Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice
Abstract
:1. Introduction
2. Results
2.1. Sargassum muticum Suppresses the Clinical Symptoms of Collagen-Induced Arthritis (CIA)
2.2. Sargassum muticum Extract Inhibits IL-6, TNF-α, and Interferon (IFN)-γ Production in CIA Mouse Serum
2.3. Sargassum muticum Extract Inhibits Splenomegaly and Suppresses IL-6, TNF-α, and IFN-γ Production in CIA Mouse Lymphocytes
2.4. Sargassum muticum Extract Suppresses Joint Degradation in CIA Mice
2.5. Sargassum muticum Extract Decreases the Expression of Inflammatory Cytokines in CIA Mice
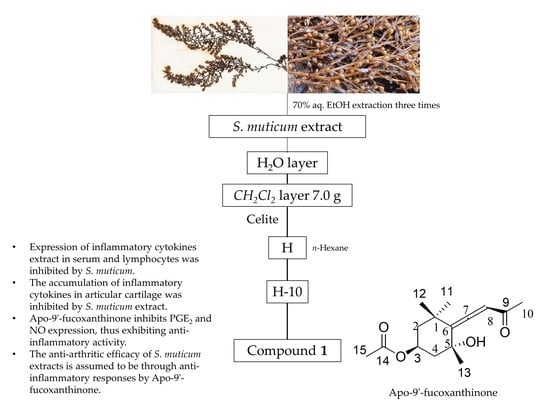
2.6. Isolation and Composition Experiments of Sargassum muticum Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Sargassum muticum Extract (SME)
4.3. Fractionation and Isolation of SME
4.4. In Vivo Study
4.4.1. Induction of Arthritis
4.4.2. Treatment of Extractions
4.4.3. Clinical Observation and Evaluation of Arthritis
4.4.4. Measurement of Paw Swelling
4.4.5. Isolation and Culture of Lymphocytes from Spleen
4.4.6. Measurement of IL-6, TNF-α, and IFN-γ Production in Serum and Splenocytes
4.4.7. Histological Staining
4.4.8. Immunohistochemical Staining
4.5. In Vitro Study
4.5.1. Cell Culture
4.5.2. Cytotoxicity Assay
4.5.3. Measurement of Nitric Oxide (NO) Production
4.5.4. Measurement of PGE2Production
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Chou, E.H.S.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Keller, T.T.; Van Gorp, E.; Ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, K.; Mihara, M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthitis 2011, 2011, 765624. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.-K. Nutritional and digestive health benefits of seaweeds. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The protein value in human nutrition of edible marine algae in Japan. Hydrobiologia 1984, 1, 513–516. [Google Scholar] [CrossRef]
- Kaehler, S.; Kennish, R. Summer and winter comparisons in the nutritional value of marine macroalgae from Hong Kong. Bot. Mar. 1996, 39, 11–17. [Google Scholar] [CrossRef]
- Murata, M.; Nakazoe, J. Production and use of marine algae in Japan. Jpn. Agr. Res. Q. 2001, 35, 281–290. [Google Scholar] [CrossRef]
- Teas, J.; Braberman, L.E.; Kurzer, M.S.; Pino, S.; Hyrley, T.G.; Herbert, J.R. Seaweed and Soy: Companion foods in Asian cuisine and their effects on thyroid function in American woman. J. Med. Food 2007, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.B.; Barbosa, M.; Pedro, R.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2015, 32, 15–45. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, S.M.; Ko, Y.D.; Mattio, L.; Boo, S.M. Molecular systematic reassessment of Sargassum (Fucales, Phaeophyceae) in Korea using four gene regions. Bot. Mar. 2012, 55, 473–484. [Google Scholar] [CrossRef]
- Villarreal-Gomez, L.J.; Soria-Mercado, I.E.; Guerra-Rivas, G.; Ayala-Sanchez, N.E. Antibacterial and anticancer activity of seaweeds and bacteria associated with their surface. Rev. Biol. Mar. Oceanorg. 2010, 45, 267–275. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.A.; Kim, K.N.; Yoon, W.J.; Lee, W.J.; Park, S.Y. Antioxidative and antimicrobial activities of Sargassum muticum extracts. J. Korean Soc. Food Sci. Nutr. 2007, 36, 663–669. [Google Scholar] [CrossRef]
- Chae, D.; Manzoor, Z.; Kim, S.C.; Kim, S.; Oh, T.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.H.; Ko, M.-H.; et al. Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway. Mar. Drugs 2013, 11, 3272–3287. [Google Scholar] [CrossRef] [Green Version]
- Zuercher, A.W.; Fritsché, R.; Corthésy, B.; Mercenier, A. Food products and allergy development, prevention and treatment. Curr. Opin. Biotechnol. 2006, 17, 198–203. [Google Scholar] [CrossRef]
- Perez, G.R.M.; Zavala, S.M.A.; Perez, G.S.; Perez, G.C. Antidiabetic effect of compounds isolated from plants. Phytomedicine 1998, 5, 55–75. [Google Scholar] [CrossRef]
- Nishino, T.; Fukuda, A.; Nagumo, T.; Fujihara, M.; Kaji, E. Inhibition of the generation of thrombin and factor Xa by a fucoidan from the brown seaweed Ecklonia kurome. Thromb. Res. 1999, 96, 37–49. [Google Scholar] [CrossRef]
- Miyashita, K. The carotenoid fucoxanthin from brown seaweed affects obesity. Lipid. Tech. 2009, 21, 186–190. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Sahashi, Y.; Watanabe, K.; Ohtsuchi, S.; Yamamoto, K.; Ando, K.; Nagata, C. Seaweed intake and blood pressure levels in healthy preschool Japanese children. Nutr. J. 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Zhu, G.; Xu, H.; Rietz, A.C.; Drake, C.G.; Matteson, E.L.; Chen, L. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS Med. 2009, 6, e1000166. [Google Scholar] [CrossRef] [PubMed]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Bronte, B.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef]
- Suresh, E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J. R. Soc. Med. 2004, 97, 421–424. [Google Scholar] [CrossRef]
- Gibofsky, A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am. J. Manag. Care 2012, 18, S292–S302. [Google Scholar]
- Betteridge, N.; Bevan, S.; Bosworth, A.; Emery, P.; Hammond, A.; Meek, R.; Nye, A.; Oliver, S.; Silman, A.; Tennant, A. The Department’s approach to rheumatoid arthritis. In Services for People with Rheumatoid Arthritis; Morse, A., Groom, C., Dixon, P., Ross, C., Xu, D., Lucas, E., Taylor, K., Eds.; National Audit Office: London, UK, 2009; pp. 5–37. [Google Scholar]
- Pincus, T. The Underestimated Long Term Medical and Economic Consequences of Rheumatoid Arthritis. Drugs 1995, 50, 1–14. [Google Scholar] [CrossRef]
- Newsome, G. Guidelines for the Management of Rheumatoid Arthritis 2002 Update. J. Am. Acad. Nurse Pract. 2002, 14, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Oak, J.H.; Lee, I.K. Taxonomy of the genus Sargassum (Fucales, Phaeophyceae) from Korea; I. Subgenus Bactrophycus section Teretia. Algae 2005, 20, 77–90. [Google Scholar] [CrossRef]
- De la Mare, J.A.; Lawson, J.C.; Chiwakata, M.T.; Beukes, D.R.; Edkins, A.L.; Blatch, G.L. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro. Investig. New Drugs 2012, 30, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.G.; Lee, B.; Joung, E.J.; Choi, M.W.; Yoon, N.; Shin, T.; Oh, C.W.; Kim, H.R. Sargaquinoic acid inhibits TNF-α-induced NF-κB signaling, thereby contributing to decreased monocyte adhesion to human umbilical vein endothelial cells (HUVECs). J. Agric. Food Chem. 2015, 63, 9053–9061. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Jung, Y.; Kim, M.C.; Kwon, H.C.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Sargahydroquinoic acid inhibits TNFα-induced AP-1 and NF-κB signaling in HaCaT cells through PPARα activation. Biochem. Biophys. Res. Commun. 2014, 450, 1553–1559. [Google Scholar] [CrossRef]
- Joung, E.J.; Lee, B.; Gwon, W.G.; Shin, T.; Jung, B.M.; Yoon, N.Y.; Choi, J.S.; Oh, C.W.; Kim, H.R. Sargaquinoic acid attenuates inflammatory responses by regulating NF-κB and Nrf2 pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2015, 29, 693–700. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, W.; Bae, G.U.; Kim, Y.K. Anti-diabetic and hypolipidemic effects of Sargassum yezoense in db/db mice. Biochem. Biophys. Res. Commun. 2012, 424, 675–680. [Google Scholar] [CrossRef]
- Joung, E.J.; Gwon, W.G.; Shin, T.; Jung, B.M.; Choi, J.S.; Kim, H.R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Mun, S.H.; Kim, H.S.; Kim, J.W.; Ko, N.Y.; Kim, D.K.; Lee, B.Y.; Kim, B.; Won, H.S.; Shin, H.S.; Han, J.W.; et al. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: inhibition of the PKCdelta/JNK/c-Jun pathway. J. Pharmacol. Sci. 2009, 111, 13–21. [Google Scholar] [CrossRef]
- Banda, N.K.; Levitt, B.; Glogowska, M.J.; Thurman, J.M.; Takahashi, K.; Stahl, G.L.; Tomlinson, S.; Arend, W.P.; Holers, V.M. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J. Immunol. 2009, 183, 5928–5937. [Google Scholar] [CrossRef] [PubMed]
- Piróg, K.A.; Jaka, O.; Katakura, Y.; Meadows, R.S.; Kadler, K.E.; Boot-Handford, R.P.; Briggs, M.D. A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Mol. Genet. 2010, 19, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.O.; So, T.; Ueda, T.; Imoto, T.; Koga, T. Prevention of collagen-induced arthritis (CIA) by treatment with polyethylene glycol-conjugated type II collagen; distinct tolerogenic property of the conjugated collagen from the native one. Clin. Exp. Immunol. 1997, 108, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.I.; Lee, S.Y.; Yoon, K.H.; Choi, K.S.; Jang, K.Y.; Yoo, W.H.; Kim, S.H.; Choi, T.H.; Park, J.G. Molecular MR imaging for visualizing ICAM-1 expression in the inflamed synovium of collagen-induced arthritic mice. Korean J. Radiol. 2009, 10, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Lee, S.R.; Jeong, Y.J.; Park, D.W.; Cho, Y.M.; Joo, H.M.; Kim, I.; Seu, Y.B.; Sohn, E.H.; Kang, S.C. Antiallergic activity of ethanol extracts of Arctium lappa L. undried roots and its active compound, oleamide, in regulating FcεRI-mediated and MAPK signaling in RBL-2H3 cells. J. Agric. Food Chem. 2016, 64, 3564–3573. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K.; et al. Fucoidan from Fucus vesiculosus Protects against Alcohol-Induced Liver Damage by Modulating Inflammatory Mediators in Mice and HepG2 Cells. Mar. Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.J.; Yoon, W.J.; Sohn, E.H.; Ham, Y.M.; Jang, S.A.; Kwon, J.E.; Jeong, Y.J.; Kwak, J.H.; Sohn, E.; Park, S.Y.; et al. The analgesic and anti-inflammatory effects of Litsea japonica fruit are mediated via suppression of NF-kappaB and JNK/p38 MAPK activation. Int. Immunopharmacol. 2014, 22, 84–97. [Google Scholar] [CrossRef]
Sample Availability: Not available. |










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.; Yoon, W.-J.; Ham, Y.-M.; Yoon, S.-A.; Kang, S.C. Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice. Molecules 2019, 24, 276. https://doi.org/10.3390/molecules24020276
Jeon H, Yoon W-J, Ham Y-M, Yoon S-A, Kang SC. Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice. Molecules. 2019; 24(2):276. https://doi.org/10.3390/molecules24020276
Chicago/Turabian StyleJeon, Hyelin, Weon-Jong Yoon, Young-Min Ham, Seon-A Yoon, and Se Chan Kang. 2019. "Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice" Molecules 24, no. 2: 276. https://doi.org/10.3390/molecules24020276