membrane technology for removal of lead ions. J. Memb. Sci. 2012, 409, 44–53. [Google Scholar] [CrossRef]
- Liang, X.-X.; Wang, N.; Qu, Y.-L.; Yang, L.-Y.; Wang, Y.-G.; Ouyang, X.-K. Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions. Molecules 2018, 23, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, M.; Ahmad, R.; Barakat, M. l-Methionine modified Dowex-50 ion-exchanger of reduced size for the separation and removal of Cu (II) and Ni (II) from aqueous solution. Chem. Eng. J. 2013, 218, 32–38. [Google Scholar] [CrossRef]
- Dolgormaa, A.; Lv, C.-j.; Li, Y.; Yang, J.; Yang, J.-x.; Chen, P.; Wang, H.-p.; Huang, J. Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, T.; Bakan, G.; Altas, L.; Buyukgungor, H. The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme Microb. Technol. 2007, 41, 98–102. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.; Smith, R. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Misra, R.; Jain, S.; Khatri, P. Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr (VI), Cd (II), Ni (II) and Pb (II) from their aqueous solutions. J. Hazard. Mater. 2011, 185, 1508–1512. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.-L. Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Keng, P.-S.; Lee, S.-L.; Ha, S.-T.; Hung, Y.-T.; Ong, S.-T. Removal of hazardous heavy metals from aqueous environment by low-cost adsorption materials. Environ. Chem. Lett. 2014, 12, 15–25. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2, 4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef]
- Ahn, C.K.; Park, D.; Woo, S.H.; Park, J.M. Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J. Hazard. Mater. 2009, 164, 1130–1136. [Google Scholar] [CrossRef]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Lăcrămioara, (N.)N.; Bulgariu, L. Optimization of process parameters for heavy metals biosorption onto mustard waste biomass. Open Chem. 2016, 14, 175–187. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. Functionalized soy waste biomass-A novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J. Clean. Prod. 2018, 197, 875–885. [Google Scholar] [CrossRef]
- Perez-Quintanilla, D.; Del Hierro, I.; Fajardo, M.; Sierra, I. Mesoporous silica functionalized with 2-mercaptopyridine: Synthesis, characterization and employment for Hg (II) adsorption. Microporous Mesoporous Mater. 2006, 89, 58–68. [Google Scholar] [CrossRef]
- Kooshki, M.; Shams, E. Selective response of dopamine in the presence of ascorbic acid on carbon paste electrode modified with titanium phosphated silica gel. Anal. Chim. Acta 2007, 587, 110–115. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Hassanien, M.M.; Abou-El-Sherbini, K.S.; GÖRLITZ, V. Controlled-pore silica glass modified with N-propylsalicylaldimine for the separation and preconcentration of trace Al (III), Ag (I) and Hg (II) in water samples. Anal. Sci. 2003, 19, 1151–1156. [Google Scholar] [CrossRef]
- Camel, V. Solid phase extraction of trace elements. Spectrochim. Acta Part B 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Toubi, Y.; Bacquet, M. Polysiloxane surface modified with bipyrazolic tripodal receptor for quantitative lead adsorption. J. Hazard. Mater. 2011, 185, 494–501. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically modified silica with pyrazole-3-carbaldehyde as a new sorbent for solid-liquid extraction of heavy metals. Molecules 2013, 19, 247–262. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Bacquet, M.; Dacquin, J.-P.; Mabkhot, Y.N.; Jodeh, S.; Warad, I.; Zaghrioui, M. Synthesis of 1-(Furan-2-yl) imine functionalized silica as a chelating sorbent and its preliminary use in metal Ion adsorption. Sep. Sci. Technol. 2015, 50, 710–717. [Google Scholar] [CrossRef]
- Radi, S.; Attayibat, A.; El-Massaoudi, M.; Bacquet, M.; Jodeh, S.; Warad, I.; Al-Showiman, S.S.; Mabkhot, Y.N. C, N-bipyrazole receptor grafted onto a porous silica surface as a novel adsorbent based polymer hybrid. Talanta 2015, 143, 1–6. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Bacquet, M.; Degoutin, S.; Mabkhot, Y.N.; Garcia, Y. An inorganic–organic hybrid material made of a silica-immobilized Schiff base receptor and its preliminary use in heavy metal removal. RSC Adv. 2016, 6, 34212–34218. [Google Scholar] [CrossRef]
- Hu, J.; Liu, L.; Xiao, Z. Adsorptions of Cd (II) and methylene blue from aqueous solution by silica hybrid hollow spheres. RSC Adv. 2015, 5, 68092–68098. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, C.; Dai, J.; Shi, W.; Yan, Y. Design of mesoporous silica hybrid materials as sorbents for the selective recovery of rare earth metals. J. Mater. Chem. A 2015, 3, 10327–10335. [Google Scholar] [CrossRef]
- Thomas, D.W.; Martell, A.E. Metal Chelates of Tetraphenylporphine and of some p-Substituted Derivatives1, 2. J. Am. Chem. Soc. 1959, 81, 5111–5119. [Google Scholar] [CrossRef]
- Di Natale, C.; Monti, D.; Paolesse, R. Chemical sensitivity of porphyrin assemblies. Mater. Today 2010, 13, 46–52. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef]
- Kadish, K.; Smith, K.; Guilard, R. The Porphyrin Handbook. In Biochemistry and Binding: Activation of Small Molecules; Academic Press: Cambridge, MA, USA, 2000; Volume 4. [Google Scholar]
- Gamelas, S.; Gomes, A.; Moura, N.; Faustino, M.; Cavaleiro, J.; Lodeiro, C.; Veríssimo, M.; Fernandes, T.; Daniel-da-Silva, A.; Gomes, M.; et al. N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy. Molecules 2018, 23, 867. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Nunez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.; Capelo, J.L.; Lodeiro, C. Synthesis, Spectroscopy Studies, and Theoretical Calculations of New Fluorescent Probes Based on Pyrazole Containing Porphyrins for Zn(II), Cd(II), and Hg(II) Optical Detection. Inorg. Chem. 2014, 53, 6149–6158. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Nunez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Paz, F.A.A.; Neves, M.; Capelo, J.L.; Lodeiro, C. A New 3,5-Bisporphyrinylpyridine Derivative as a Fluorescent Ratiometric Probe for Zinc Ions. Chem. Eur. J. 2014, 20, 6684–6692. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Sarkar, T.; Kakoullis, J., Jr.; Myung, N.V.; Konnanath, B.; Spanias, A.; Mulchandani, A. Porphyrin-functionalized single-walled carbon nanotube chemiresistive sensor arrays for VOCs. J. Phys. Chem. C 2012, 116, 3845–3850. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.-H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of porphyrin science. World Sci. Singap. 2010, 2014, 1–35. [Google Scholar]
- Cerqueira, A.; Moura, N.; Serra, V.; Faustino, M.; Tomé, A.; Cavaleiro, J.; Neves, M. β-Formyl- and β-Vinylporphyrins: Magic Building Blocks for Novel Porphyrin Derivatives. Molecules 2017, 22, 1269. [Google Scholar] [CrossRef]
- Amao, Y.; Miyashita, T.; Okura, I. Platinum tetrakis(pentafluorophenyl)porphyrin immobilized in polytrifluoroethylmethacrylate film as a photostable optical oxygen detection material. J. Fluor. Chem. 2001, 107, 101–106. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dyes Pigm. 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Huang, G.; Mo, L.-Q.; Cai, J.-L.; Cao, X.; Peng, Y.; Guo, Y.-A.; Wei, S.-J. Environmentally friendly and efficient catalysis of cyclohexane oxidation by iron meso-tetrakis(pentafluorophenyl)porphyrin immobilized on zinc oxide. Appl. Catal. B 2015, 162, 364–371. [Google Scholar] [CrossRef]
- Barbosa, I.A.; de Sousa Filho, P.C.; da Silva, D.L.; Zanardi, F.B.; Zanatta, L.D.; de Oliveira, A.J.A.; Serra, O.A.; Iamamoto, Y. Metalloporphyrins immobilized in Fe3O4@SiO2 mesoporous submicrospheres: Reusable biomimetic catalysts for hydrocarbon oxidation. J. Colloid Interface Sci. 2016, 469, 296–309. [Google Scholar] [CrossRef]
- Zanatta, L.D.; Barbosa, I.A.; Zanardi, F.B.; de Sousa Filho, P.C.; Bolzon, L.B.; Ramos, A.P.; Serra, O.A.; Iamamoto, Y. Hydrocarbon oxidation by iron-porphyrin immobilized on SBA-15 as biomimetic catalyst: Role of silica surface. RSC Adv. 2016, 6, 104886–104896. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Fernandes, A.; Faustino, M.A.F.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Nakagaki, S.; Almeida, A.; Cunha, Â.; Silvestre, A.J.D.; et al. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dyes Pigm. 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Costa, J.I.; Tomé, A.C.; Neves, M.G.; Cavaleiro, J.A. 5, 10, 15, 20-tetrakis (pentafluorophenyl) porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Mutneja, R.; Singh, R.; Kaur, V.; Wagler, J.; Fels, S.; Kroke, E. Schiff base tailed silatranes for the fabrication of functionalized silica based magnetic nano-cores possessing active sites for the adsorption of copper ions. New J. Chem. 2016, 40, 1640–1648. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Tütem, E.; Hügül, M.; Hizal, J. Heavy metal cation retention by unconventional sorbents (red muds and fly ashes). Water Res. 1998, 32, 430–440. [Google Scholar] [CrossRef]
- Şen, P.; Hirel, C.; Andraud, C.; Aronica, C.; Bretonnière, Y.; Mohammed, A.; Ågren, H.; Minaev, B.; Minaeva, V.; Baryshnikov, G. Fluorescence and FTIR spectra analysis of trans-A2B2-substituted di-and tetra-phenyl porphyrins. Materials 2010, 3, 4446–4475. [Google Scholar] [CrossRef]
- Arakaki, L.; Filha, V.A.; Germano, A.; Santos, S.; Fonseca, M.; Sousa, K.; Espínola, J.; Arakaki, T. Silica gel modified with ethylenediamine and succinic acid-adsorption and calorimetry of cations in aqueous solution. Thermochim. Acta 2013, 556, 34–40. [Google Scholar] [CrossRef]
- Yin, P.; Tian, Y.; Wang, Z.; Qu, R.; Liu, X.; Xu, Q.; Tang, Q. Synthesis of functionalized silica gel with poly (diethylenetriamine bis (methylene phosphonic acid)) and its adsorption properties of transition metal ions. Mater. Chem. Phys. 2011, 129, 168–175. [Google Scholar] [CrossRef]
- Dai, B.; Cao, M.; Fang, G.; Liu, B.; Dong, X.; Pan, M.; Wang, S. Schiff base-chitosan grafted multiwalled carbon nanotubes as a novel solid-phase extraction adsorbent for determination of heavy metal by ICP-MS. J. Hazard. Mater. 2012, 219, 103–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, R.; Sun, C.; Wang, C.; Ji, C.; Chen, H.; Yin, P. Chemical modification of silica-gel with diethylenetriamine via an end-group protection approach for adsorption to Hg (II). Appl. Surf. Sci. 2009, 255, 5818–5826. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Sánchez, A.; del Hierro, I.; Fajardo, M.; Sierra, I. Preparation, characterization, and Zn 2+ adsorption behavior of chemically modified MCM-41 with 5-mercapto-1-methyltetrazole. J. Colloid Interface Sci. 2007, 313, 551–562. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Banerjee, I.A.; Yu, L.; Matsui, H. Cu nanocrystal growth on peptide nanotubes by biomineralization: Size control of Cu nanocrystals by tuning peptide conformation. PNAS 2003, 100, 14678–14682. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yin, P.; Liu, X.; Dong, X.; Zhang, J.; Xu, Q. Thermodynamics, kinetics, and isotherms studies for gold (III) adsorption using silica functionalized by diethylenetriaminemethylenephosphonic acid. Chem. Eng. Res. Des. 2013, 91, 2748–2758. [Google Scholar] [CrossRef]
- Fang, G.-Z.; Tan, J.; Yan, X.-P. An ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique combined with a sol− gel process for selective solid-phase extraction of cadmium (II). Anal. Chem. 2005, 77, 1734–1739. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Miao, J.; Qian, J.; Wang, X.; Zhang, Y.; Yang, H.; He, P. Synthesis and characterization of ordered mesoporous silica by using polystyrene microemulsion as templates. Mater. Lett. 2009, 63, 989–990. [Google Scholar] [CrossRef]
- Mureseanu, M.; Reiss, A.; Cioatera, N.; Trandafir, I.; Hulea, V. Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg (II) adsorption from aqueous media. J. Hazard. Mater. 2010, 182, 197–203. [Google Scholar] [CrossRef]
- Synytsya, A.; Synytsya, A.; Blafková, P.; Ederová, J.; Spěvaček, J.; Slepička, P.; Král, V.; Volka, K. pH-Controlled Self-Assembling of meso-Tetrakis(4-sulfonatophenyl)porphyrin−Chitosan Complexes. Biomacromolecules 2009, 10, 1067–1076. [Google Scholar] [CrossRef]
- Roumeliotis, P.; Kurganov, A.; Davankov, V. Effect of the hydrophobic spacer in bonded [Cu (l-hydroxyprolyl) alkyl]+ silicas on retention and enantioselectivity of α-amino acids in high-performance liquid chromatography. J. Chromatogr. A 1983, 266, 439–450. [Google Scholar] [CrossRef]
- Kudryavtsev, G.; Mil’chenko, D.; Bernadyuk, S.; Vertinskaya, T.; Lisichkin, G. Synthesis and properties of phosphate cation-exchangers based on silica. Theor. Exp. Chem. 1988, 23, 658–663. [Google Scholar] [CrossRef]
- Radi, S.; El Abiad, C.; Moura, N.M.; Faustino, M.A.; Neves, M.G.P. New Hybrid Adsorbent Based on Porphyrin Functionalized Silica for Heavy Metals Removal: Synthesis, Characterization, Isotherms, Kinetics and Thermodynamics Studies. J. Hazard. Mater. 2017. [Google Scholar] [CrossRef]
- Sanders, J.K.; Bampos, N.; Clyde-watson, Z.; Darling, S.L.; Hawley, J.C.; KIM, H.-J.; Mak, C.C.; Webb, S.J. Axial Coordination Chemistry 15 of Metalloporphyrins. Porphyr. Handb. Inorg. Organomet. Coord. Chem. 2000, 3, 1. [Google Scholar]
- De Luca, G.; Romeo, A.; Scolaro, L.M.; Ricciardi, G.; Rosa, A. Sitting-atop metallo-porphyrin complexes: Experimental and theoretical investigations on such elusive species. Inorg. Chem. 2009, 48, 8493–8507. [Google Scholar] [CrossRef]
- Munro, O.Q.; Bradley, J.C.; Hancock, R.D.; Marques, H.M.; Marsicano, F.; Wade, P.W. Molecular mechanics study of the ruffling of metalloporphyrins. J. Am. Chem. Soc. 1992, 114, 7218–7230. [Google Scholar] [CrossRef]
- Sanders, J.K.M.; Bampos, N.; Clyde-Watson, Z.; Darling, S.L.; Hawley, J.C.; Kim, H.-J.; Ching, C.; Webb, S.J. Axial Coordination Chemistry of Metalloporphyrins; Academic Press: Singapore, 2000; Volume 3. [Google Scholar]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Shi, H.; Li, W.; Zhong, L.; Xu, C. Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: Equilibrium, kinetics, and thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 1108–1118. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Djaafri, A.; Mokaddem, H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2010, 184, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mangwandi, C.; Ala’a, H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the correct evaluation of sorption thermodynamic parameters from equilibrium isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Hamad, H.; Ezzeddine, Z.; Kanaan, S.; Lakis, F.; Hijazi, A.; Moussawi, M.-A. A novel modification and selective route for the adsorption of Pb2+ by oak charcoal functionalized with glutaraldehyde. Adv. Powder Technol. 2016, 27, 631–637. [Google Scholar] [CrossRef]
- Koorepazan Moftakhar, M.; Dousti, Z.; Yaftian, M.R.; Ghorbanloo, M. Investigation of heavy metal ions adsorption behavior of silica-supported Schiff base ligands. Desalin. Water Treat. 2016, 57, 27396–27408. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Janus, L.; Mabkhot, Y.N. Fabrication and covalent modification of highly chelated hybrid material based on silica-bipyridine framework for efficient adsorption of heavy metals: Isotherms, kinetics and thermodynamics studies. RSC Adv. 2016, 6, 82505–82514. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Baquet, M.; Zaghrioui, M. New adsorbent material based on nitrothiophene-functionalized silica particles for aqueous heavy metals removal. J. Sulfur Chem. 2016, 37, 296–306. [Google Scholar] [CrossRef]
- Fan, H.-T.; Sun, X.-T.; Zhang, Z.-G.; Li, W.-X. Selective removal of lead (II) from aqueous solution by an ion-imprinted silica sorbent functionalized with chelating N-donor atoms. J. Chem. Eng. Data 2014, 59, 2106–2114. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; El Massaoudi, M.; Bacquet, M.; Degoutin, S.; Revel, B.; Mabkhot, Y.N. Thermodynamics and kinetics of heavy metals adsorption on silica particles chemically modified by conjugated β-ketoenol furan. J. Chem. Eng. Data 2015, 60, 2915–2925. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Wu, G.; Wu, D.; Wu, A. Colorimetric detection of copper and efficient removal of heavy metal ions from water by diamine-functionalized SBA-15. Dalton Trans. 2014, 43, 8461–8468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. Preparation and characterization of functionalized silica spheres for removal of Cu (II), Pb (II), Cr (VI) and Cd (II) from aqueous solutions. RSC Adv. 2015, 5, 28624–28632. [Google Scholar] [CrossRef]
- Wang, F.P.; Li, G.F.; Zhou, Q.Q.; Yang, C.X.; Wang, Q.Z. Removal of metal ions from aqueous solution with β-cyclodextrin-based hydrogels. Mater. Express 2016, 6, 394–402. [Google Scholar] [CrossRef]
- Parambadath, S.; Mathew, A.; Park, S.S.; Ha, C.-S. Pentane-1, 2-dicarboxylic acid functionalized spherical MCM-41: A simple and highly selective heterogeneous ligand for the adsorption of Fe 3+ from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1918–1927. [Google Scholar] [CrossRef]
- He, S.; Zhao, C.; Yao, P.; Yang, S. Chemical modification of silica gel with multidentate ligands for heavy metals removal. Desalin. Water Treat. 2016, 57, 1722–1732. [Google Scholar] [CrossRef]
- Liang, Z.; Shi, W.; Zhao, Z.; Sun, T.; Cui, F. The retained templates as “helpers” for the spherical meso-silica in adsorption of heavy metals and impacts of solution chemistry. J. Colloid Interface Sci. 2017, 496, 382–390. [Google Scholar] [CrossRef]
- Tamez, C.; Hernandez, R.; Parsons, J. Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem J. 2016, 125, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Nowee, S.M.; Ramezanian, N.; Raji, F. A new nanostructured material amino functionalized mesoporous silica synthesized via co-condensation method for Pb (II) and Ni (II) ion sorption from aqueous solution. Hydrometallurgy 2016, 161, 117–126. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Bacquet, M.; Degoutin, S.; Zaghrioui, M.; Jodeh, S.; Warad, I. Removal efficiency of Pb (II), Zn (II), Cd (II) and Cu (II) from aqueous solution and natural water by ketoenol–pyrazole receptor functionalized silica hybrid adsorbent. Sep. Sci. Technol. 2017, 52, 608–621. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Garcia, Y. New hybrid material based on a silica-immobilised conjugated β-ketoenol-bipyridine receptor and its excellent Cu (ii) adsorption capacity. Anal. Methods 2016, 8, 6923–6931. [Google Scholar] [CrossRef]
- Xue, X.; Li, F. Removal of Cu (II) from aqueous solution by adsorption onto functionalized SBA-16 mesoporous silica. Microporous Mesoporous Mater. 2008, 116, 116–122. [Google Scholar] [CrossRef]
Sample Availability: Sample of the hybrid SiTF5PP is available from the authors. |


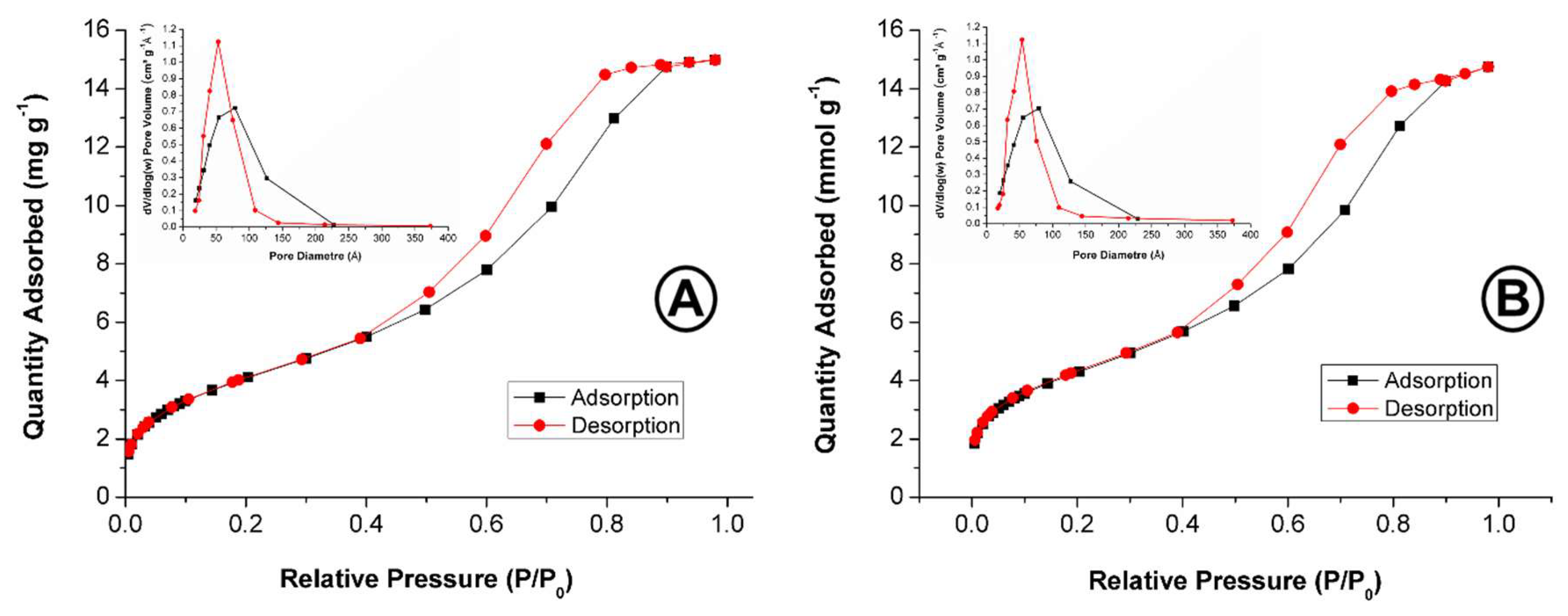




| Materials | Specific Surface SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (Å) |
|---|---|---|---|
| Si | 434.6526 ± 4.6197 | 0.671 ± 0.002 | 47.882 |
| SiPn | 328.7584 ± 5.5689 | 0.482 ± 0.003 | 46.358 |
| SiTF5PP | 341.8908 ± 4.0805 | 0.477 ± 0.010 | 46.307 |
| Material | qe ± 1.0 (mg g−1) | |||
|---|---|---|---|---|
| Pb(II) | Cu(II) | Cd(II) | Zn(II) | |
| Si | 4.35 | 4.58 | 3.26 | 6.89 |
| SiPn | 6.34 | 5.32 | 6.57 | 7.86 |
| SiTF5PP | 187.36 | 125.16 | 82.44 | 56.23 |
| Parameters | Metals | |||
|---|---|---|---|---|
| Pb(II) (1.19 Å) | Cu(II) (0.73 Å) | Cd(II) (0.95 Å) | Zn(II) (0.74 Å) | |
| qe (exp) (mg g−1) | 187.36 ± 1 | 125.17 ± 1 | 82.45 ± 1 | 56.23 ± 1 |
| qe (exp) (mmol g−1) | 0.90 | 1.97 | 0.73 | 0.86 |
| Pseudo-first-order | ||||
| qe (mg g−1) | 182.695 ± 2.095 | 123.393 ± 1.223 | 81.634 ± 0.568 | 55.390 ± 0.412 |
| qe (mmol g−1) | 0.881 | 1.943 | 0.726 | 0.847 |
| k1 (min−1) | 0.447 ± 0.50 | 0.340 ± 0.024 | 0.365 ± 0.019 | 0.322 ± 0.016 |
| R2 | 0.996 | 0.997 | 0.998 | 0.998 |
| Pseudo-second-order | ||||
| qe (mg −1g) | 191.205 ± 1.613 | 132.122 ± 0.651 | 83.641 ± 0.572 | 59.644 ± 0.455 |
| qe (mmol g−1) | 0.922 | 2.080 | 0.744 | 0.912 |
| k2 (g mg−1 min−1) | (6.11 ± 0.77) 10−3 | (5.27 ± 0.26) 10−3 | (9.40 ± 0.70) 10−3 | (10.53 ± 0.75) 10−3 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 |
| Metal | ΔH° (kJ mol−1) | ΔS° (J K−1 mol−1) | T (K) ± 1 K | ΔG° (kJ mol−1) |
|---|---|---|---|---|
| Pb(II) | 9.883 ± 0.100 | 35.52 ± 0.321 | 299.15 309.15 319.15 | −0.742 −1.098 −1.453 |
| Cu(II) | 21.834 ± 0.206 | 81.26 ± 0.659 | 299.15 309.15 319.15 | −2.477 −3.290 −4.103 |
| Cd(II) | 14.485 ± 0.149 | 55.63 ± 0.477 | 299.15 309.15 319.15 | −2.158 −2.714 −3.271 |
| Zn(II) | 19.275 ± 0.237 | 74.50 ± 0.759 | 299.15 309.15 319.15 | −3.010 −3.756 −4.500 |
| Metal | Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|---|
| q (mg g−1) | q (mmol g−1) | KL (L mg−1) | R2 | KF (mg g−1) | N | R2 | |
| Pb(II) | 206.282 ± 0.992 | 0.995 | 0.082 ± 0.001 | 0.999 | 48.209 ± 6.486 | 3.407 ± 0.383 | 0.952 |
| Cu(II) | 135.374 ± 0.880 | 2.131 | 0.115 ± 0.004 | 0.998 | 38.920 ± 5.789 | 3.945 ± 0.529 | 0.944 |
| Cd(II) | 85.384 ± 0.444 | 0.759 | 0.212 ± 0.008 | 0.998 | 33.282 ± 4.043 | 5.060 ± 0.723 | 0.944 |
| Zn(II) | 59.997 ± 0.400 | 0.917 | 0.101 ± 0.003 | 0.998 | 17.724 ± 1.637 | 4.080 ± 0.354 | 0.974 |
| Water Samples | Metal Ion | Cfound ± 0.05 (mg L−1) | Metal Ions Concentration after Adsorption on SiTF5PP (mg g−1) |
|---|---|---|---|
| Moulouya River | Pb(II) | 0.7506 | 0.6199 |
| Cd(II) | 0.3133 | 0.2048 | |
| Cu(II) | 0.9794 | 0.1726 | |
| Zn(II) | Not detectable | - |
| Silica gel - Ligand | Ref | Adsorption Capacity (mg g−1) | |||
|---|---|---|---|---|---|
| Pb(II) | Cu(II) | Cd(II) | Zn(II) | ||
| 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin | This work | 187.36 | 125.17 | 82.45 | 56.23 |
| 5,10,15,20-Tetraphenylporphyrin | [68] | 55.17 | 19.08 | 26.46 | 34.62 |
| N,N-bipyrazole amine | [25] | 123 | - | - | - |
| Glutaraldehyde | [79] | 29.32 | - | - | - |
| 2-Hydroxy-3-methoxybenzaldehyde | [80] | 2.27 | 4.70 | - | - |
| Bipyridine tripodal receptor | [81] | 99.68 | 64.84 | 42.18 | 82.68 |
| Nitrothiophene | [82] | 52.41 | 61.52 | 38.45 | 35.72 |
| PMAEEDA | [83] | 61.90 | 19.96 | 19.34 | 17.16 |
| Ketoenol Furane | [84] | 18.75 | 32.08 | 52.15 | 23.36 |
| N-[3(Trimethoxysilyl)propyl]ethyl-ene-diamine | [85] | 96.43 | 27.22 | - | 12.16 |
| 3-(2-Aminoethylamino)pro-pyldimethoxymethylsilane | [86] | 100.58 | 74.69 | 121.43 | - |
| SiO2-g-GMA/β-CD-Ac. | [87] | 51.02 | 110.0 | 15.20 | - |
| Pentane-1,2-dicarboxylic acid | [88] | 122.0 | 38.0 | 30.0 | 28.0 |
| (E)-4-((Pyridin-2-yl-methyl-ene)amino)phenol | [29] | 36.38 | - | 32.38 | 92.00 |
| Dithiocarbamate | [89] | 42.16 | 25.00 | 10.01 | 26.01 |
| Cetyltrimethylammonium bromide | [90] | - | 32.20 | 8.00 | - |
| Nano-sized Fe3O4 | [91] | 166.67 | 37.04 | - | - |
| MCM-41/N-(3-trimethoxysilyl)-propyl) diethylenetriamine | [92] | 77.52 | - | - | - |
| (2Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxybut-2-en-1-one | [93] | 94.18 | 71.40 | 40.18 | 83.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Abiad, C.; Radi, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium. Molecules 2019, 24, 669. https://doi.org/10.3390/molecules24040669
El Abiad C, Radi S, Faustino MAF, Neves MGPMS, Moura NMM. Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium. Molecules. 2019; 24(4):669. https://doi.org/10.3390/molecules24040669
Chicago/Turabian StyleEl Abiad, Chahrazad, Smaail Radi, Maria A. F. Faustino, M. Graça P. M. S. Neves, and Nuno M. M. Moura. 2019. "Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium" Molecules 24, no. 4: 669. https://doi.org/10.3390/molecules24040669
APA StyleEl Abiad, C., Radi, S., Faustino, M. A. F., Neves, M. G. P. M. S., & Moura, N. M. M. (2019). Supramolecular Hybrid Material Based on Engineering Porphyrin Hosts for an Efficient Elimination of Lead(II) from Aquatic Medium. Molecules, 24(4), 669. https://doi.org/10.3390/molecules24040669