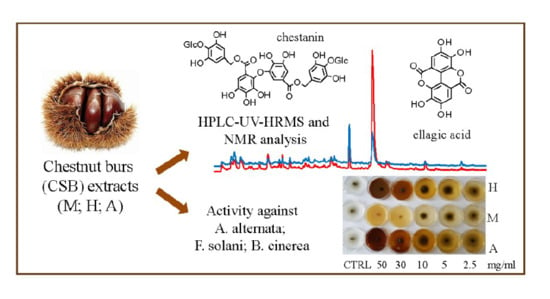
Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenol Content and Antioxidant Activity of CSB Extracts
2.2. UHPLC-UV-(−)-HRMS Profiling of CSB Extracts
2.2.1. Hydrolysable Tannins
2.2.2. Flavonoids
2.3. Isolation and Identification of Compounds
2.4. Quantitative Analysis of CSB Extracts
2.5. In Vitro Antifungal Activity
3. Experimental Section
3.1. Chemicals and Reagents
3.2. General Experimental Procedures
3.3. Chestnut Spiny Burs Material
3.4. Preparation of Chestnut Spiny Bur Extracts
3.5. Quantitative Determination of Total Phenol Content
3.6. Bleaching of the Free-radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH Test)
3.7. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.8. UHPLC-UV-ESI-HRMS Analysis
3.9. Quantitative HPLC Analysis
3.10. Isolation and Identification of Compounds
3.11. Antifungal Activity
3.11.1. Fungal Pathogens
3.11.2. In Vitro Antifungal Assays
3.11.3. Spore Germination Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vella, F.M.; Laratta, B.; La Cara, F.; Alessandra, M. Recovery of bioactive molecules from chestnut (Castanea sativa Mill) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, T.; Hu, G.; Guo, K.; Wei, C. Comparison of Physicochemical Properties of Starches from Nine Chinese Chestnut Varieties. Molecules 2018, 23, 3248. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crop. Prod. J. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Fernández-agulló, A.; Freire, M.S.; Antorrena, G.; Pereira, A.J.; González-álvarez, J. Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from Chestnut (Castanea sativa) Bur and Shell Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from. Sep. Sci. Technol. 2014, 49, 267–277. [Google Scholar] [CrossRef]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential anticancer effects of polyphenols from chestnut shell extracts: Modulation of cell growth, and cytokinomic and metabolomic profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa by-productss: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef]
- Pinto, D.; Rodrigues, F.; Braga, N.; Santos, J.; Pimentel, F.B.; Oliveira, M.B.P.P. Function ingredient for nutraceutical and cosmetic. Food Funct. 2017, 8, 201–208. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Álvarez, J.; Freire, M.S.; Fernández-Agulló, A.; Santos, J.; Antorrena, G. Chestnut Burs as a Source of Natural Antioxidants. Chem. Eng. Trans. 2009, 17, 855–860. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects. A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yin, P.; Wang, Y.; Yang, L.; Sui, J.; Liu, Y. Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid, Kaempferol and Their Derivatives. Molecules 2018, 23, 1046. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal Activity of Ellagic Acid In Vitro and In Vivo. Phytother. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites : A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Romeo, F.V.; Ballistreri, G.; Fabroni, S.; Pangallo, S.; Li Destri Nicosia, M.G.; Schena, L.; Rapisarda, P. Chemical characterization of different sumac and pomegranate extracts effective against Botrytis cinerea rots. Molecules 2015, 20, 11941–11958. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, S.; Tsao, R.; Charles, M.T.; Yang, R.; Khanizadeh, S. Archives of Phytopathology and Plant Protection In vitro antifungal activity and mode of action of selected polyphenolic antioxidants on Botrytis cinerea. Arch. Phytopathol. Plant Prot. 2010, 43, 1564–1578. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Parisi, M.; Nazzaro, F.; Zaccardelli, M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016, 84, 81–87. [Google Scholar] [CrossRef]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial Identi fi cation of Antifungal Compounds from Punica granatum Peel Extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R.; Aguilar, C.N. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind. Crop. Prod. 2010, 31, 153–157. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Pane, C.; Villecco, D.; Roscigno, G.; De Falco, E.; Zaccardelli, M. Screening of plant-derived antifungal substances useful for the control of seedborne pathogens. Arch. Phytopathol. Plant Prot. 2013, 46, 1533–1539. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-rich Fraction with Antioxidant and Anti-Candida albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.Y.; Chen, S.Y.; Shi, L.L.; Liu, Y.J.; Ma, C. Antioxidant potential of polyphenols and tannins from burs of Castanea mollissima Blume. Molecules 2011, 16, 8590–8600. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Salminen, J. Two-Dimensional Tannin Fingerprints by Liquid Chromatography Tandem Mass Spectrometry Offer a New Dimension to Plant Tannin Analyses and Help To Visualize the Tannin Diversity in Plants. J. Agric. Food Chem. 2018, 66, 9162–9171. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Sinkkonen, J.; Salminem, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass spectrom. 2004, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Vukics, V.; Introduction, I. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef]
- García-villalba, R.; Carlos, J.; Tomás-barberán, F.A.; Rocha-guzmán, N.E. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food Compos. Anal. 2017, 63, 38–46. [Google Scholar] [CrossRef]
- Yang, B.; Kortesniemi, M.; Liu, P.; Karonen, M.; Salminen, J. Analysis of Hydrolyzable Tannins and Other Phenolic Compounds in Emblic Leafflower (Phyllanthus emblica L.) Fruits by High Performance Liquid Chromatography−Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 8672–8683. [Google Scholar] [CrossRef]
- Tanaka, T.; Ueda, N.; Shinohara, H.; Nonaka, G.; Fujioka, T.; Mihashi, K.; Kouno, I. C-Glycosidic ellagitannin metabolites in the heartwood of japanese chestnut tree (Castanea crenata SIEB. et ZUCC.). Chem. Pharm. Bull. 1996, 44, 2236–2242. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-gonzález, C.; Vallverdú-queralt, A.; Simal-gándara, J.; Lamuela-raventós, R.; Izquierdo-pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap—Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D.; Kim, W.H.; Yang, H.; Sung, S.H. Phytochemistry Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their a -glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Tiago, C.; Montserrat, D.; Silva, S.; Oliveira, R.; Carvalho, A.M.; Henriques, M.; Santos-buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds in wild medicinal flowers from Portugal by HPLC – DAD – ESI/MS and evaluation of antifungal properties. Ind. Crop. Prod. 2013, 44, 104–110. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Michele, L.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La, F.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Mendoza, L.; Ya, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind. Crop. Prod. 2013, 43, 360–364. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Song, X.; Liu, C.; Chen, P.; Zhang, H.; Sun, R. Natural product-based pesticide discovery: Design, synthesis and bioactivity studies of N-amino-maleimide derivatives. Molecules 2018, 23, 1521. [Google Scholar] [CrossRef]
- Thomidis, T.; Michailides, T.; Exadaktylou, E. Contribution of pathogens to peach fruit rot in northern Greece and their sensitivity to iprodione, carbendazim, thiophanate-methyl and tebuconazole fungicides. J. Phytopathol. 2009, 157, 194–200. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Di Liberto, M.; Raimondi, M.; Postigo, A.; Sortino, M. Phytomedicine Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana l.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Mansour, A.; Celano, R.; Mencherini, T.; Picerno, P.; Piccinelli, A.L.; Foudil-Cherif, Y.; Csupor, D.; Rahili, G.; Yahi, N.; Nabavi, S.M.; et al. A new cineol derivative, polyphenols and norterpenoids from Saharan myrtle tea (Myrtus nivellei): Isolation, structure determination, quantitative determination and antioxidant activity. Fitoterapia 2017, 119, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Nicoletta, P.; Proteggente, A.; Ananth, P.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radical. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chemam, Y.; Benayache, S.; Marchioni, E.; Zhao, M.; Mosset, P.; Benayache, F.; McPhee, D.J. On-line screening, isolation and identification of antioxidant compounds of Helianthemum ruficomum. Molecules 2017, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.; Regasini, L.O.; Skrzek, S.C.G.; Queiroz, M.M.F.; Silva, D.H.S.; Da Silva Bolzani, V. Free radical scavenging activity of Kielmeyera variabilis (Clusiaceae). Molecules 2013, 18, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Takino, Y. Carbon-13 Nuclear Magnetic Resonance Spectra of Phenolic Glycosides Isolated from Chestnut Galls. Agric. Biol. Chern. 1979, 43, 1173–1177. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Nonaka, G.; Nishioka, I. Hydrolysable tannins and related compounds Castanea mollissima. Phytochemistry 1988, 27, 1185–1189. [Google Scholar] [CrossRef]
- Jaramillo, K.; Dawid, C.; Hofmann, T.; Fujimoto, Y.; Osorio, C. Identification of Antioxidative Flavonols and Anthocyanins in Sicana odorifera Fruit Peel. J. Agric. Food Chem. 2011, 59, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.L.M.; Carli, C.B.A.; Rodrigues, C.M.; Maia, D.C.G.; Carlos, I.Z.; Eberlin, M.N.; Hiruma-Lima, A.; Vilegas, W. Identification of Ellagic Acid Derivatives in Methanolic Extracts from Qualea Species. Z Naturforsch C 2008, 63, 794–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Shen, L.; Muraoka, O.; Cheng, M. Synthesis of Quercetin 3-O-[6″-O-(trans-p- Coumaroyl)]-β-d-Glucopyranoside. J. Carbohydr. Chem. 2011, 30, 119–131. [Google Scholar] [CrossRef]
- Benahmed, M.; Akkal, S.; Elomri, A.; Laouer, H.; Vérité, P.; Seguin, E. Constituents from Bupleurum montanum (Coss. & Dur.) (Apiaceae). Arabian J. Chem. 2014, 7, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Merfort, I.; Wendisch, D. Flavonoiglucuronide aus den Bluten von Arnica montana. Planta Med. 1988, 16, 98–101. [Google Scholar]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
Sample Availability: Samples of CSB extracts and isolated compounds are available from the authors. |


| DPPH Test (EC50 a µg/mL Extract or Phenol) b | TEAC Value c (mM Trolox/mg Extract or mM Compound) b | TPC b g GAE d/100 g Extract | Chestanin e (mg/g) | EA e (mg/g) | |
|---|---|---|---|---|---|
| CSB-H | 24.94 ± 0.46 | 3.00 ± 0.22 | 20.60 ± 0.85 | 3.21 | 7.40 |
| CSB-M | 12.64 ± 0.12 | 3.52 ± 0.13 | 26.42 ± 0.95 | 13.34 | 79.32 |
| CSB-A | 22.38 ± 2.80 | 2.71 ± 0.71 | 20.26 ± 0.14 | 1.10 | 5.26 |
| quercetin 3-O-β-d-glucopyranoside (25) | 2.98 ± 0.84 | 3.39 ± 0.11 | |||
| EA (27) | 2.40 ± 0.24 | 4.98 ± 0.21 | |||
| Chestanin (21) | 16.62 ± 0.84 | 1.05 ± 0.14 | |||
| gallic acid f | 1.23 ± 0.15 | 3.49 ± 0.21 |
| N a | RT (min) | [M − H]− (m/z) | Molecular Formula | Error ppm | Diagnostic Product Ions (m/z) b | Compound | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1.97 | 1101.0672 | C48H30O31 | −1.4 | 1057 [M − H − CO2]− | valoneoyl–NHTP–glucose (vescavaloninic/castavaloninic acid) | [24] |
| 2 | 2.45 | 933.0613 | C41H26O26 | −1.6 | 915 [M − H − H2O]−, 889 [M − H − CO2]−, 871 [M − H − H2O − CO2]−, 631 [M − H − EA]−, 613 [M − H − EA − H2O]−, 587 [M − H − EA − CO2]−, 569 [M − H − EA − H2O − CO2]− | HHDP–NHTP–glucose (castalagin/vescalagin) | [28] |
| 3 | 2.59 | 783.0663 | C34H24O22 | −1.6 | 481 [M − H − EA]−, 301 [EA − H]− (C14H5O8 1.8 ppm) | diHHDP–glucose (pedunculagin) | [28] |
| 4 | 3.16 | 947.0772 | C42H28O26 | −1.4 | 915 [M − H − CH3OH]− | methyl–HHDP–NHTP–glucose (methylvescalagin) | [24] |
| 5 | 4.01 | 783.0661 | C34H24O22 | −1.9 | 481 [M − H − EA]−, 301 [EA − H]− (C14H5O8 1.8 ppm) | diHHDP-glucose (pedunculagin) | [28] |
| 6 | 4.71 | 953.0882 | C41H30O27 | −0.9 | 909 [M − H − CO2]−, 785 [M − H − C7H4O5]− | galloyl-chebuloyl-HHDP-glucose (chebulagic acid) | [29] |
| 7 | 4.74 | 613.0454 | C27H18O17 | −0.9 | 595 [M − H − H2O]−, 523 [M − H − C3H6O3]−, 493 [M − H − C 4H8O4]− | castacrenin C | [30] |
| 8 | 5.32 | 785.0820 | C34H26O22 | −1.4 | 633 [M − H − galloyl]−, 615 [M − H − GA]−, 483 [M − H − EA]−, 301 (C14H5O8 2.1 ppm) | digalloyl-HHDP-glucose (tellimagrandin I) | [31] |
| 9 | 5.58 | 613.0455 | C27H18O17 | −0.8 | 595 [M − H − H2O]−, 523 [M − H − C3H6O3]−, 493 [M − H − C4H8O4]− | castacrenin B f | [30] |
| 10 | 5.71 | 1115.0825 | C49H32O31 | −1.3 | 1097 [M − H − H2O]−, 1071 [M − H − CO2]−, 1053 [M − H − H2O − CO2]−, 933 [M − H − C8H6O5]−, 569 [M − H − C8H6O5 − EA − CO2 − H2O]− | methylvaloneoyl–NHTP–glucose (vescavaloninic/castavaloninic acid methyl ester) | |
| 11 | 5.98 | 613.0454 | C27H18O17 | −0.9 | 523 [M − H − C3H6O3]−, 493 [M − H − C4H8O4]− | Castacrenin A f | [30] |
| 12 | 6.20 | 935.0769 | C41H28O26 | −1.8 | 917 [M − H − H2O]−, 873 [M − H − H2O − CO2]−, 783 [M − H − GA]− 633 [M − H − EA]−, | galloyl-diHHDP-glucose (stachyurin/casuarinin) | [31] |
| 13 | 6.53 | 933.0609 d | C82H52O52 | 1.0 | 1565 [M − H − EA]−, 915 [HHDP-NHTP-glucose − H2O]−, 633 [galloyl-diHHDP-glucose − EA]−, 631 [HHDP-NHTP-glucose − EA]− | HHDP-NHTP-glucose-galloyl-diHHDP-glucose (cocciferin d2) | [24] |
| 14 | 6.93 | 967.1035 | C42H32O27 | −1.3 | 785 [M − H − C8H6O5]− | galloyl-methylchebuloyl-HHDP-glucose (chebulagic acid methyl ester) | [32] |
| 15 | 7.76 | 785.0822 | C34H26O22 | −1.3 | 633 [M − H − galloyl]−, 615 [M − H − GA]−, 483 [M − H − EA]−, 301 (C14H5O8 2.2 ppm) | digalloyl-HHDP-glucose (tellimagrandin I) | [31] |
| 16 | 7.97 | 953.0882 | C41H30O27 | −0.9 | 909 [M − H − CO2]−, 785 [M − H − C7H4O5]− | galloyl-chebuloyl-HHDP-glucose (chebulagic acid) | [29] |
| 17 | 8.18 | 637.1028 | C27H26O18 | −1.2 | 467 [M − H − GA]−, 305 [M − H − GA − hex]− | Chesnatin f | |
| 18 | 8.65 | 967.1038 | C42H32O27 | −1.0 | 785 [M − H − C8H6O5]− | galloyl-methylchebuloyl-HHDP-glucose (chebulagic acid methyl ester) | [32] |
| 19 | 9.49 | 637.1032 | C27H26O18 | −1.0 | 593 [M − H − CO2]−, 469 [M − H − C7H6O5]− | isochesnatin f | |
| 20 | 10.35 | 469.0972 | C20H22O13 | −1.0 | 169 [GA − H]− (C7H5O5 1.8 ppm), | cretanin f | |
| 21 | 11.61 | 937.1871 | C40H42O26 | −1.0 | 637 [M − H − C13H16O8]−, 467 [M − H − C20H22O13]− | chestanin f | |
| 22 | 12.25 | 351.1076 | C17H20O8 | 0.5 | 163 [M − H − C8H12O5]− | methyl coumaroyl quinate | |
| 23 | 12.43 | 615.0977 | C28H24O16 | −0.5 | 463 [M − H − galloyl]−, 301 [Ag − H]− (C15H9O7 0.4 ppm) | quercetin-galloyl-hexoside | [28] |
| 24 | 12.64 | 937.1865 | C40H42O26 | 1.7 | 467 [M − H − C20H22O13]− | chestanin isomer f | |
| 25 | 13.48 | 463.0867 | C21H20O12 | −1.5 | 301 [Ag − H]− (C15H9O7 1.2 ppm) | quercetin 3-O-β-d-glucopyranoside f | |
| 26 | 13.33 | 477.0660 | C21H18O13 | −0.8 | 301 [Ag − H]− (C15H9O7 0.8 ppm) | quercetin hexuronoside | [33] |
| 27 | 13.62 | 300.9982 | C14H6O8 | 1.1 | - | ellagic acid c | |
| 28 | 14.78 | 551.1026 e | C23H22O13 | −1.0 | 343 [M − H − Hex]− | Ellagic acid 3,3′,4-trimethoxy 4′-O-β-d-glucopyranoside f | |
| 29 | 14.91 | 447.0916 | C21H20O11 | −1.3 | 327 [M − H − C4H8O4]−, 285 [Ag − H]− (C15H9O6 1.1 ppm) | Astragalin f | |
| 30 | 14.94 | 491.0815 | C22H20O13 | −1.0 | 315 [Ag − H]− (C16H11O7 0.6 ppm), 301 [M − H − Hexu−CH3]− | Isorhamnetin hexuronoside f | |
| 31 | 15.11 | 477.1024 | C22H22O12 | −0.6 | 315 [Ag − H]− (C16H11O7 1.8 ppm) | isorhamnetin 3-O-β-d-glucopyranoside f | |
| 32 | 15.29 | 623.1599 | C28H32O16 | −1.2 | 315 [Ag − H]− (C16H11O7 1.6 ppm) | isorhamnetin-rhamnoside-hexoside | [28] |
| 33 | 16.8 | 609.1231 | C30H26O14 | −1.3 | 463 [M − H − coumaroyl]−, 301 [Ag − H]− (C15H9O7 0.8 ppm)] | quercetin 3-O-(6″-O-trans-p-coumaroyl)-β-d-glucopyranoside f | |
| 34 | 17.84 | 593.128 | C30H26O13 | −1.5 | 447 [M − H − coumaroyl]−, 285 [Ag − H]− (C15H10O6 1.2 ppm) | kaempherol coumaroyl hexoside | [28] |
| 35 | 18.00 | 593.12748 | C30H26O13 | −2.5 | 447 [M − H − coumaroyl]−, 285 [Ag − H]− (C15H10O6 1.7 ppm) | Tiliroside c | |
| 36 | 18.04 | 535.1076 e | C23H22O12 | −1.1 | 343 [M−H dHex]− | Ellagic acid 3,3′,4-trimethoxy 4′-O-α-l-rhamnopyranoside f | |
| 37 | 18.22 | 623.1388 | C31H28O14 | −1.1 | 477 [M − H − coumaroyl]−, 315 [Ag − H]− (C16H12O7 1.5 ppm) | isorhamnetin coumaroyl hexoside | [28] |
| 38 | 19.16 | 593.1284 | C30H26O13 | −1.0 | 285 [Ag − H]− (C15H10O6 1.5 ppm) | kaempherol coumaroyl hexoside | [28] |
| 39 | 19.74 | 635.1282 | C32H28O14 | −2.1 | 575 [M − H − acetyl]−, 285 [Ag − H]− (C15H10O6 2.7 ppm) | kaempherol acetyl coumaroyl hexoside | [28] |
| 40 | 21.09 | 739.1648 | C39H32O15 | −1.3 | 593 [M − H − coumaroyl]−, 453 [M − H − Kaempferol]−, 285 [Ag − H]− (C15H10O6 2.4 ppm) | kaempferol dicoumaroyl hexoside | [28] |
| 41 | 21.9 | 781.1753 | C41H34O16 | −1.3 | 635 [M − H − coumaroyl]−, 495 [M − H − Kaempferol]−, 285 [Ag − H]− (C15H10O6 2.2 ppm) | kaempherol acetyl dicoumaroyl hexoside | [28] |
| 42 | 22.11 | 781.1747 | C41H34O16 | −2.0 | 635 [M − H − coumaroyl]−, 495 [M − H−Kaempferol]−, 285 [Ag − H]− (C15H10O6 2.2 ppm) | kaempherol acetyl dicoumaroyl hexoside | [28] |
| EC50 Growth Inhibition (mg mL−1) | 95% Fiducial Limits | Chi-square Test (p Value) a | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Alternaria alternata | ||||
| CSB-H | 8.71 | 7.16 | 10.26 | 1.00 |
| CSB-M | 6.29 | 5.71 | 6.87 | 0.88 |
| CSB-A | 14.53 | 13.59 | 18.17 | 0.99 |
| Botrytis cinerea | ||||
| CSB-H | >70 | |||
| CSB-M | 64.98 | 61.85 | 68.11 | 0.88 |
| CSB-A | >70 | |||
| Fusarium solani | ||||
| CSB-H | 14.13 | 11.35 | 16.91 | 0.55 |
| CSB-M | 6.04 | 5.22 | 6.85 | 0.99 |
| CSB-A | 15.51 | 11.19 | 19.83 | 0.93 |
| EC50 Germination Inhibition (mg mL −1) | 95% Fiducial Limits | Chi-square Test (p Value) a | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Alternaria alternata | ||||
| CSB-H | 11.17 | 8.91 | 27.77 | 0.53 |
| CSB-M | 2.66 | 1.48 | 8.70 | 1.00 |
| CSB-A | 5.48 | 1.14 | 9.82 | 0.24 |
| Botrytis cinerea | ||||
| CSB-H | >50 | |||
| CSB-M | 16.33 | 4.85 | 27.81 | 0.61 |
| CSB-A | >50 | |||
| Fusarium solani | ||||
| CSB-H | 10.52 | 5.28 | 15.76 | 0.72 |
| CSB-M | 2.22 | 1.84 | 2.60 | 0.95 |
| CSB-A | 6.80 | 5.18 | 8.42 | 0.99 |
| EC50 Growth Inhibition (µg mL−1) | 95% Fiducial Limits | Chi-square Test (p value) a | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Alternaria alternata | ||||
| EA (27) | 13.33 | 12.77 | 13.90 | 0.99 |
| Chestanin (21) | 561.56 | 544.57 | 578.54 | 0.92 |
| Iprodione | 0.85 | 0.70 | 0.99 | 1.00 |
| Botrytis cinerea | ||||
| EA (27) | 112.64 | 8.89 | 219.11 | 1.00 |
| Chestanin (21) | >2000 | |||
| Iprodione | 37.36 | 18.90 | 58.10 | 0.99 |
| Fusarium solani | ||||
| EA (27) | 21.27 | 15.57 | 26.43 | 1.00 |
| Chestanin (21) | >2000 | |||
| Carbendazim | 14.29 | 6.03 | 21.97 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. https://doi.org/10.3390/molecules24020302
Esposito T, Celano R, Pane C, Piccinelli AL, Sansone F, Picerno P, Zaccardelli M, Aquino RP, Mencherini T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules. 2019; 24(2):302. https://doi.org/10.3390/molecules24020302
Chicago/Turabian StyleEsposito, Tiziana, Rita Celano, Catello Pane, Anna Lisa Piccinelli, Francesca Sansone, Patrizia Picerno, Massimo Zaccardelli, Rita Patrizia Aquino, and Teresa Mencherini. 2019. "Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi" Molecules 24, no. 2: 302. https://doi.org/10.3390/molecules24020302