MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease
Abstract
:1. Introduction
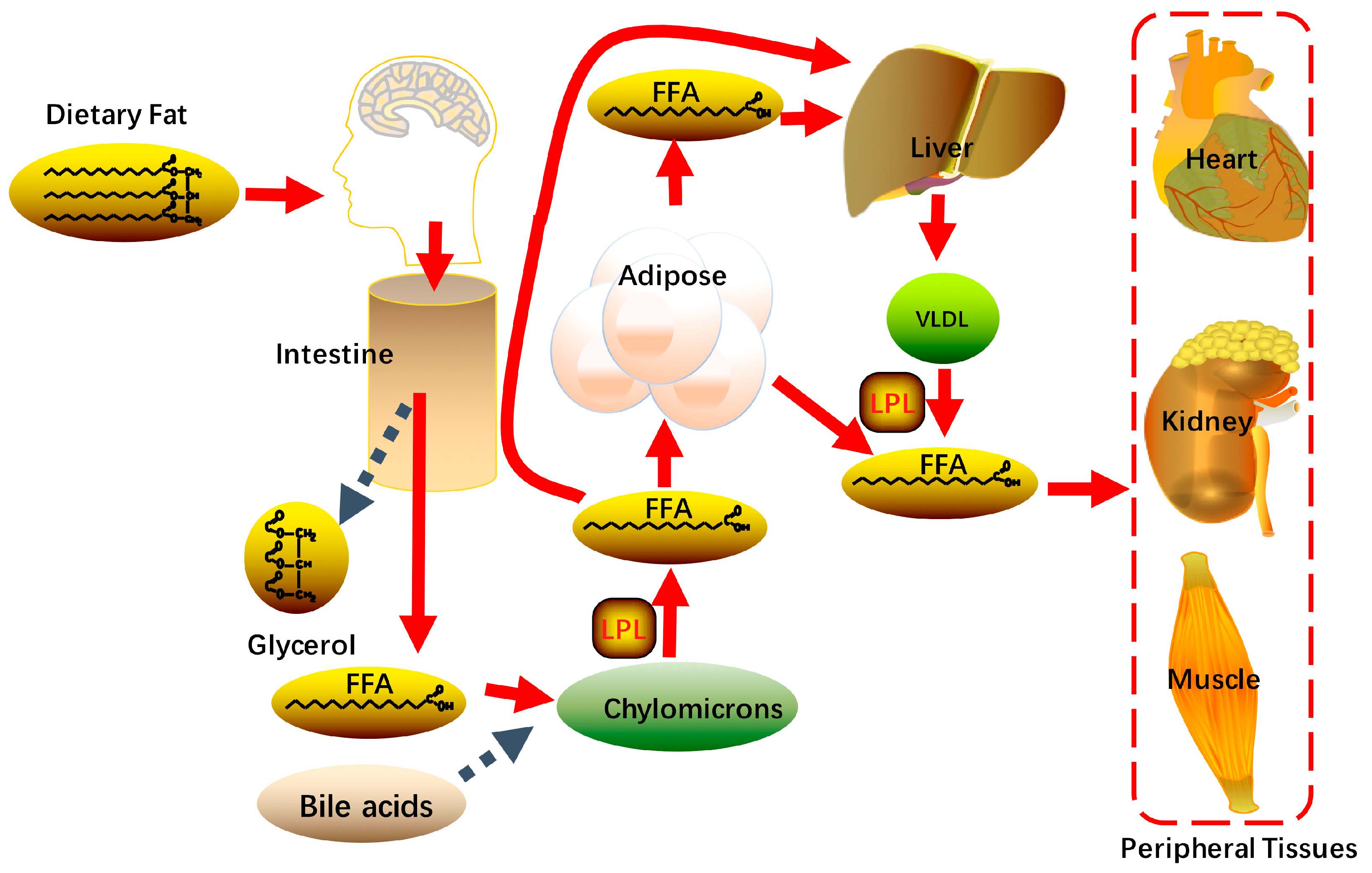
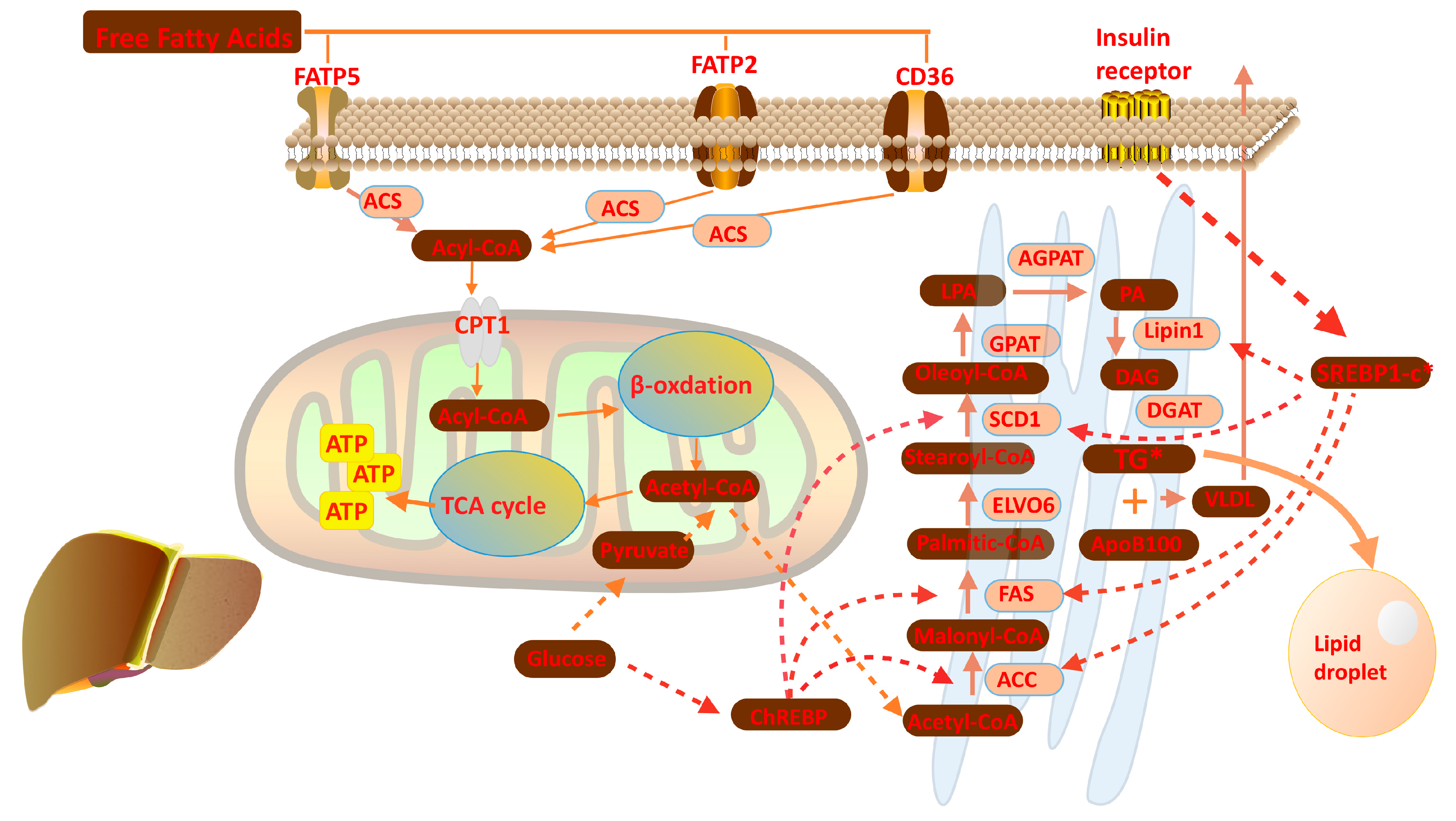
2. Overview of Liver Lipid Metabolism
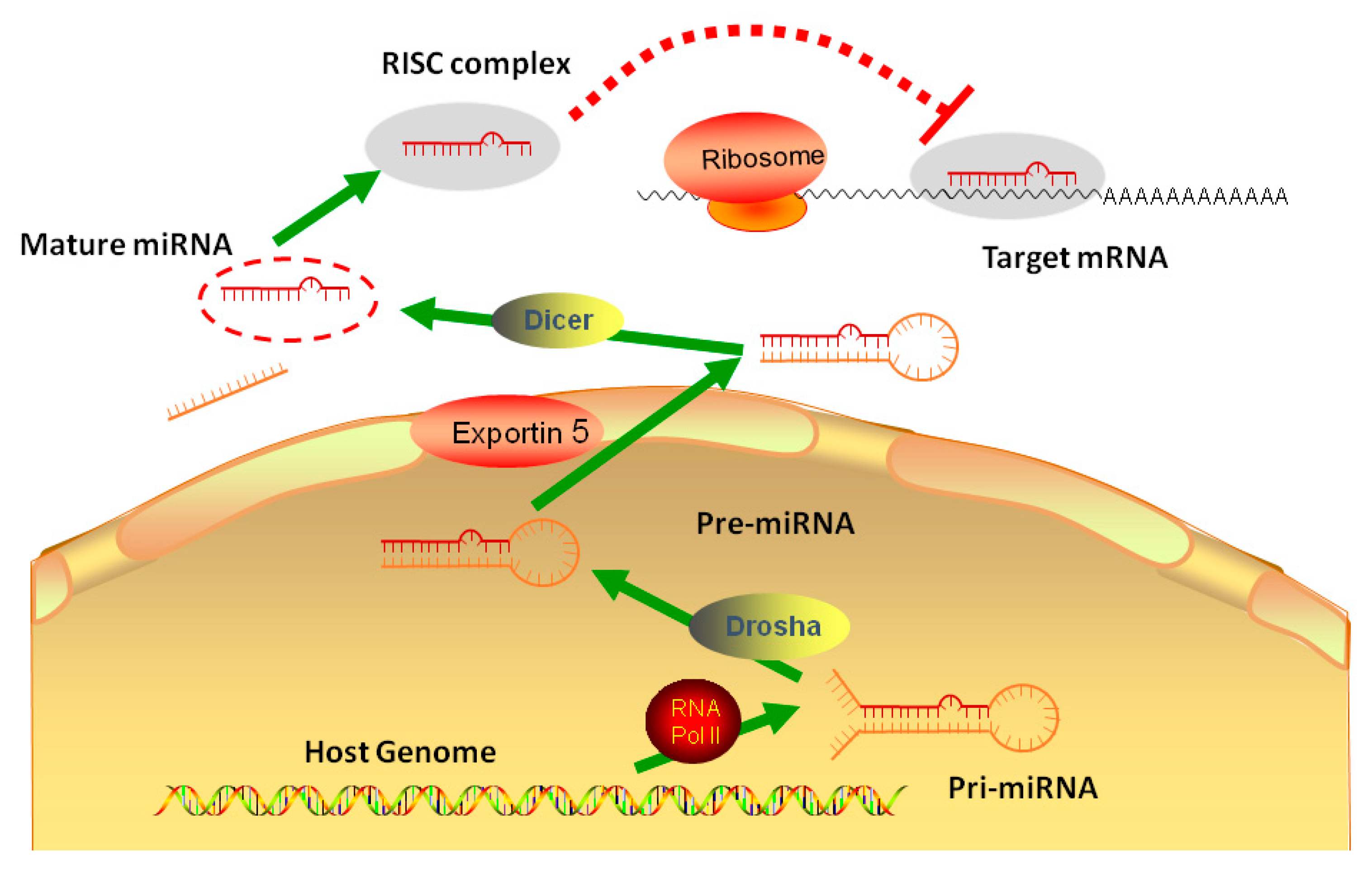
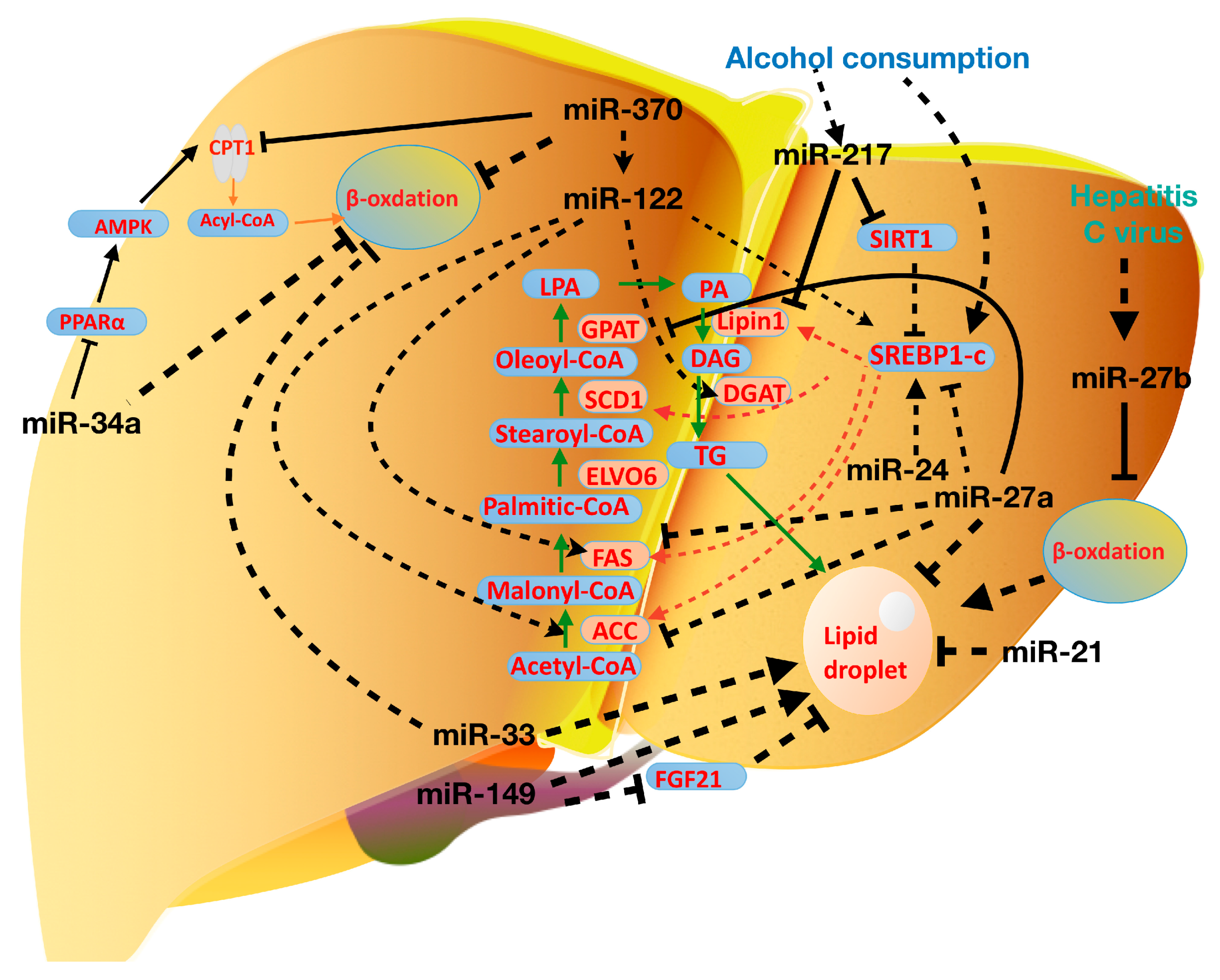
3. miRNAs in Lipid Metabolism
4. miRNAs in Nonalcoholic Fatty Liver Disease
5. miRNAs in Alcohol and Virus-Induced Fatty Liver
6. Novel Diagnostic Tools and Treatments for Fatty Liver Diseases
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Diehl-Jones, W.L.; Askin, D.F. The neonatal liver, Part 1: Embryology, anatomy, and physiology. Neonatal Netw. 2002, 21, 5–12. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.; Bloomston, M. Liver anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef]
- Peng, J.; Yu, J.; Xu, H.; Kang, C.; Shaul, P.W.; Guan, Y.; Zhang, X.; Su, W. Enhanced Liver Regeneration after Partial Hepatectomy in Sterol Regulatory Element-Binding Protein (SREBP)-1c-Null Mice is Associated with Increased Hepatocellular Cholesterol Availability. Cell Physiol. Biochem. 2018, 47, 784–799. [Google Scholar] [CrossRef]
- Ai, R.; Laragione, T.; Hammaker, D.; Boyle, D.L.; Wildberg, A.; Maeshima, K.; Palescandolo, E.; Krishna, V.; Pocalyko, D.; Whitaker, J.W.; et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat. Commun. 2018, 9, 1921. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Rottiers, V.; Najafi-Shoushtari, S.H.; Kristo, F.; Gurumurthy, S.; Zhong, L.; Li, Y.; Cohen, D.E.; Gerszten, R.E.; Bardeesy, N.; Mostoslavsky, R.; et al. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 225–233. [Google Scholar] [CrossRef]
- Saunders, M.A.; Liang, H.; Li, W.H. Human polymorphism at microRNAs and microRNA target sites. Proc. Natl. Acad. Sci. USA 2007, 104, 3300–3305. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar] [PubMed]
- Ashbrook, J.D.; Spector, A.A.; Santos, E.C.; Fletcher, J.E. Long chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1975, 250, 2333–2338. [Google Scholar] [PubMed]
- Ashbrook, J.D.; Spectro, A.A.; Fletcher, J.E. Medium chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1972, 247, 7038–7042. [Google Scholar]
- Krammer, J.; Digel, M.; Ehehalt, F.; Stremmel, W.; Fullekrug, J.; Ehehalt, R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int. J. Med. Sci. 2011, 8, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Bian, Y.; Yuan, S.; Chen, K.; Sheng, Y.; Fu, T.; Wei, L.; Pei, Y.; Sun, H.J.P. Identification of 4-aminoquinoline core for the design of new cholinesterase inhibitors. PeerJ. 2016, 4. [Google Scholar] [CrossRef]
- Hajri, T.; Han, X.X.; Bonen, A.; Abumrad, N.A. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Investig. 2002, 109, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Pei, K.; Cai, H.; Tu, S.; Zhang, Z.; Cheng, X.; Qiao, F.; Fan, K.; Qin, K.; Liu, X.; et al. Bioactivity evaluation-based ultra high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole-time-of-flight mass spectrometry and novel distinction of multi-subchemome compatibility recognition strategy with Astragali Radix-Fructus Corni herb-pair as a case study. J. Pharm. Biomed. Anal. 2016, 129, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Smith, A.J.; Bernlohr, D.A. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 2003, 278, 43008–43013. [Google Scholar] [CrossRef]
- Richards, M.R.; Harp, J.D.; Ory, D.S.; Schaffer, J.E. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 2006, 47, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA: Diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Q.; Hong, L.; Chen, K.; Yang, L.; Guo, T.; Ke, S.; Yan, C.; Cheng, G.; Lee, R.J. Lipid Nanoparticles Loaded with an Antisense Oligonucleotide Gapmer Against Bcl-2 for Treatment of Lung Cancer. Pharm. Res. 2017, 34, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R.; Haystead, T.A.; Holland, R.; Carling, D.A.; Hardie, D.G. The role of phosphorylation/dephosphorylation of acetyl-CoA carboxylase in the regulation of mammalian fatty acid biosynthesis. Biochem. Soc. Trans. 1986, 14, 559–562. [Google Scholar] [CrossRef] [Green Version]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Huang, K.; Ai, R.; Wang, M.; Wang, W.J.G. Predicting CpG methylation levels by integrating Infinium HumanMethylation450 BeadChip array data. Genomics 2016, 107, 132–137. [Google Scholar] [CrossRef]
- Fan, S.; Chi, W. Methods for genome-wide DNA methylation analysis in human cancer. Brief Funct. Genom. 2016, 15, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Ferre, P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: A role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 2002, 366, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Sun, Y.; Chen, K.; Sun, H.; Wei, W. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J. Drug Target. 2016, 25, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, C.; Ai, R.; Firestein, G.S.; Wang, W. Computationally expanding Infinium HumanMethylation450 BeadChip array data to reveal distinct DNA methylation patterns of rheumatoid arthritis. Bioinformatics 2016, 32, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.J.; Wang, M.; Zou, S.B.; Moon, K.M.; Foster, L.J.; Ibba, M.; Navarre, W.W. Divergent protein motifs direct elongation factor P-mediated translational regulation in Salmonella enterica and Escherichia coli. MBio 2013, 4, e00180-13. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Hernandez, V.A.; Hu, K. Functional interaction of the two-pore domain potassium channel TASK-1 and caveolin-3. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Ion channels, protein kinase C and caveolae in cardioprotection. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Ferre, P.; Dugail, I. ADD-1/SREBP-1 is a major determinant of tissue differential lipogenic capacity in mammalian and avian species. J. Lipid Res. 2001, 42, 106–113. [Google Scholar] [PubMed]
- Kang, C.; Qin, J.; Osei, W.; Hu, K. Age-dependent Mitochondrial Targeting Of Protein Kinase C Epsilon In Cardioprotection. FASEB J. 2017. [Google Scholar] [CrossRef]
- Willy, P.J.; Mangelsdorf, D.J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997, 11, 289–298. [Google Scholar] [CrossRef]
- Kang, C.; Sun, Y.; Zhu, J.; Li, W.; Zhang, A.; Kuang, T.; Xie, J.; Yang, Z. Delivery of Nanoparticles for Treatment of Brain Tumor. Curr. Drug Metab. 2016, 17, 745–754. [Google Scholar] [CrossRef]
- Kang, C.; Qin, J.; Osei, W.; Hu, K. Regulation of protein kinase C-epsilon and its age-dependence. Biochem. Biophys. Res. Commun. 2017, 482, 1201–1206. [Google Scholar] [CrossRef]
- Dentin, R.; Denechaud, P.D.; Benhamed, F.; Girard, J.; Postic, C. Hepatic gene regulation by glucose and polyunsaturated fatty acids: A role for ChREBP. J. Nutr. 2006, 136, 1145–1149. [Google Scholar] [CrossRef]
- Kang, C.N.A.; Hu, K. Role of caveolin-3 in adenosine-induced increase in mitochondrial PKCε. FASEB J. 2013. [Google Scholar] [CrossRef]
- Adeli, K.; Taghibiglou, C.; Van Iderstine, S.C.; Lewis, G.F. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc. Med. 2001, 11, 170–176. [Google Scholar] [CrossRef]
- Taghibiglou, C.; Carpentier, A.; Van Iderstine, S.C.; Chen, B.; Rudy, D.; Aiton, A.; Lewis, G.F.; Adeli, K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J. Biol. Chem. 2000, 275, 8416–8425. [Google Scholar]
- Lei, S.; Chen, K.; Yuan, S.; Huang, W.; Wei, L.; Qian, Z. Biochemistry. Crocetin Inhibits Lipopolysaccharide-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. 2016, 40, 443–452. [Google Scholar]
- White, D.A.; Bennett, A.J.; Billett, M.A.; Salter, A.M. The assembly of triacylglycerol-rich lipoproteins: An essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 1998, 80, 219–229. [Google Scholar] [PubMed]
- Li, Q.; Yang, H.; Mo, J.; Chen, Y.; Wu, Y.; Kang, C.; Sun, Y.; Sun, H. Identification by shape-based virtual screening and evaluation of new tyrosinase inhibitors. PeerJ. 2018, 6. [Google Scholar] [CrossRef]
- Esau, C.; Kang, X.L.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.Q.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Qiao, H.; Dong, F.; Jing, C.; Yuan, S.; Chen, K.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Sun, Y.; Kang, C.; Zhu, H. Pegylated drug delivery systems: From design to biomedical applications. Nano LIFE 2016, 6, 1642002. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Song, L. Delivery of Antipsychotics with Nanoparticles. Drug Dev. Res. 2016, 77, 393–399. [Google Scholar] [CrossRef]
- Qiao, H.; Zhu, Z.; Fang, D.; Sun, Y.; Kang, C.; Di, L.; Zhang, L.; Gao, Y. Redox-triggered mitoxantrone prodrug micelles for overcoming multidrug-resistant breast cancer. J. Drug Target. 2017, 26, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Zhang, H.; Liang, X.; Xiang, Y.; Yu, C.; Zen, K.; Li, Y.; Zhang, C.Y. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J. Lipid Res. 2009, 50, 1756–1765. [Google Scholar] [CrossRef] [Green Version]
- Tome, M.; Lopez-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011, 18, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Yao, Z.; Liu, F.; Zhou, Y. Peptide-Based Ligand for Active Delivery of Liposomal Doxorubicin. Nano Life 2016, 6. [Google Scholar] [CrossRef]
- Shirasaki, T.; Honda, M.; Shimakami, T.; Horii, R.; Yamashita, T.; Sakai, Y.; Sakai, A.; Okada, H.; Watanabe, R.; Murakami, S.; et al. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J. Virol. 2013, 87, 5270–5286. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.P.; George, M.; Kalyanasundaram, A.; Chen, K.; Periasamy, M.; Hu, K.; Lacombe, V.A. GLUT12 functions as a basal and insulin-independent glucose transporter in the heart. Biochim. Biophys. Acta. 2013, 1832, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Dou, S.; Xiong, M.; Sun, T.; Nano, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, N.Y.; Yu, H.T.; Sun, Y.; Kang, C.; Huang, Q.B.; Sun, H.P.; Wang, X.L.; Li, N.G. Discovery of novel inhibitors disrupting HIF-1α/von Hippel–Lindau interaction through shape-based screening and cascade docking. PeerJ. 2016, 4. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmen, J.; Lindow, M.; Schutz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjarn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Control. Release 2016, 243, 160–171. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.Y.; Hsiao, J.K.; Wang, Y.P.; Lan, C.H.; Wu, H.C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feinberg, M.W. MicroRNA-management of lipoprotein homeostasis. Circ. Res. 2014, 115, 2–6. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Naar, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef]
- Brown, M.S.; Ye, J.; Goldstein, J.L. HDL miR-ed down by SREBP introns. Science 2010, 328, 1495–1496. [Google Scholar] [CrossRef]
- Yung, B.C.; Li, J.; Zhang, M.; Cheng, X.; Li, H.; Yung, E.M.; Kang, C.; Cosby, L.E.; Liu, Y.; Teng, L.; et al. Lipid nanoparticles composed of quaternary amine-tertiary amine cationic lipid combination (QTsome) for therapeutic delivery of antimiR-21 for lung cancer. Mol. Pharm. 2016, 13, 653–662. [Google Scholar] [CrossRef]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b Is a Regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; William-Olsson, L.; Ahnmark, A.; Ekroos, K.; Hallberg, C.; Sjogren, H.P.; Becker, B.; Svensson, L.; Clapham, J.C.; Oscarsson, J.; et al. Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J. 2006, 20, 434–443. [Google Scholar] [CrossRef]
- van Ree, J.H.; van den Broek, W.J.; Dahlmans, V.E.; Groot, P.H.; Vidgeon-Hart, M.; Frants, R.R.; Wieringa, B.; Havekes, L.M.; Hofker, M.H. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis 1994, 111, 25–37. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C. Self-Assembly of Peptides into Hydrogel. J. Org. Inorg. Chem. 2016, 2. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Arrese, M.; Zamora-Valdes, D.; Uribe, M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007, 27, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Kechagias, S. Low clinical relevance of the nonalcoholic fatty liver disease activity score (NAS) in predicting fibrosis progression. Scand. J. Gastroenterol. 2012, 47, 108–115. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Design 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Youssef, W.; McCullough, A.J. Diabetes mellitus, obesity, and hepatic steatosis. Semin. Gastrointest. Dis. 2002, 13, 17–30. [Google Scholar]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ye, Y.F.; Chen, S.H.; Yu, C.H.; Liu, J.; Li, Y.M. MicroRNA expression pattern in different stages of nonalcoholic fatty liver disease. Dig. Liver Dis. 2009, 41, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Chung, C.H.; Ha, T. High fat diet induced downregulation of microRNA-467b increased lipoprotein lipase in hepatic steatosis. Biochem. Biophys. Res. Commun. 2011, 414, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, A.; Zhu, L.; Gupta, N.; Chang, Y.; Fang, F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 2007, 21, 2785–2794. [Google Scholar] [CrossRef]
- Xu, J.; Wu, C.; Che, X.; Wang, L.; Yu, D.; Zhang, T.; Huang, L.; Li, H.; Tan, W.; Wang, C.; et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol. Carcinog. 2011, 50, 136–142. [Google Scholar] [CrossRef]
- Hoekstra, M.; van der Sluis, R.J.; Kuiper, J.; Van Berkel, T.J.C. Nonalcoholic fatty liver disease is associated with an altered hepatocyte microRNA profile in LDL receptor knockout mice. J. Nutr. Biochem. 2012, 23, 622–628. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef]
- Lee, J.; Padhye, A.; Sharma, A.; Song, G.; Miao, J.; Mo, Y.Y.; Wang, L.; Kemper, J.K. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J. Biol. Chem. 2010, 285, 12604–12611. [Google Scholar] [CrossRef] [PubMed]
- Xiuyun, H.; Shanqin, X.; Maitland-Toolan, K.A.; Kaori, S.; Bingbing, J.; Yasuo, I.; Fan, L.; Kenneth, W.; Michel, W.; Verbeuren, T.J. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar]
- Ding, J.; Li, M.; Wan, X.; Jin, X.; Chen, S.; Yu, C.; Li, Y. Effect of miR-34a in regulating steatosis by targeting PPARalpha expression in nonalcoholic fatty liver disease. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef]
- Nakajima, T.; Tanaka, N.; Kanbe, H.; Hara, A.; Kamijo, Y.; Zhang, X.; Gonzalez, F.J.; Aoyama, T. Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: A novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol. Pharmacol. 2009, 75, 782–792. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic micro RNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Wu, H.; Xiao, H.; Chen, X.; Willenbring, H.; Steer, C.J.; Song, G. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology 2014, 60, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Hornby, R.J.; Starkey Lewis, P.; Dear, J.; Goldring, C.; Park, B.K. MicroRNAs as potential circulating biomarkers of drug-induced liver injury: Key current and future issues for translation to humans. Expert Rev. Clin. Pharmacol. 2014, 7, 349–362. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Marzolf, B.; Troisch, P.; Brightman, A.; Hu, Z.; Hood, L.E.; Galas, D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA 2009, 106, 4402–4407. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Suzuki, K.; Ichino, N.; Ando, Y.; Sawada, A.; Osakabe, K.; Sugimoto, K.; Ohashi, K.; Teradaira, R.; Inoue, T. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta 2013, 424, 99–103. [Google Scholar] [CrossRef]
- Sun, C.; Huang, F.; Liu, X.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L. miR-21 regulates triglyceride and cholesterol metabolism in non-alcoholic fatty liver disease by targeting HMGCR. Int. J. Mol. Med. 2015, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lv, D.; Zhao, Y.; Chen, X.; Song, M.; Liu, J.; Bei, Y.; Wang, F.; Yang, W.; Yang, C. miR-149 controls non-alcoholic fatty liver by targeting FGF-21. J. Cell. Mol. Med. 2016, 20, 1603–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Bei, Y.; Liu, J.; Dimitrova-Shumkovska, J.; Kuang, D.; Zhou, Q.; Li, J.; Yang, Y.; Xiang, Y.; Wang, F.; et al. miR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J. Cell. Mol. Med. 2016, 20, 204–216. [Google Scholar] [CrossRef]
- Murata, Y.; Konishi, M.; Itoh, N. FGF21 as an Endocrine Regulator in Lipid Metabolism: From Molecular Evolution to Physiology and Pathophysiology. J. Nutr. Metabol. 2011, 2011. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, J.; Dai, Y.; Zhou, N.; Ji, C.; Li, X. MiR-146b attenuates high-fat diet-induced non-alcoholic steatohepatitis in mice. J. Gastroenterol. Hepatol. 2015, 30, 933–943. [Google Scholar] [CrossRef]
- Miller, A.M.; Gilchrist, D.S.; Nijjar, J.; Araldi, E.; Ramirez, C.M.; Lavery, C.A.; Fernándezhernando, C.; Mcinnes, I.B.; Kurowskastolarska, M. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS ONE 2013, 8, e72324. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lv, G.C.; Sheng, J.; Yang, Y.D. Effect of miRNA-10b in regulating cellular steatosis level by targeting PPAR-alpha expression, a novel mechanism for the pathogenesis of NAFLD. J. Gastroenterol. Hepatol. 2010, 25, 156–163. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Costinean, S.; Croce, C.M. MicroRNAs, the immune system and rheumatic disease. Nat. Clin. Pract. Rheum. 2008, 4, 534–541. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Starlard-Davenport, A.; Tryndyak, V.P.; Han, T.; Ross, S.A.; Rusyn, I.; Beland, F.A. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab. Investig. 2010, 90, 1437–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csak, T.; Bala, S.; Lippai, D.; Kodys, K.; Catalano, D.; Iracheta-Vellve, A.; Szabo, G. MicroRNA-155 deficiency attenuates liver steatosis and fibrosis without reducing inflammation in a mouse model of steatohepatitis. PLoS ONE 2015, 10, e0129251. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, N.; Wang, Z.; Ai, D.M.; Cao, Z.Y.; Pan, H.P. Decreased MiR-155 level in the peripheral blood of non-alcoholic fatty liver disease patients may serve as a biomarker and may influence LXR activity. Cell. Physiol. Biochem. 2016, 39, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. New pathway of ethanol metabolism in the liver. Gastroenterology 1970, 59, 930–937. [Google Scholar] [PubMed]
- Lieber, C.S. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol. Clin. Exp. Res. 1991, 15, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Song, Z.; Lambert, J.C.; McClain, C.J.; Kang, Y.J. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am. J. Pathol. 2003, 163, 1137–1146. [Google Scholar] [CrossRef]
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Marcos, M.; Kodys, K.; Csak, T.; Catalano, D.; Mandrekar, P.; Szabo, G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 2011, 286, 1436–1444. [Google Scholar] [CrossRef]
- Bala, S.; Szabo, G. MicroRNA Signature in Alcoholic Liver Disease. Int. J. Hepatol. 2012, 2012. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar]
- Ponugoti, B.; Kim, D.H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.Y.; Chiang, C.M.; Veenstra, T.D.; Kemper, J.K. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef] [Green Version]
- You, M.; Jogasuria, A.; Taylor, C.; Wu, J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 88–100. [Google Scholar]
- Liang, X.M.; Hu, M.; Rogers, C.Q.; Shen, Z.; You, M. Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid. Redox Signal. 2011, 15, 425–435. [Google Scholar] [CrossRef]
- Yin, H.; Hu, M.; Zhang, R.; Shen, Z.; Flatow, L.; You, M. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J. Biol. Chem. 2012, 287, 9817–9826. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Wang, R.; Tuo, H.; Guo, Y.; Yi, L.; Wang, D.; Wang, J. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS ONE 2013, 8, e83620. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liang, X.; Rogers, C.Q.; Rideout, D.; You, M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G364–G374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, T.E.; Finck, B.N. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrin. Met. 2011, 22, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Wang, F.M.; Li, X.; Rogers, C.Q.; Liang, X.M.; Finck, B.N.; Mitra, M.S.; Zhang, R.; Mitchell, D.A.; You, M. Regulation of hepatic lipin-1 by ethanol: Role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology 2012, 55, 437–446. [Google Scholar] [CrossRef]
- Khalil, M.B.; Sundaram, M.; Zhang, H.Y.; Links, P.H.; Raven, J.F.; Manmontri, B.; Sariahmetoglu, M.; Tran, K.; Reue, K.; Brindley, D.N.; et al. The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL. J. Lipid Res. 2009, 50, 47–58. [Google Scholar] [CrossRef]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Naoki, T.; Kyoji, M.; Kendo, K.; Kazuhiko, K.; Gonzalez, F.J.; Toshifumi, A. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J. Clin. Investig. 2008, 118, 683–694. [Google Scholar]
- Ferguson, D.; Zhang, J.; Davis, M.A.; Helsley, R.N.; Vedin, L.L.; Lee, R.G.; Crooke, R.M.; Graham, M.J.; Parini, P.; Brown, J.M. The Lipid Droplet Associated Protein Perilipin 3 facilitates hepatitis C Virus-Driven Hepatic Steatosis. J. Lipid Res. 2016. [Google Scholar] [CrossRef]
- Sarnow, P.; Jopling, C.L.; Norman, K.L.; Schutz, S.; Wehner, K.A. MicroRNAs: Expression, avoidance and subversion by vertebrate viruses. Nat. Rev. Microbiol. 2006, 4, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, R.; Chen, R.; Lyn, R.K.; Jones, D.M.; O’Hara, S.; Rouleau, Y.; Cheng, J.; Srinivasan, P.; Nasheri, N.; Russell, R.S.; et al. Hepatitis C Virus Induced Up-Regulation of MicroRNA-27: A Novel Mechanism for Hepatic Steatosis. Hepatology 2014, 59, 98–108. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Liu, S.A.; Zhang, J.Q.; Ju, W.; Quan, M.; Feng, S.H.; Dong, J.L.; Gao, P.; Cheng, J. MicroRNA-185-5p mediates regulation of SREBP2 expression by hepatitis C virus core protein. World J. Gastroenterol. 2015, 21, 4517–4525. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wang, Y.P.; Borlak, J.; Tong, W.D. Mechanistically linked serum miRNAs distinguish between drug induced and fatty liver disease of different grades. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ge, G.; Pan, T.; Wen, D.; Gan, J. A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e105192. [Google Scholar] [CrossRef]
- Pirola, C.J.; Gianotti, T.F.; Castano, G.O.; Sookoian, S. Circulating MicroRNA-122 signature in nonalcoholic fatty liver disease and cardiovascular disease: A new endocrine system in metabolic syndrome. Hepatology 2013, 57, 2545–2547. [Google Scholar] [CrossRef] [Green Version]
- Miyaaki, H.; Ichikawa, T.; Kamo, Y.; Taura, N.; Honda, T.; Shibata, H.; Milazzo, M.; Fornari, F.; Gramantieri, L.; Bolondi, L.; et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014, 34, e302–e307. [Google Scholar] [CrossRef]
- Bala, S.; Petrasek, J.; Mundkur, S.; Catalano, D.; Levin, I.; Ward, J.; Alao, H.; Kodys, K.; Szabo, G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012, 56, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K.; Gerhard, G.S. Circulating microRNAs in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Venugopal, S.K. Circulating microRNAs: Possible role as non-invasive diagnostic biomarkers in liver disease. Clin. Res. Hepatol. Gastroenterol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M.; et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012, 126. [Google Scholar] [CrossRef]
- Uzumcu, A.; Tekguc, M.; Sevli, S.; Bayrak, O.F.; Ozen, M. The role of microRNA (miRNA)s as a novel tool in cancer diagnosis and therapy. Int. Un. Biochem. Mol. Biol. Life 2009, 61, 298. [Google Scholar]
- Xu, S.; Kang, C.; Chen, M.; Zhou, P.; He, G.; Cui, Y.; Yang, D.; Wu, Y. Dynamic expression of AQP4 in early stage of ischemia/reperfusion rats and cerebral edema. Chin. Pharmacol. Bull. 2016, 32, 1433–1441. [Google Scholar]
- Sun, Y.; Kang, C.; Zhang, A.; Liu, F.; Hu, J.; Zhong, X.; Xie, J. Co-delivery of dual-drugs with nanoparticle to overcome multidrug resistance. Eur. J. Biomed. Res. 2016, 2, 12–18. [Google Scholar] [CrossRef]
- Jing, X.; Yang, Z.; Zhou, C.; Jing, Z.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Sun, Y.; Wang, M.; Cheng, X. Nanosized camptothecin conjugates for single and combined drug delivery. Eur. J. Biomed. Res. 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Kang, C.; Hu, K. Modulation of the two-pore domain potassium channel TASK-1 by caveolin-3. FASEB J. 2015. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for Cancer Therapy. FASEB J. 2010, 18, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.B.; Zhu, L.Y.; Wang, Y.G.; Li, F.; Zhang, X.Y.; Dai, W.J. Systemic transcriptome analysis of hepatocellular carcinoma. Tumour Biol. 2016, 37, 13323–13331. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Sun, Y.; Kang, C.; Wan, G. The theory of dielectrophoresis and its applications on medical and materials research. Eur. J. BioMed. Res. 2017, 2, 7–11. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, Y.; Kang, C. Structure and self-assembly of multicolored naphthalene diimides semiconductor. Nano Life 2016, 6, 6. [Google Scholar] [CrossRef]
- Yan, G.; Du, Q.; Wei, X.; Miozzi, J.; Kang, C.; Wang, J.; Han, X.; Pan, J.; Xie, H.; Chen, J.; et al. Application of Real-Time Cell Electronic Analysis System in Modern Pharmaceutical Evaluation and Analysis. Molecules 2018, 23, 3280. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, Y.; Kang, C. Controlling amphiphilic functional block copolymers’ self-assembly: From structure to size. Glob. J. Nanomed. 2016. [Google Scholar] [CrossRef]
| miRNA | Species | Disease-Association | Expression | Target Genes | Findings | Pathologic (+) or Protective (−) | Reference |
|---|---|---|---|---|---|---|---|
| miR-122 | Human, mouse | NAFLD | ↑ (circulation) ↓ | HMGCR, MTTP, HMGCS1PGC1-α | Upregulate the expression of SREBP1-c, DGAT2, FAS and ACC1 | + | [37,112,113] |
| miR-370 | Human | NAFLD | ↑ | SREBP-1c, DGAT2 | Upregulate the expression of genes involved in lipogenesis Upregulate the expression of miR-122; | + | [39] |
| miR-29c | Human | NAFLD | ↑ | HMGR, Sirt1 | Regulate insulin resistance and lipid metabolism | − | [84] |
| miR-216 | Mouse | NAFLD | ↓ | FAS, SREBP-1c | Regulate the lipid synthesis | / | [61] |
| miR-302a | Mouse | NAFLD | ↓ | ELOVL; ABCA1 | Regulate hepatic lipid accumulation | / | [61] |
| miR-34a | Mouse, Human | NAFLD | ↑ | PPARα, SIRT1 | Decrease FA β-oxidation | − | [68,75,84] |
| miR-24 | Human, Mouse | NAFLD | ↑ | Insig1 | Downregulate Insig1 expression; Promote SREBP-1 processing | − | [72] |
| miR-21 | Mouse | NAFLD | ↓ | HMGCR | Regulate liver TG and cholesterol metabolism | + | [76] |
| miR-149 | Mouse | NAFLD | ↑ | FGF21 | Regulate lipogenesis in HepG2 cells | − | [77] |
| miR-10b | Human | NAFLD | ↑ | PPARα | Overexpression of miR-10b increases the triglyceride levels in hepatocytes | − | [82] |
| miR-155 | Mouse, Human | NAFLD; Alcoholic fatty liver | ↓ | LXRα | Regulate LXRα/SREBP-1c signaling and influence liver lipid accumulation. | +/− | [117,118,119,124,125] |
| miR-467 | Mouse | NAFLD | ↓ | LPL | Regulate lipid metabolism through target LPL | + | [86] |
| miR-217 | Human | Alcoholic fatty liver | ↑ | SIRT1; Lipin1 | Promote ethanol induced -fat accumulation in hepatocytes | +/− | [133] |
| miR-27 | Human | DyslipidemiaVirus hepatitis | ↑ | PPARα; ANGPTL3 | Promote triglyceride accumulation in hepatocytes and inhibit hepatitis C virus replication dyslipidemia animal model | +/− | [60,81,136] |
| miR-185-5p | Human | Virus hepatitis | ↓ | SREBP2 | Regulate cholesterol homeostasis in liver | − | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Peng, J.; Luan, Z.; Zheng, F.; Su, W. MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules 2019, 24, 230. https://doi.org/10.3390/molecules24020230
Yu J, Peng J, Luan Z, Zheng F, Su W. MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules. 2019; 24(2):230. https://doi.org/10.3390/molecules24020230
Chicago/Turabian StyleYu, Jingwei, Jun Peng, Zhilin Luan, Feng Zheng, and Wen Su. 2019. "MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease" Molecules 24, no. 2: 230. https://doi.org/10.3390/molecules24020230




