Moose and Caribou as Novel Sources of Functional Lipids: Fatty Acid Esters of Hydroxy Fatty Acids, Diglycerides and Monoacetyldiglycerides
Abstract
:1. Introduction
2. Experimental Section
2.1. Meat and Antler Acquisition
2.2. Lipid Analysis
Mass Spectrometry
2.3. Lipid Standards
3. Results and Discussions
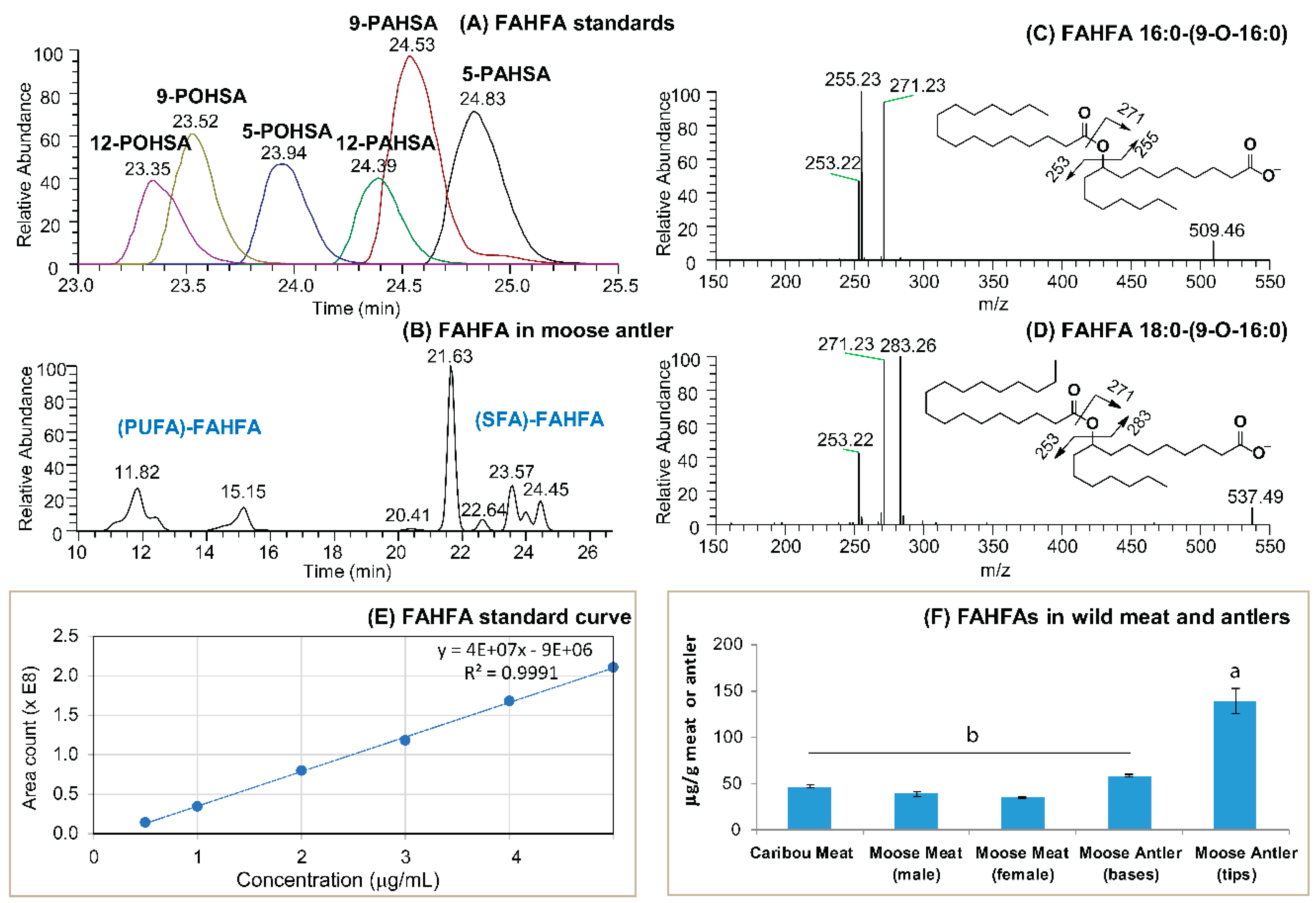
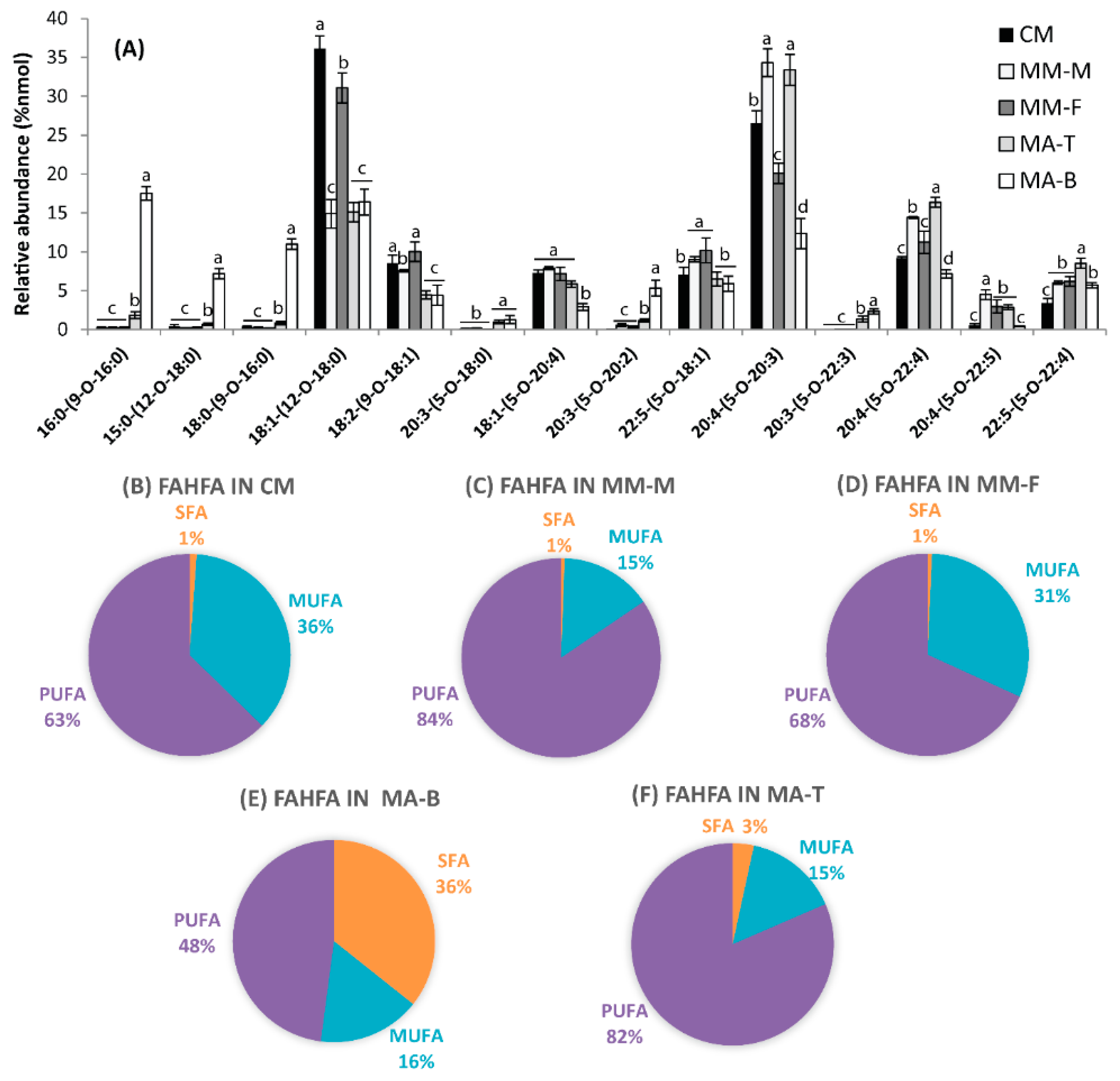
3.1. FAHFAs as Functional Lipids in Moose and Caribou
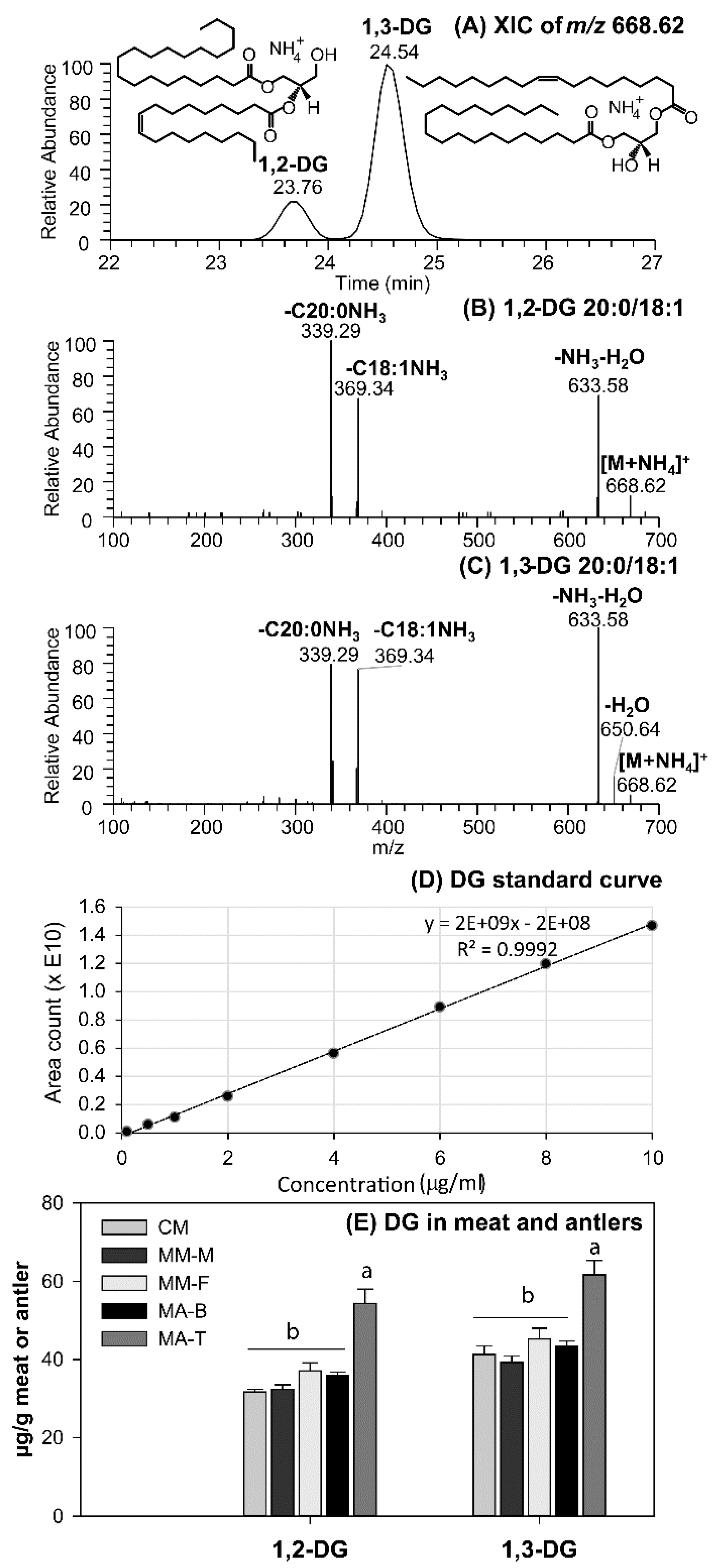
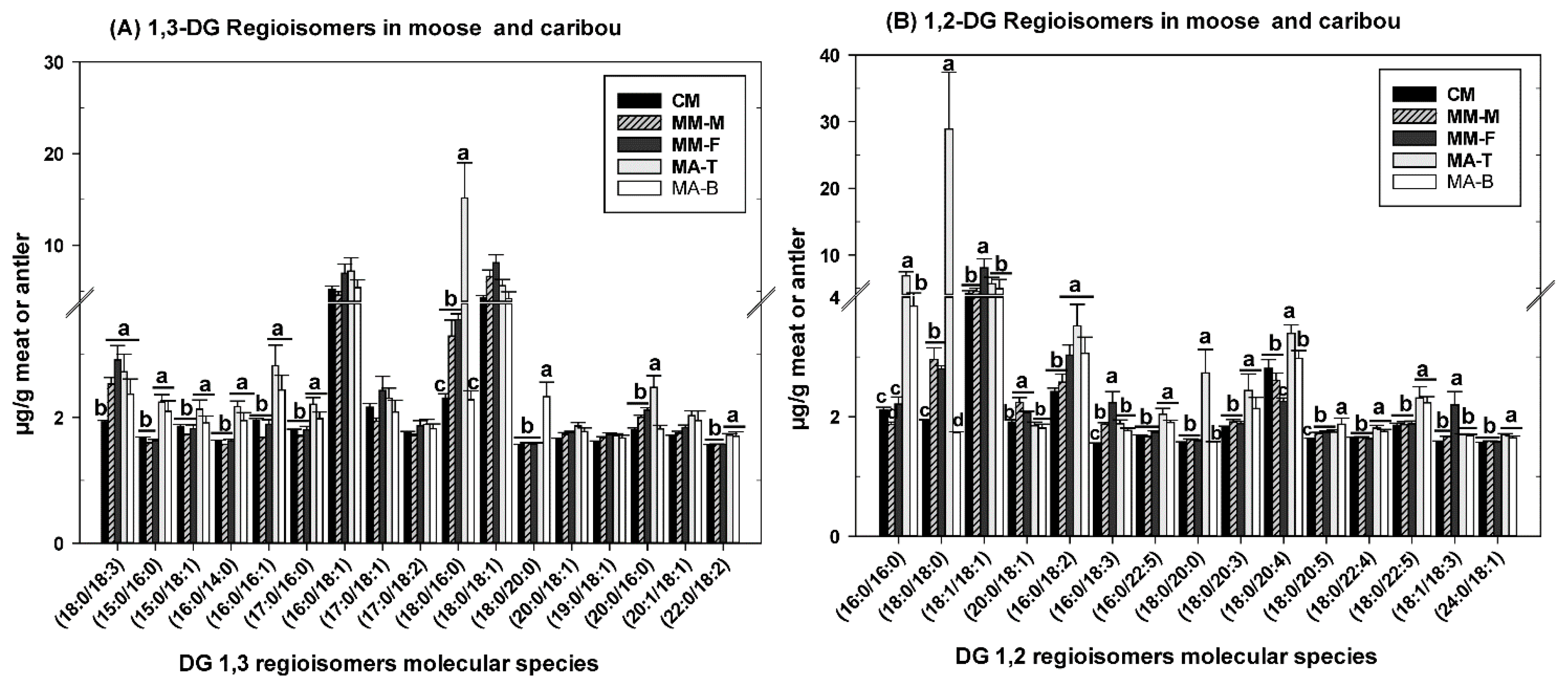
3.2. DG as Functional Lipid in Moose and Caribou
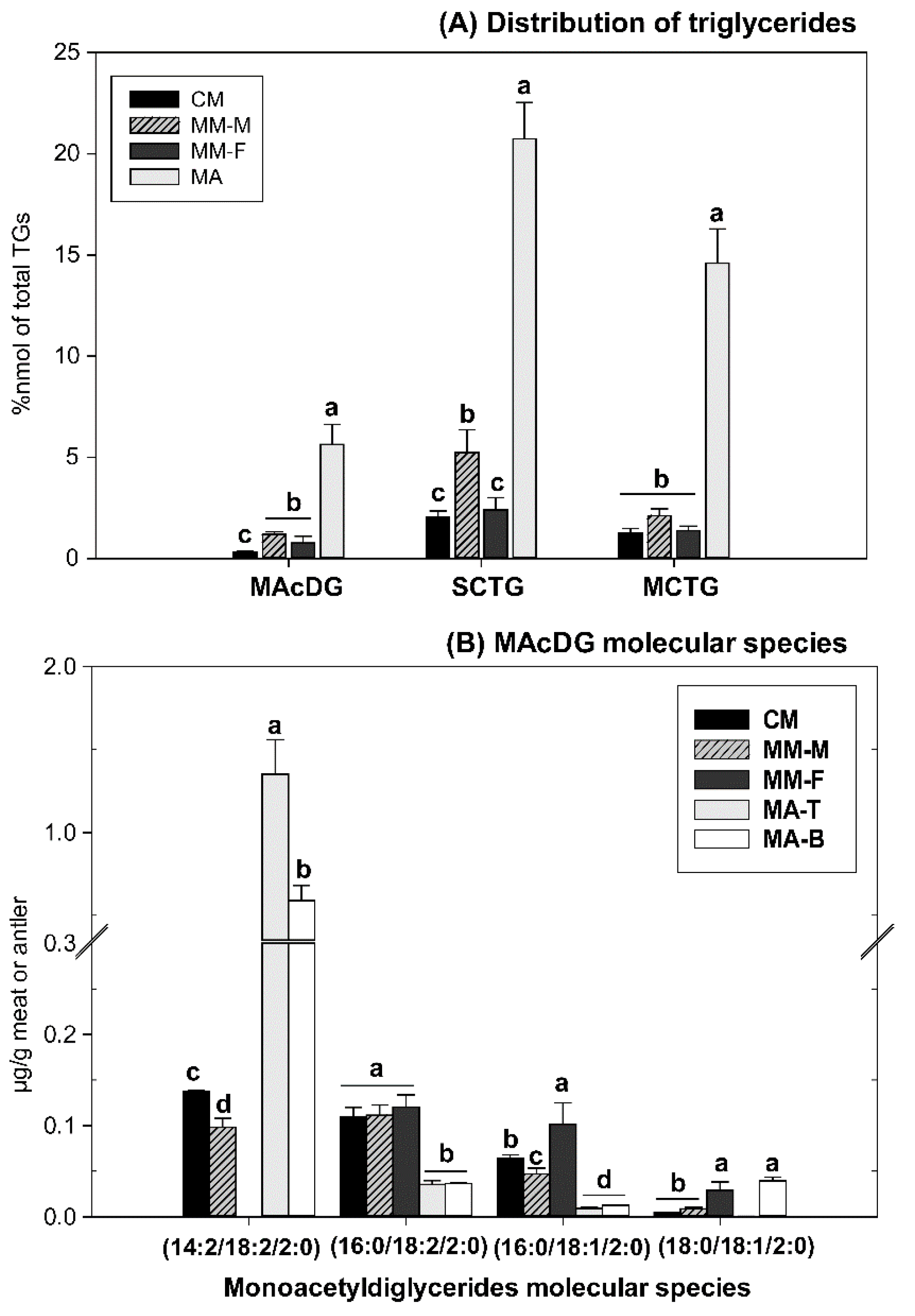
3.3. MAcDG as Functional Lipids in Moose and Caribou
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C30RPLC | C30 reverse phase liquid chromatography |
| FAHFA | fatty acid esters of hydroxy fatty acid |
| DG | diglyceride |
| MAcDG | monoacetyldiglyceride |
| TG | triglyceride or commonly known as triacylglycerol |
| SCTG | short-chain triglyceride |
| MCTG | medium-chain triglyceride |
| PAHSA | palmitoyl hydroxy stearic acids |
| POHSA | palmitoleyl hydroxy stearic acids |
| OAHSA | oleoyl hydroxy stearic acids |
| ARA-5-HERA | arachidonyl 5-hydroxyeicosatrienoic acid |
| DHA-13-HLA | docosahexaenoyl 13-hydroxylinoleic acid |
| SFA | saturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| CM | Caribou Meat |
| MM-M | Moose Meat (male) |
| MM-F | Moose meat (female) |
| MA-T | Moose Antler (tips) |
| MA-B | Moose Antler (bases) |
References
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Li, D.; Siriamornpun, S.; Wahlqvist, M.L.; Mann, N.J.; Sinclair, A. Lean meat and heart health. Asia Pac. J. Clin. Nutr. 2005, 14, 113–119. [Google Scholar] [PubMed]
- Troy, D.J.; Tiwari, B.K.; Joo, S.T. Health implications of beef intramuscular fat consumption. Korean J. Food Sci. Anim. Resour. 2016, 36, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Hochegger, R.; Cichna-Markl, M. Sika deer (Cervus nippon)-specific real-time PCR method to detect fraudulent labelling of meat and meat products. Sci. Rep. 2018, 8, 7236. [Google Scholar] [CrossRef]
- Parker, K.L.; Barboza, P.S.; Gillingham, M.P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 2009, 23, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Oh, S.R.; Ahn, K.S.; Kang, H.B.; Park, B.S.; Lee, T.S.; Kang, J.K.; Jung, Y.S.; Yong-Hae, H.; Ki-Young, S. Compositions Containing Monoacetyldiacylglycerol Compound as an Active Ingredient for Preventing or Treating Rheumatoid Arthritis. U.S. Patent 20160166528A1, 16 June 2016. [Google Scholar]
- Shoji, K.; Mizuno, T.; Shiiba, D.; Kawagoe, T.; Mitsui, Y. Effects of a meal rich in 1,3-diacylglycerol on postprandial cardiovascular risk factors and the glucose-dependent insulinotropic polypeptide in subjects with high fasting triacylglycerol concentrations. J. Agric. Food Chem. 2012, 60, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Balas, L.; Feillet-Coudray, C.; Durand, T. Branched fatty acyl esters of hydroxyl fatty acids (FAHFAs), appealing beneficial endogenous fat against obesity and type-2 diabetes. Chem. A Eur. J. 2018, 24, 9463–9476. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H. Immunomodulating Agent, Anti-Cancer Agent and Health Food Containing Monoacetyldiacylglycerol Derivatives. U.S. Patent 20080200543A1, 21 August 2008. [Google Scholar]
- Oh, S.-R.; Ahn, K.S.; LEE, S.U.; SHIN, I.S.; Shin, N.-R.; Lee, T.-S.; JongKoo, K.; Jung, Y.-S.; Yong-Hae, H.; SOHN, K.Y. Composition Containing Monoacetyldiacylglycerol Compound as Active Ingredient for Preventing or Treating Asthma. U.S. Patent 20160199339A1, 14 July 2016. [Google Scholar]
- Sui, Z.; Zhang, L.; Huo, Y.; Zhang, Y. Bioactive components of velvet antlers and their pharmacological properties. J. Pharm. Biomed. Anal. 2014, 87, 229–240. [Google Scholar] [CrossRef]
- Yang, H.O.; Kim, S.H.; Cho, S.-H.; Kim, M.-G.; Seo, J.-Y.; Park, J.-S.; Jhon, G.-J.; Han, S.-Y. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem. Pharm. Bull. 2004, 52, 874–878. [Google Scholar] [CrossRef]
- Limb, J.-K.; Kim, Y.H.; Han, S.-Y.; Jhon, G.-J. Isolation and characterization of monoacetyldiglycerides from bovine udder. J. Lipid Res. 1999, 40, 2169–2176. [Google Scholar] [PubMed]
- Marshall, K.E.; Thomas, R.H.; Roxin, Á.; Chen, E.K.Y.; Brown, J.C.L.; Gillies, E.R.; Sinclair, B.J. Seasonal accumulation of acetylated triacylglycerols by a freeze-tolerant insect. J. Exp. Biol. 2014, 217, 1580–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Tjellström, H.; McGlew, K.; Shaw, V.; Rice, A.; Simpson, J.; Kosma, D.; Ma, W.; Yang, W.; Strawsine, M.; et al. Field production, purification and analysis of high-oleic acetyl-triacylglycerols from transgenic Camelina sativa. Ind. Crops Prod. 2015, 65, 259–268. [Google Scholar] [CrossRef]
- Kim, S. Monoacetyldiacylglycerol Derivative for the Treatment of Sepsis. U.S. Patent 2010013137435A1, 13 May 2010. [Google Scholar]
- Maki, K.C.; Davidson, M.H.; Tsushima, R.; Matsuo, N.; Tokimitsu, I.; Umporowicz, D.M.; Dicklin, M.R.; Foster, G.S.; Ingram, K.A.; Anderson, B.D.; et al. Consumption of diacylglycerol oil as part of a reduced-energy diet enhances loss of body weight and fat in comparison with consumption of a triacylglycerol control oil. Am. J. Clin. Nutr. 2002, 76, 1230–1236. [Google Scholar] [CrossRef]
- Zheng, M.-M.; Huang, Q.; Huang, F.-H.; Guo, P.-M.; Xiang, X.; Deng, Q.-C.; Li, W.-L.; Wan, C.-Y.; Zheng, C. Production of novel “functional oil” rich in diglycerides and phytosterol esters with “one-pot” enzymatic transesterification. J. Agric. Food Chem. 2014, 62, 5142–5148. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Shoji, K.; Hase, T.; Tokimitsu, I. Effect of diacylglycerol on postprandial lipid metabolism in non-diabetic subjects with and without insulin resistance. Atherosclerosis 2005, 180, 197–204. [Google Scholar] [CrossRef]
- Murase, T.; Aoki, M.; Wakisaka, T.; Hase, T.; Tokimitsu, I. Anti-obesity effect of dietary diacylglycerol in C57BL/6J mice: Dietary diacylglycerol stimulates intestinal lipid metabolism. J. Lipid Res. 2002, 43, 1312–1319. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Etherton, T.D.; Carlson, J.; Gardner, C. Recent discoveries in inclusive food-based approaches and dietary patterns for reduction in risk for cardiovascular disease. Curr. Opin. Lipidol. 2002, 13, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.R.; Mela, D.J. Metabolically active functional food ingredients for weight control. Obes. Rev. 2006, 7, 59–78. [Google Scholar] [CrossRef]
- Bates, P.; Browse, J. The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef]
- Nelson, A.T.; Kolar, M.J.; Chu, Q.; Syed, I.; Kahn, B.B.; Saghatelian, A.; Siegel, D. Stereochemistry of endogenous palmitic acid ester of 9-hydroxystearic acid and relevance of absolute configuration to regulation. J. Am. Chem. Soc. 2017, 139, 4943–4947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Syed, I.; Stahlman, M.; Kolar, M.J.; Homan, E.A.; Chu, Q.; Smith, U.; Borén, J.; Kahn, B.B.; et al. LC-MS–based workflow for measurement of branched fatty acid esters of hydroxy fatty acids. Nat. Protoc. 2016, 11, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J.; Kudova, E.; et al. Docosahexaenoic acid–derived fatty acid esters of hydroxy fatty acids (FAHFAs) with anti-inflammatory properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Rivas, M.; Zhang, Q. Comprehensive untargeted lipidomic analysis using core-shell C30 particle column and high field orbitrap mass spectrometer. J. Chromatogr. A 2016, 1440, 123–134. [Google Scholar] [CrossRef]
- Kalbfleisch, T.S.; Murdoch, B.M.; Smith, T.P.L.; Murdoch, J.D.; Heaton, M.P.; McKay, S.D. A SNP resource for studying North American moose. F1000 Res. 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Center for Food Security and Public Health. Available online: http://www.cfsph.iastate.edu/Species/cervids.php (accessed on 20 November 2018).
- Poławska, E.; Cooper, R.G.; Jóźwik, A.; Pomianowski, J. Meat from alternative species—nutritive and dietetic value, and its benefit for human health—A review. CyTA J. Food 2013, 11, 37–42. [Google Scholar] [CrossRef]
- Ma, Y.; Kind, T.; Vaniya, A.; Gennity, I.; Fahrmann, J.F.; Fiehn, O. An in silico MS/MS library for automatic annotation of novel FAHFA lipids. J. Chem. 2015, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- McAnoy, A.M.; Wu, C.C.; Murphy, R.C. Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap. J. Am. Soc. Mass Spectrom. 2005, 16, 1498–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, R.C.; James, P.F.; McAnoy, A.M.; Krank, J.; Duchoslav, E.; Barkley, R.M. Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry. Anal. Biochem. 2007, 366, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, R.C.; Leiker, T.J.; Barkley, R.M. Glycerolipid and cholesterol ester analyses in biological samples by mass spectrometry. Biochim. Biophys. Acta 1811, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium chain triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS One 2018, 13, 191182. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (moose and caribou meats, moose antlers) are available from the authors. |

| RT | Lipid Molecules | Molecular Species | Precursor m/z | Peak 1 m/z | Peak 2 m/z | Peak 3 m/z | Peak 4 m/z | Peak 5 m/z |
|---|---|---|---|---|---|---|---|---|
| 21.66 | FAHFA(32:0) | 16:0-(9-O-16:0) | 509.4575 | 255.2330 | 271.2279 | 253.2173 | 127.1128 | 127.1128 |
| 22.09 | FAHFA(33:0) | 15:0-(12-O-18:0) | 523.4732 | 241.2173 | 299.2592 | 281.2486 | 169.1598 | 113.0972 |
| 22.53 | FAHFA(34:0) | 18:0-(9-O-16:0) | 537.4888 | 283.2643 | 271.2279 | 253.2173 | 127.1128 | 127.1128 |
| 14.14 | FAHFA(36:1) | 18:1-(12-O-18:0) | 563.5045 | 281.2486 | 299.2592 | 281.2486 | 169.1598 | 113.0972 |
| 11.81 | FAHFA(36:3) | 18:2-(9-O-18:1) | 559.4732 | 279.2330 | 297.2435 | 279.233 | 127.1128 | 153.1285 |
| 13.70 | FAHFA(38:3) | 20:3-(5-O-18:0) | 587.5045 | 305.2486 | 299.2592 | 281.2486 | 71.0502 | 211.2067 |
| 11.07 | FAHFA(38:5) | 20:4-(5-O-18:1) | 583.4732 | 303.2330 | 297.2435 | 279.233 | 71.0502 | 209.1911 |
| 12.74 | FAHFA(40:5) | 20:3-(5-O-20:2) | 611.5045 | 305.2486 | 323.2592 | 305.2486 | 71.0502 | 235.2067 |
| 11.62 | FAHFA(40:6) | 22:5-(5-O-18:1) | 609.4888 | 329.2486 | 297.2435 | 279.233 | 71.0502 | 209.1911 |
| 11.05 | FAHFA(40:7) | 20:4-(5-O-20:3) | 607.4732 | 303.2330 | 321.2435 | 303.2330 | 71.0502 | 233.1911 |
| 13.51 | FAHFA(42:6) | 20:3-(5-O-22:3) | 637.5201 | 305.2486 | 349.2748 | 331.2643 | 71.0502 | 261.2224 |
| 11.16 | FAHFA(42:8) | 20:4-(5-O-22:4) | 633.4888 | 303.2330 | 347.2592 | 329.2486 | 71.0502 | 259.2067 |
| 10.28 | FAHFA(42:9) | 20:4-(5-O-22:5) | 631.4732 | 303.2330 | 345.2435 | 327.2330 | 71.0502 | 257.1911 |
| 11.56 | FAHFA(44:9) | 22:5-(5-O-22:4) | 659.5045 | 329.2486 | 347.2592 | 329.2486 | 71.0502 | 259.2067 |
| RT | Lipid Molecules | Molecular Species | Precursor m/z | Peak 1 m/z | Peak 2 m/z | Peak 3 m/z | Peak 4 m/z | Peak 5 m/z |
|---|---|---|---|---|---|---|---|---|
| 22.50 | MAcDG(34:4) | 14:2/18:2/2:0 | 620.49 | 543.45 | 379.29 | 323.22 | 207.17 | 263.24 |
| 21.42 | MAcDG(36:2) | 16:0/18:2/2:0 | 652.55 | 575.51 | 379.29 | 355.29 | 239.24 | 263.24 |
| 22.10 | MAcDG(36:1) | 16:0/18:1/2:0 | 654.57 | 577.52 | 381.30 | 355.29 | 239.24 | 265.25 |
| 22.69 | MAcDG(38:1) | 18:0/18:1/2:0 | 682.60 | 605.56 | 381.30 | 383.32 | 267.27 | 265.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.H.; Vidal, N.P.; Manful, C.F.; Fillier, T.A.; Pumphrey, R.P.; Doody, K.M.; Thomas, R.H. Moose and Caribou as Novel Sources of Functional Lipids: Fatty Acid Esters of Hydroxy Fatty Acids, Diglycerides and Monoacetyldiglycerides. Molecules 2019, 24, 232. https://doi.org/10.3390/molecules24020232
Pham TH, Vidal NP, Manful CF, Fillier TA, Pumphrey RP, Doody KM, Thomas RH. Moose and Caribou as Novel Sources of Functional Lipids: Fatty Acid Esters of Hydroxy Fatty Acids, Diglycerides and Monoacetyldiglycerides. Molecules. 2019; 24(2):232. https://doi.org/10.3390/molecules24020232
Chicago/Turabian StylePham, Thu Huong, Natalia P. Vidal, Charles F. Manful, Tiffany A. Fillier, Ryley P. Pumphrey, Karen M. Doody, and Raymond H. Thomas. 2019. "Moose and Caribou as Novel Sources of Functional Lipids: Fatty Acid Esters of Hydroxy Fatty Acids, Diglycerides and Monoacetyldiglycerides" Molecules 24, no. 2: 232. https://doi.org/10.3390/molecules24020232