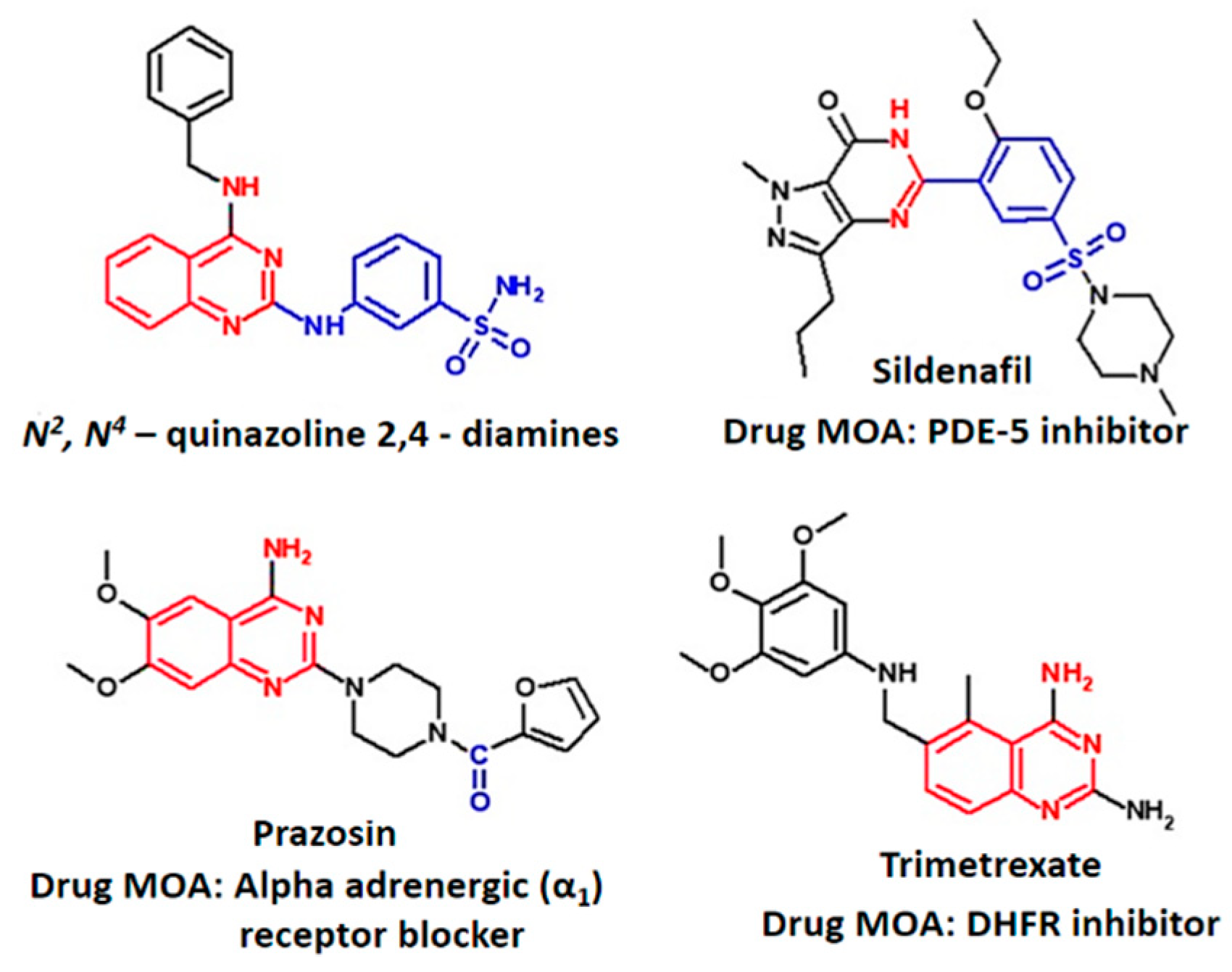
Elucidation of Vasodilation Response and Structure Activity Relationships of N2,N4-Disubstituted Quinazoline 2,4-Diamines in a Rat Pulmonary Artery Model
Abstract
:1. Introduction
2. Results
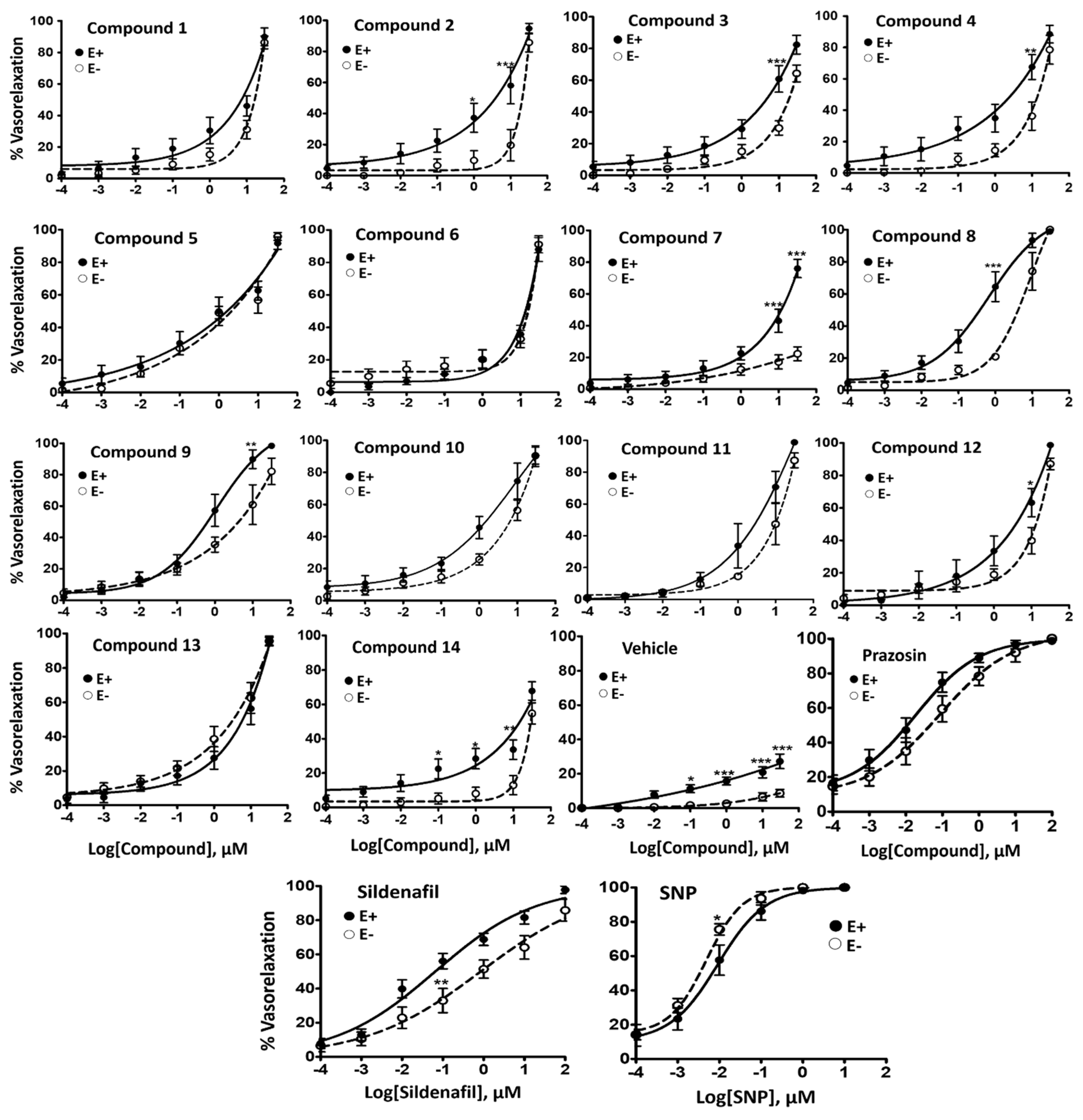
2.1. Vasorelaxant Effects of N2,N4-Diamino Quinazoline Analogues
2.2. Inhibitory Effect on Phenylephrine—Induced Contractile Response
2.3. Cytotoxicity and Solubility
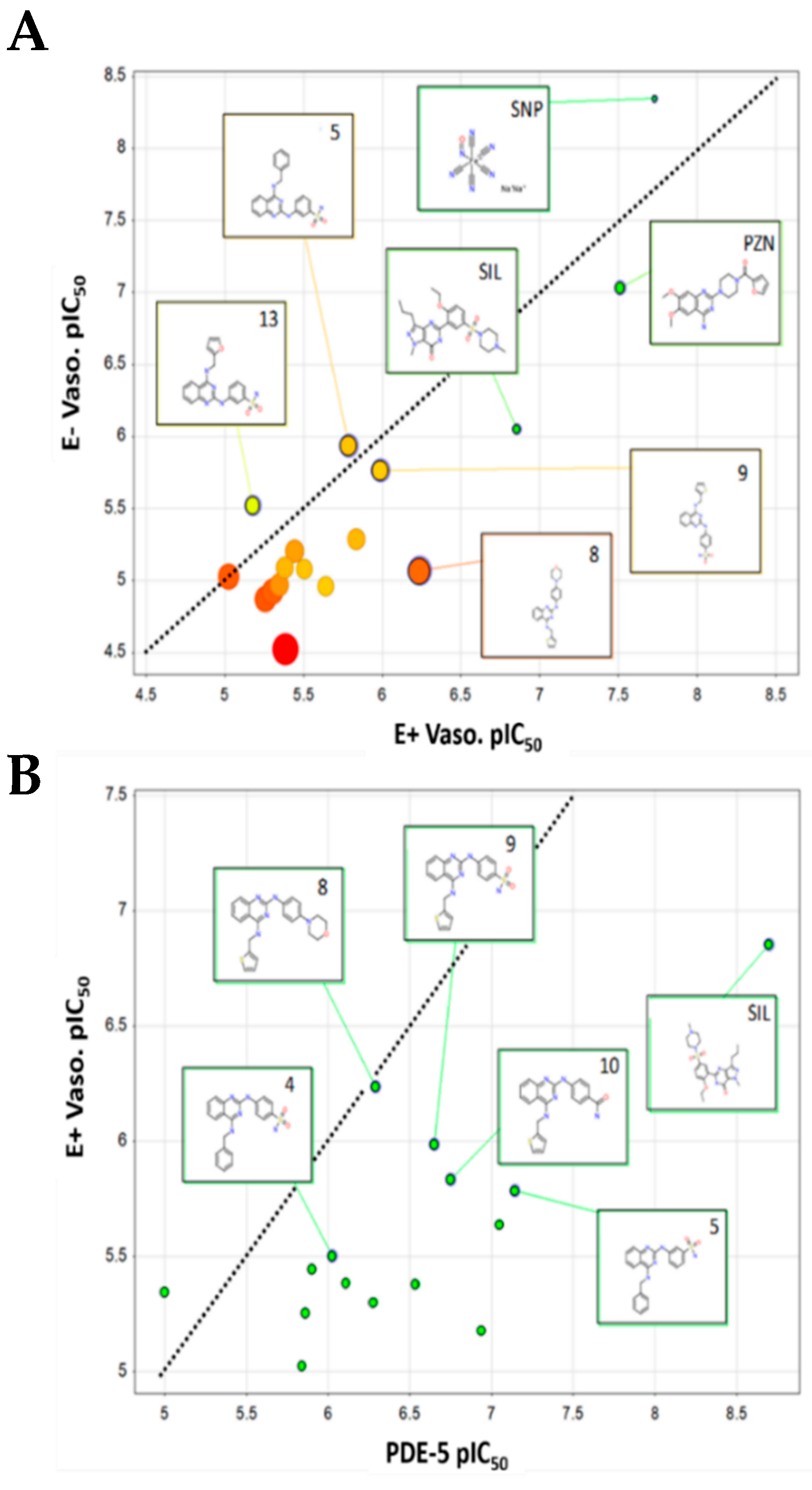
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.1.1. Animals
4.1.2. Compounds and Solutions
4.2. Experimental Protocols
4.2.1. Vascular Reactivity
4.2.2. Vasodilator Effects of Various Quinazoline Analogues
4.2.3. Determination of Inhibitory Role of Quinazoline Analogues on PE-Induced Contraction
4.2.4. Cytotoxicity
4.2.5. Solubility
4.2.6. Cheminformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.-F.; Chabot, F. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ribeiro, D.; Mendes-Ferreira, P.; Maia-Rocha, C.; Adao, R.; Leite-Moreira, A.F.; Brás-Silva, C. Pulmonary arterial hypertension: Basic knowledge for clinicians. Arch. Cardiovasc. Dis. 2016, 109, 550–561. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Gibbs, J.S.R. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur. Respir. Rev. 2014, 23, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef]
- Christman, B.W.; McPherson, C.D.; Newman, J.H.; King, G.A.; Bernard, G.R.; Groves, B.M.; Loyd, J.E. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992, 327, 70–75. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Q.; Luo, Q.; Qiao, H.; Wang, P.; Yu, J.; Cao, Y.; Lu, B.; Qu, L. Norepinephrine stimulation of alpha1D-adrenoceptor promotes proliferation of pulmonary artery smooth muscle cells via ERK-1/2 signaling. Int. J. Biochem. Cell Biol. 2017, 88, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Sahara, M.; Takahashi, T.; Imai, Y.; Nakajima, T.; Yao, A.; Morita, T.; Hirata, Y.; Nagai, R. New insights in the treatment strategy for pulmonary arterial hypertension. Cardiovasc. Drugs Ther. 2006, 20, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Humbert, M.; Jagot, J.; Taravella, O.; Fartoukh, M.; Parent, F.; Herve, P.; Simonneau, G. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium-channel blockers in primary pulmonary hypertension. Eur. Respir. J. 1998, 12, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badlam, J.B.; Bull, T.M. Steps forward in the treatment of pulmonary arterial hypertension: Latest developments and clinical opportunities. Ther. Adv. Chronic Dis. 2017, 8, 47–64. [Google Scholar] [CrossRef]
- McLaughlin, V.; Sitbon, O.; Badesch, D.; Barst, R.; Black, C.; Galie, N.; Rainisio, M.; Simonneau, G.; Rubin, L. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur. Respir. J. 2005, 25, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Rubin, L.J.; Galie, N.; Barst, R.J.; Fleming, T.R.; Frost, A.E.; Engel, P.J.; Kramer, M.R.; Burgess, G.; Collings, L. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann. Intern. Med. 2008, 149, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Tymchak, W.; Noga, M.; Webster, L.; Wu, X.-C.; Lien, D.; Wang, S.-H.; Modry, D.; Archer, S.L. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation 2003, 108, 2066–2069. [Google Scholar] [CrossRef]
- Sebkhi, A.; Strange, J.W.; Phillips, S.C.; Wharton, J.; Wilkins, M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003, 107, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, J.; Archer, S.L.; Soliman, D.; Gurtu, V.; Haromy, A.; Rebeyka, I.M.; Ross, D.B.; Light, P.E.; Michelakis, E.D. Phosphodiesterase-5 is highly expressed in the hypertrophied human right ventricle and acute inhibition causes increased contractility via cGMP-inhibition of phosphodiesterase-3. Circulation 2007, 116, 238–248. [Google Scholar] [CrossRef]
- Corbin, J.D.; Francis, S.H. Cyclic GMP phosphodiesterase-5: Target of sildenafil. J. Biol. Chem. 1999, 274, 13729–13732. [Google Scholar] [CrossRef]
- Czarniecki, M.; Ahn, H.-S.; Sybertz, E.J. Inhibitors of types I and V phosphodiesterase: Elevation of cGMP as a therapeutic strategy. Ann. Rep. Med. Chem. 1996, 31, 61–70. [Google Scholar]
- Raiesdana, A.; Loscalzo, J. Pulmonary arterial hypertension. Ann. Med. 2006, 38, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995, 75, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Ückert, S.; Hedlund, P.; Andersson, K.-E.; Truss, M.C.; Jonas, U.; Stief, C.G. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: Present and future. Eur. Urol. 2006, 50, 1194–1207. [Google Scholar] [CrossRef]
- Rotella, D.P. Phosphodiesterase type 5 inhibitors: Discovery and therapeutic utility. Drugs Future 2001, 26, 153–162. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, P.; Dumètre, A.; Castera-Ducros, C.; Hutter, S.; Laget, M.; Fersing, C.; Prieri, M.; Yzombard, J.; Sifredi, F.; Rault, S. 4-Thiophenoxy-2-trichloromethyquinazolines display in vitro selective antiplasmodial activity against the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2011, 21, 6003–6006. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.-F.; Liao, J.-F.; Shum, A.Y.-C.; Chen, C.-F. Mechanisms of vasorelaxant effect of dehydroevodiamine: A bioactive isoquinazolinocarboline alkaloid of plant origin. J. Cardiovas. Pharmacol. 1996, 27, 845–853. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Abouzid, K.; El Ella, D.A.A. Molecular modeling study and synthesis of quinazolinone-arylpiperazine derivatives as α1-adrenoreceptor antagonists. Eur. J. Med. Chem. 2011, 46, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, H.; Lee, J.; Hwang, I.-C.; Moon, S.K.; Kim, S.J.; Lee, H.W.; Lee, S.S.; Ahn, S.K.; Kim, S.W. Quinazolines as potent and highly selective PDE5 inhibitors as potential therapeutics for male erectile dysfunction. Bioorg. Med. Chem. Lett. 2008, 18, 6279–6282. [Google Scholar] [CrossRef]
- Pobsuk, N.; Urooj Paracha, T.; Chaichamnong, N.; Salaloya, N.; Suphakun, P.; Hannongbua, S.; Choowongkomon, K.; Pekthong, P.; Chootip, C.; Ingkaninan, K.; et al. Design, synthesis and evaluation of N2,N4-diaminoquinazoline-based inhibitors of phosphodiesterase type 5. Bioorg. Med. Chem. Lett. 2018. [CrossRef]
- Toviwek, B.; Suphakun, P.; Choowongkomon, K.; Hannongbua, S.; Gleeson, M.P. Synthesis and evaluation of the NSCLC anti-cancer activity and physical properties of 4-aryl-N-phenylpyrimidin-2-amines. Bioorg. Med. Chem. Lett. 2017, 27, 4749–4754. [Google Scholar] [CrossRef]
- Kaneda, T.; Sasaki, N.; Urakawa, N.; Shimizu, K. Endothelium-dependent and-independent vasodilator effects of dimethyl sulfoxide in rat aorta. Pharmacology 2016, 97, 171–176. [Google Scholar] [CrossRef]
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 15 September 2018).
- Cai, Z.; Lu, Q.; Ding, Y.; Wang, Q.; Xiao, L.; Song, P.; Zou, M.-H. Endothelial nitric oxide synthase-derived nitric oxide prevents dihydrofolate reductase degradation via promoting S-nitrosylation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2366–2373. [Google Scholar] [CrossRef]
- Capettini, L.S.A.; Cortes, S.F.; Lemos, V.S. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur. J. Pharmacol. 2010, 643, 260–266. [Google Scholar] [CrossRef]
- El-Daly, M.; Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Kang, S.; Fedak, P.W.M.; Alston, L.A.; Hirota, S.A.; Ding, H.; Triggle, C.R.; et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-transporter-2 and minimizing oxidative stress. Vascul. Pharmacol. 2018, 109, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Page, N.A.; Fung, S.M.; Fung, H.-L. In vitro organic nitrate bioactivation to nitric oxide by recombinant aldehyde dehydrogenase 3A1. Nitric Oxide 2013, 35, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruangtip, O.; Chootip, K.; Temkitthawon, P.; Changwichit, K.; Chuprajob, T.; Changtam, C.; Suksamrarn, A.; Khorana, N.; Scholfield, C.N.; Ingkaninan, K. Curcumin analogues inhibit phosphodiesterase-5 and dilate rat pulmonary arteries. J. Pharm. Pharmacol. 2015, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wisutthathum, S.; Demougeot, C.; Totoson, P.; Adthapanyawanich, K.; Ingkaninan, K.; Temkitthawon, P.; Chootip, K. Eulophia macrobulbon extract relaxes rat isolated pulmonary artery and protects against monocrotaline-induced pulmonary arterial hypertension. Phytomedicine 2018, 50, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Moohammadaree, A.; Changtam, C.; Wicha, P.; Suksamrarn, A.; Tocharus, J.; Tocharus, C. Mechanisms of vasorelaxation induced by hexahydrocurcuminin isolated rat thoracic aorta. Phytother. Res. 2015, 29, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Pantan, R.; Onsa-ard, A.; Tocharus, J.; Wonganan, O.; Suksamrarn, A.; Tocharus, C. Endothelium-independent vasorelaxation effects of 16-O-acetyldihydroisosteviol on isolated rat thoracic aorta. Life Sci. 2014, 116, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Phuangsawai, O.; Beswick, P.; Ratanabunyong, S.; Tabtimmai, L.; Suphakun, P.; Obounchoey, P.; Srisook, P.; Horata, N.; Chuckowree, I.; Hannongbua, S. Evaluation of the anti-malarial activity and cytotoxicity of 2, 4-diamino-pyrimidine-based kinase inhibitors. Eur. J. Med. Chem. 2016, 124, 896–905. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon JChem. Available online: www.chemaxon.com (accessed on 13 October 2018).
- Umetrics. Available online: www.umetrics.com (accessed on 15 September 2018).
Sample Availability: Samples of the quinazoline compounds are available from M. Paul Gleeson. |




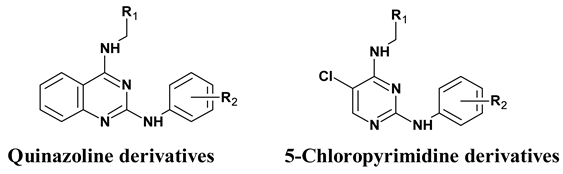
| Compound ID | Core Structure | R1 | R2 | MW |
|---|---|---|---|---|
| 1 | Quinazoline | –phenyl | –H | 326.4 |
| 2 | –phenyl | –4-morpholino | 411.5 | |
| 3 | –phenyl | –4-SO2N(CH3)2 | 433.5 | |
| 4 | –phenyl | –4-SO2NH2 | 405.5 | |
| 5 | –phenyl | –3-SO2NH2 | 405.5 | |
| 6 | –2-thiophene | –H | 332.4 | |
| 7 | –2-thiophene | –4-NHCONHPh | 466.6 | |
| 8 | –2-thiophene | –4-morpholino | 417.5 | |
| 9 | –2-thiophene | –4-SO2NH2 | 411.5 | |
| 10 | –2-thiophene | –4-CONH2 | 375.5 | |
| 11 | –2-thiophene | –3-SO2NH2 | 411.5 | |
| 12 | –2-thiophene | –3-CONH2 | 375.5 | |
| 13 | –furan | –3-SO2NH2 | 395.4 | |
| 14 | 5-chloropyrimidine | –phenyl | –H | 310.8 |
| Compound ID | Vaso E+ EC50 µM (SEM) | E+ Emax (%) | Vaso E− EC50 µM (SEM) | E− Emax (%) | % PE-Contraction Inhibition (SEM) | Sol. pH7.4 mg/mL (µM) | PDE-5 IC50, µM (S.D.) | A549 IC50, µM (S.D.) | SI E+ vs. A549 (PDE-5 vs. A549) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.54 * (2.25) | 89.98 ± 5.64 | 13.46 (1.48) | 86.41 ± 4.03 | - | 0.13 (0.41) | 1.38 (0.169) | 12.29 (1.2) | 2.2 (8.9) |
| 2 | 4.98 (2.38) | 94.69 ± 3.33 | 11.96 (3.17) | 85.67 ± 6.05 | 56.62 (5.90) | 0.25 (0.60) | 0.53 (0.066) | 12.1 (3.6) | 2.4 (22.8) |
| 3 | 3.59 (1.42) | 82.36 ± 5.90 | 6.25 (1.80) | 64.27 ± 5.31 | - | 0.38 (0.88) | 1.26 (0.092) | 18.32 (0.82) | 5.1 (14.5) |
| 4 | 3.13 * (1.52) | 88.48 ± 5.58 | 8.29 (1.38) | 78.53 ± 9.19 | - | 0.01(0.03) | 0.95 (0.035) | 22.97 (4.55) | 7.3 (24.2) |
| 5 | 1.63 (0.72) | 91.68 ± 3.77 | 1.15 (0.18) | 95.83 ± 2.40 | - | 0.56 (1.37) | 0.07 (0.008) | 11.15 (1.22) | 6.8 (159.3) |
| 6 | 9.42 (2.54) | 88.03 ± 7.39 | 9.51 (1.28) | 91.16 ± 5.26 | - | 0.13 (0.40) | 1.44 (0.17) | 27.78 (6) | 2.9 (19.3) |
| 7 | 4.10 (1.46) | 76.03 ± 5.73 | >30 | 22.31 ± 4.40 | 43.62 (7.02) | 0.10 (0.22) | 0.78 (0.101) | 2.91 (0.73) | 0.7 (3.7) |
| 8 | 0.58 *** (0.22) | 98.88 ± 0.79 | 8.50 (1.6) | 100 | 42.43 (5.99) | 0.20 (0.48) | 0.51 (0.021) | 11.37 (3.07) | 19.6 (22.3) |
| 9 | 1.03 (0.33) | 98.40 ± 1.40 | 1.72 (0.63) | 82.10 ± 8.43 | - | 0.06 (0.16) | 0.22 (0.038) | 32.67 (3.61) | 31.7 (148.5) |
| 10 | 1.46 (0.43) | 90.15 ± 6.48 | 5.15 (1.57) | 90.42 ± 5.30 | - | 0.45 (1.20) | 0.18 (0.016) | 28.29 (2.58) | 19.4 (157.2) |
| 11 | 2.28 *** (0.74) | 98.74 ± 1.26 | 10.89 (0.51) | 87.42 ± 4.66 | - | 0.14 (0.34) | 0.09 (0.011) | 15.04 (4.44) | 6.6 (167.1) |
| 12 | 4.15 (1.38) | 98.69 ± 1.31 | 8.15 (1.43) | 87.11 ± 3.63 | - | 0.21(0.56) | 0.29 (0.098) | 15.44 (2.14) | 3.7 (53.2) |
| 13 | 6.61 (2.39) | 95.46 ± 2.17 | 3.02 (1.29) | 95.61 ± 2.75 | 45.93 (11.69) | 0.15 (0.38) | 0.12 (0.023) | 26.92 (2.16) | 4.1 (224.3) |
| 14 | 4.49 (1.96) | 67.80 ± 5.34 | 10.69 (2.20) | 54.74 ± 6.12 | - | 1.02 (3.27) | >10 | >100 | >22.3 (>10) |
| Sildenafil (SIL) | 0.14 (0.05) | 97.82 ± 2.19 | 0.89 (0.48) | 85.84 ± 6.41 | 58.33 (1.77) | 20.83 (43.88) | 0.002 (0.0008) | - | - |
| Sodium nitro-prusside (SNP) | 0.02 (0.01) | 99.58 ± 0.42 | 0.0045 (0.0007) | 100 | - | - | - | - | - |
| Prazosin (PZN) | 0.03 (0.01) | 98.72 ± 1.28 | 0.093 (0.03) | 100 | 98.01 (1.02) | 0.55 (1.42) | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paracha, T.U.; Pobsuk, N.; Salaloy, N.; Suphakun, P.; Pekthong, D.; Hannongbua, S.; Choowongkomon, K.; Khorana, N.; Temkitthawon, P.; Ingkaninan, K.; et al. Elucidation of Vasodilation Response and Structure Activity Relationships of N2,N4-Disubstituted Quinazoline 2,4-Diamines in a Rat Pulmonary Artery Model. Molecules 2019, 24, 281. https://doi.org/10.3390/molecules24020281
Paracha TU, Pobsuk N, Salaloy N, Suphakun P, Pekthong D, Hannongbua S, Choowongkomon K, Khorana N, Temkitthawon P, Ingkaninan K, et al. Elucidation of Vasodilation Response and Structure Activity Relationships of N2,N4-Disubstituted Quinazoline 2,4-Diamines in a Rat Pulmonary Artery Model. Molecules. 2019; 24(2):281. https://doi.org/10.3390/molecules24020281
Chicago/Turabian StyleParacha, Tamkeen Urooj, Nattakarn Pobsuk, Nattapas Salaloy, Praphasri Suphakun, Dumrongsak Pekthong, Supa Hannongbua, Kiattawee Choowongkomon, Nantaka Khorana, Prapapan Temkitthawon, Kornkanok Ingkaninan, and et al. 2019. "Elucidation of Vasodilation Response and Structure Activity Relationships of N2,N4-Disubstituted Quinazoline 2,4-Diamines in a Rat Pulmonary Artery Model" Molecules 24, no. 2: 281. https://doi.org/10.3390/molecules24020281






