Well-Defined Pre-Catalysts in Amide and Ester Bond Activation
Abstract
:1. Introduction
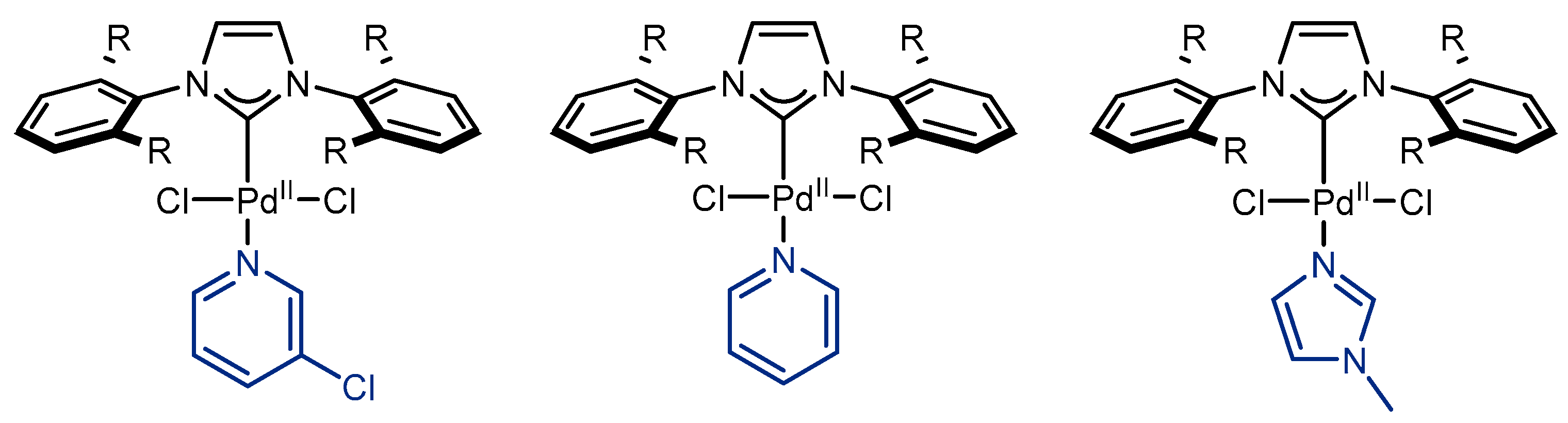
2. Pd(allyl)(NHC)Cl Pre-Catalysts in Suzuki-Miyaura Cross-Coupling of Amides and Esters
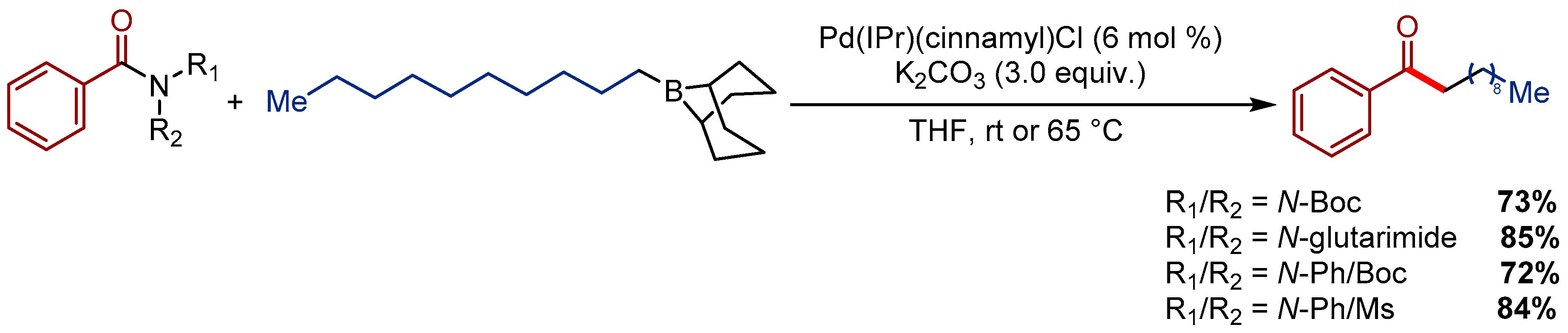
2.1. Pd(allyl)(NHC)Cl Pre-Catalysts in Suzuki-Miyaura Cross-Coupling of Amides
2.2. Pd(η3-1-t-Bu-indenyl) (NHC)Cl Pre-Catalysts in Suzuki-Miyaura Cross-Coupling of Amides
2.3. Pd(allyl)(NHC)Cl Pre-Catalysts in Suzuki-Miyaura Cross-Coupling of Esters
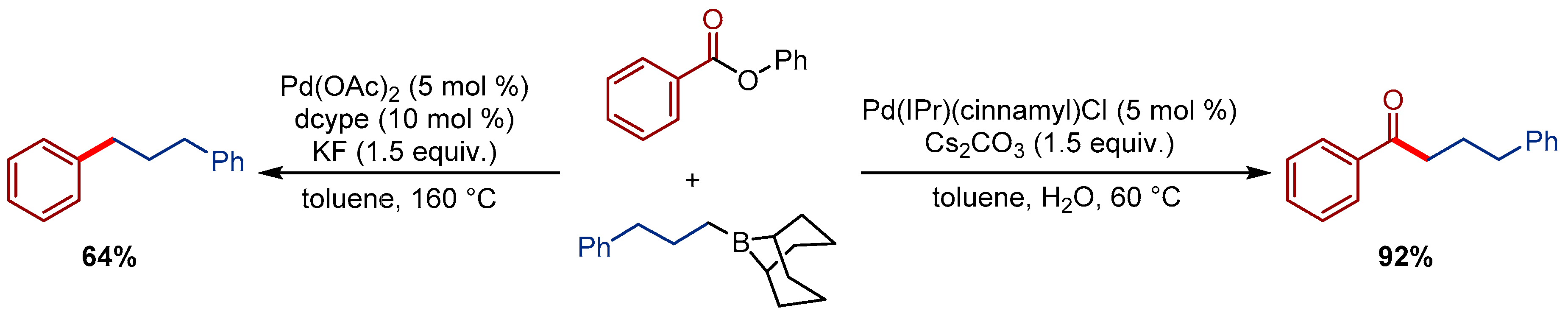
2.4. Pd(η3-1-t-Bu-indenyl) (NHC)Cl Pre-Catalysts in Suzuki-Miyaura Cross-Coupling of Esters
3. Pd(allyl)(NHC)Cl Pre-Catalysts in Buchwald-Hartwig Amination of Amides and Esters
3.1. Pd(cinnamyl)(IPr)Cl Pre-Catalyst for the Transamidation of Amides
3.2. Pd(allyl)(IPr)Cl Pre-Catalyst for the Transamidation of Esters
3.3. Pd(indenyl)(SIPr)Cl Pre-Catalyst for the Transamidation of Esters
4. Pd-PEPPSI Pre-Catalysts in the Suzuki-Miyaura Cross-Coupling of Amides and Esters
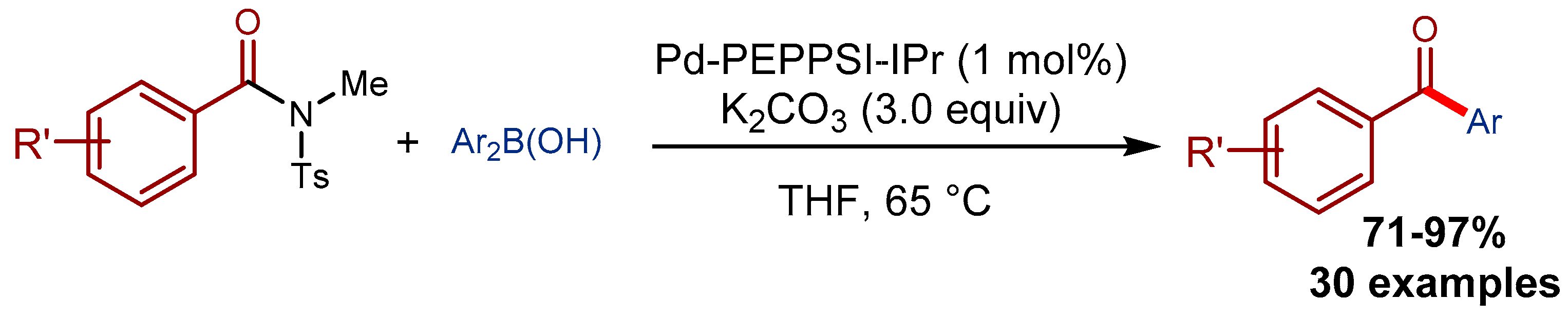
4.1. Pd-PEPPSI Pre-Catalysts in the Suzuki-Miyaura Cross-Coupling of Amides
4.2. Pd-PEPPSI Pre-Catalysts in the Suzuki-Miyaura Cross-Coupling of Esters
5. Pd−PEPPSI Pre-Catalysts in Buchwald–Hartwig Amidation of Esters and Amides
6. Palladium(II)/N-Heterocyclic Carbene-Catalyzed Direct C–H Acylation of Heteroarenes with N-Acylsaccharins
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl−Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.R.; Fagnou, K. The Catalytic Cross-Coupling of Unactivated Arenes. Science 2007, 316, 1172–1175. [Google Scholar] [CrossRef]
- Kumar, D.; Vemula, S.R.; Cook, G.R. Merging C–H Bond Functionalization with Amide Alcoholysis: En Route to 2-Aminopyridines. ACS Catal. 2016, 6, 3531–3536. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vemula, S.R.; Balasubramanian, N.; Cook, G.R. Indium-Mediated Stereoselective Allylation. Acc. Chem. Res. 2016, 49, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vemula, S.R.; Cook, G.R. Recent Advances in the Catalytic Synthesis of α-Ketoamides. ACS Catal. 2016, 6, 4920–4945. [Google Scholar] [CrossRef]
- Kumar, D.; Vemula, S.R.; Cook, G.R. Highly Chemo- and Regioselective Allylic Substitution with Tautomerizable Heteroarenes. Green Chem. 2015, 17, 4300–4306. [Google Scholar] [CrossRef]
- Vemula, S.R.; Kumar, D.; Cook, G.R. Palladium-Catalyzed Allylic Amidation with N-Heterocycles via Sp 3 C–H Oxidation. ACS Catal. 2016, 6, 5295–5301. [Google Scholar] [CrossRef]
- Satoh, T.; Miura, M. Catalytic Direct Arylation of Heteroaromatic Compounds. Chem. Lett. 2007, 36, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Masse, J.P. Activation of Grignard Reagents by Transition-Metal Complexes. A New and Simple Synthesis of Trans-Stilbenes and Polyphenyls. J. Chem. Soc. Chem. Commun. 1972, 144a. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. A General, Selective, and Facile Method for Ketone Synthesis from Acid Chlorides and Organotin Compounds Catalyzed by Palladium. J. Am. Chem. Soc. 1978, 100, 3636–3638. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A New Stereospecific Cross-Coupling by the Palladium-Catalyzed Reaction of 1-Alkenylboranes with 1-Alkenyl or 1-Alkynyl Halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Trost, B.M.; Fullerton, T.J. New Synthetic Reactions. Allylic Alkylation. J. Am. Chem. Soc. 1973, 95, 292–294. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes−Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Canseco-González, D.; Germán-Acacio, J.M.; Morales-Morales, D. Xanthine Based N-Heterocyclic Carbene (NHC) Complexes. J. Organomet. Chem. 2018, 867, 51–54. [Google Scholar] [CrossRef]
- Hazari, N.; Melvin, P.R.; Beromi, M.M. Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem. 2017, 1, 0025. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G.R.J. Metal Complexes of N-Heterocyclic Carbenes—A New Structural Principle for Catalysts in Homogeneous Catalysis. Angew. Chem. Int. Ed. English 1995, 34, 2371–2374. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)–NHC Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective N–C/O–C Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef]
- Takise, R.; Muto, K.; Yamaguchi, J. Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev. 2017, 46, 5864–5888. [Google Scholar] [CrossRef]
- Vemula, S.R.; Kumar, D.; Cook, G.R. N-Boc-Glycine-Assisted Indium-Mediated Allylation Reaction: A Sustainable Approach. Tetrahedron Lett. 2015, 56, 3322–3325. [Google Scholar] [CrossRef]
- Hie, L.; Fine Nathel, N.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.-F.; Liu, P.; Houk, K.N.; Garg, N.K. Conversion of Amides to Esters by the Nickel-Catalysed Activation of Amide C–N Bonds. Nature 2015, 524, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, M. Sterically Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N–C Cleavage in Twisted Amides. Org. Lett. 2015, 17, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, G. Acylative Suzuki Coupling of Amides: Acyl-Nitrogen Activation via Synergy of Independently Modifiable Activating Groups. Chem. Commun. 2015, 51, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Navarro, O.; Mei, J.; Stevens, E.D.; Scott, N.M.; Nolan, S.P. Modified (NHC)Pd(Allyl)Cl (NHC = N -Heterocyclic Carbene) Complexes for Room-Temperature Suzuki−Miyaura and Buchwald−Hartwig Reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. [Google Scholar] [CrossRef] [PubMed]
- Navarro, O.; Marion, N.; Mei, J.; Nolan, S.P. Rapid Room Temperature Buchwald–Hartwig and Suzuki–Miyaura Couplings of Heteroaromatic Compounds Employing Low Catalyst Loadings. Chem. A Eur. J. 2006, 12, 5142–5148. [Google Scholar] [CrossRef]
- Chartoire, A.; Lesieur, M.; Falivene, L.; Slawin, A.M.Z.; Cavallo, L.; Cazin, C.S.J.; Nolan, S.P. [Pd(IPr*)(Cinnamyl)Cl]: An Efficient Pre-Catalyst for the Preparation of Tetra-Ortho-Substituted Biaryls by Suzuki-Miyaura Cross-Coupling. Chem. A Eur. J. 2012, 18, 4517–4521. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Nolan, S.P. An Efficient Palladium-NHC (NHC=N-Heterocyclic Carbene) and Aryl Amination Pre-Catalyst: [Pd(IPr*)(Cinnamyl)Cl]. Adv. Synth. Catal. 2012, 354, 1897–1901. [Google Scholar] [CrossRef]
- Szostak, M.; Aubé, J. Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev. 2013, 113, 5701–5765. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Szostak, M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N−C Amide Bond Activation. Chem. A Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally Inverted Acyclic Amides. J. Am. Chem. Soc. 2018, 140, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Szostak, R.; Szostak, M. Suzuki-Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N-C Cleavage. Org. Lett. 2017, 19, 3596–3599. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. N-Methylamino Pyrimidyl Amides (MAPA): Highly Reactive, Electronically-Activated Amides in Catalytic N-C(O) Cleavage. Org. Lett. 2017, 19, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lei, P.; Szostak, M.; Casals-Cruañas, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki–Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(Allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, T.; Guo, W.; Wang, Z.; Huang, J.; Zhu, Y.; Zeng, Z. N -Acyl-5,5-Dimethylhydantoin, a New Mild Acyl-Transfer Reagent in Pd Catalysis: Highly Efficient Synthesis of Functionalized Ketones. Org. Proc. Res. Dev. 2018, 22, 1188–1199. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Nolan, S.P.; Szostak, M. General Method for the Suzuki-Miyaura Cross-Coupling of Primary Amide-Derived Electrophiles Enabled by [Pd(NHC)(Cin)Cl] at Room Temperature. Org. Lett. 2017, 19, 6510–6513. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, G.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. Acyl and Decarbonylative Suzuki Coupling of N -Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon–Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar] [CrossRef]
- Meng, G.; Szostak, M. Palladium/NHC (NHC = N -Heterocyclic Carbene)-Catalyzed B-Alkyl Suzuki Cross-Coupling of Amides by Selective N–C Bond Cleavage. Org. Lett. 2018, 20, 6789–6793. [Google Scholar] [CrossRef]
- Hruszkewycz, D.P.; Balcells, D.; Guard, L.M.; Hazari, N.; Tilset, M. Insight into the Efficiency of Cinnamyl-Supported Precatalysts for the Suzuki–Miyaura Reaction: Observation of Pd(I) Dimers with Bridging Allyl Ligands During Catalysis. J. Am. Chem. Soc. 2014, 136, 7300–7316. [Google Scholar] [CrossRef]
- Hruszkewycz, D.P.; Guard, L.M.; Balcells, D.; Feldman, N.; Hazari, N.; Tilset, M. Effect of 2-Substituents on Allyl-Supported Precatalysts for the Suzuki–Miyaura Reaction: Relating Catalytic Efficiency to the Stability of Palladium(I) Bridging Allyl Dimers. Organometallics 2015, 34, 381–394. [Google Scholar] [CrossRef]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η 3 -1- t Bu-Indenyl) 2 (μ-Cl) 2 Pd 2. ACS Catal. 2015, 5, 3680–3688. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Shi, S.; Ling, Y.; An, J.; Szostak, R.; Szostak, M. Suzuki–Miyaura Cross-Coupling of Amides and Esters at Room Temperature: Correlation with Barriers to Rotation around C–N and C–O Bonds. Chem. Sci. 2017, 8, 6525–6530. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, J.; Yamamoto, T.; Yamamoto, A. Selective Cleavage of C–O Bonds In Esters Through Oxidative Addition To Nickel(0) Complexes. Chem. Lett. 1976, 5, 1091–1094. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Muto, K.; Itami, K. Recent Progress in Nickel-Catalyzed Biaryl Coupling. Eur. J. Org. Chem. 2013, 2013, 19–30. [Google Scholar] [CrossRef]
- Ben Halima, T.; Vandavasi, J.K.; Shkoor, M.; Newman, S.G. A Cross-Coupling Approach to Amide Bond Formation from Esters. ACS Catal. 2017, 7, 2176–2180. [Google Scholar] [CrossRef]
- Masson-Makdissi, J.; Vandavasi, J.K.; Newman, S.G. Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of Esters. Org. Lett. 2018, 20, 4094–4098. [Google Scholar] [CrossRef]
- Dardir, A.H.; Melvin, P.R.; Davis, R.M.; Hazari, N.; Mohadjer Beromi, M. Rapidly Activating Pd-Precatalyst for Suzuki-Miyaura and Buchwald-Hartwig Couplings of Aryl Esters. J. Org. Chem. 2018, 83, 469–477. [Google Scholar] [CrossRef]
- Meng, G.; Lei, P.; Szostak, M. A General Method for Two-Step Transamidation of Secondary Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (N-Heterocyclic Carbene) Complexes. Org. Lett. 2017, 19, 2158–2161. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd–N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki–Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem. 2017, 82, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- Pace, V.; Holzer, W.; Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Corrigendum to: Structures of Highly Twisted Amides Relevant to Amide N−C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. A Eur. J. 2017, 23, 3496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, L.; Wang, F.; Zou, G. Highly Efficient Synthesis of Aryl Ketones by PEPPSI-Palladium Catalyzed Acylative Suzuki Coupling of Amides with Diarylborinic Acids. Tetrahedron Lett. 2018, 59, 2299–2301. [Google Scholar] [CrossRef]
- Shi, W.; Zou, G. Palladium-Catalyzed Room Temperature Acylative Cross-Coupling of Activated Amides with Trialkylboranes. Molecules 2018, 23, 2412. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.R.; Kumar, D.; Cook, G.R. Bismuth-Catalyzed Synthesis of 2-Substituted Quinazolinones. Tetrahedron Lett. 2018, 59, 3801–3805. [Google Scholar] [CrossRef]
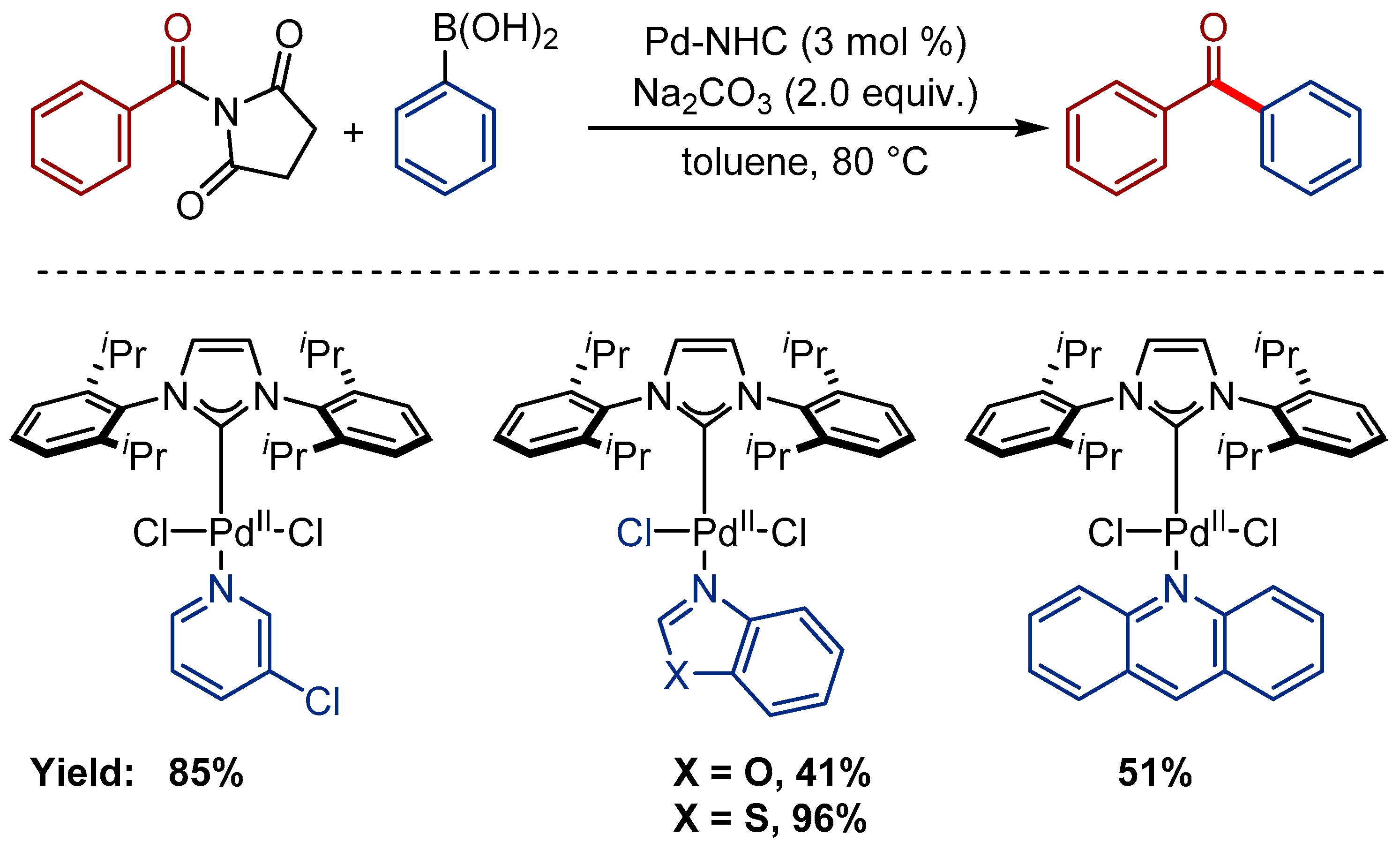
- Wang, T.; Guo, J.; Wang, H.; Guo, H.; Jia, D.; Zhang, W.; Liu, L. N-Heterocyclic Carbene Palladium(II)-Catalyzed Suzuki-Miyaura Cross Coupling of N-Acylsuccinimides by C–N Cleavage. J. Organomet. Chem. 2018, 877, 80–84. [Google Scholar] [CrossRef]
- Shi, S.; Lei, P.; Szostak, M. Pd-PEPPSI: A General Pd-NHC Precatalyst for Suzuki–Miyaura Cross-Coupling of Esters by C–O Cleavage. Organometallics 2017, 36, 3784–3789. [Google Scholar] [CrossRef]
- Li, G.; Shi, S.; Lei, P.; Szostak, M. Pd-PEPPSI: Water-Assisted Suzuki−Miyaura Cross-Coupling of Aryl Esters at Room Temperature Using a Practical Palladium-NHC (NHC=N-Heterocyclic Carbene) Precatalyst. Adv. Synth. Catal. 2018, 360, 1538–1543. [Google Scholar] [CrossRef]
- Buchspies, J.; Pyle, D.J.; He, H.; Szostak, M. Pd-Catalyzed Suzuki-Miyaura Cross-Coupling of Pentafluorophenyl Esters. Molecules 2018, 23, 3134. [Google Scholar] [CrossRef]
- Shi, S.; Szostak, M. Pd–PEPPSI: A General Pd–NHC Precatalyst for Buchwald–Hartwig Cross-Coupling of Esters and Amides (Transamidation) under the Same Reaction Conditions. Chem. Commun. 2017, 53, 10584–10587. [Google Scholar] [CrossRef]
- Karthik, S.; Gandhi, T. Palladium(II)/ N-Heterocyclic Carbene-Catalyzed Direct C–H Acylation of Heteroarenes with N -Acylsaccharins. Org. Lett. 2017, 19, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



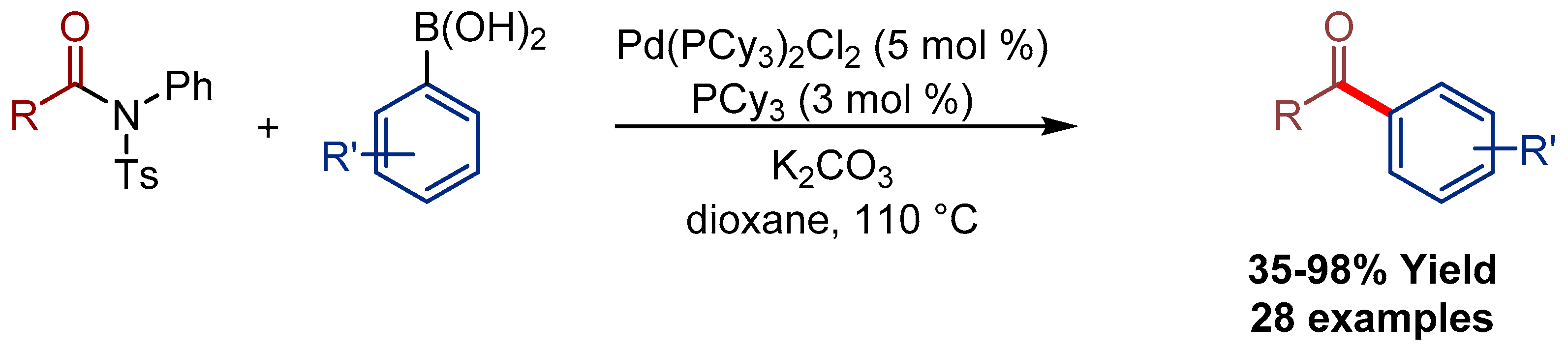
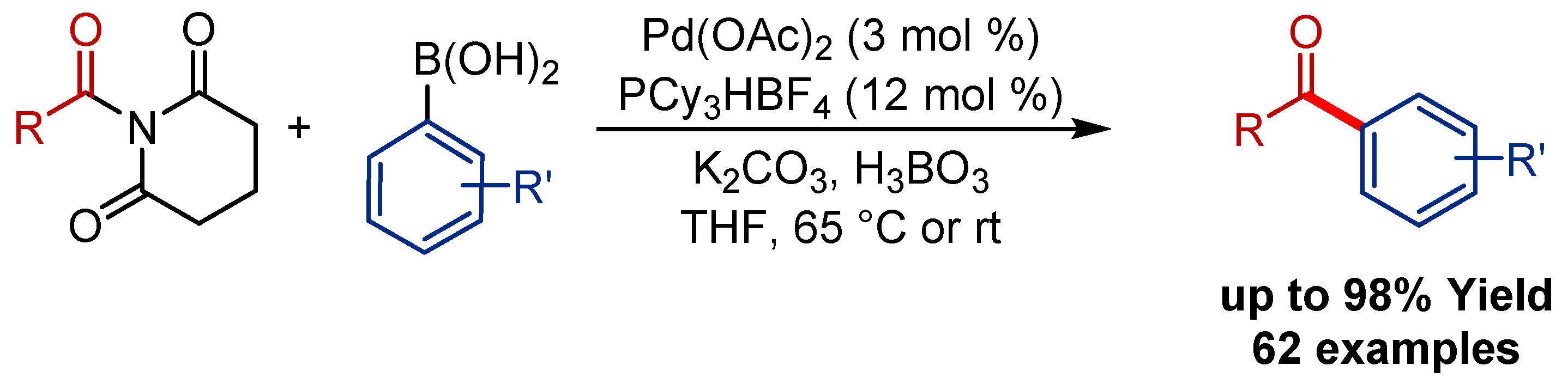
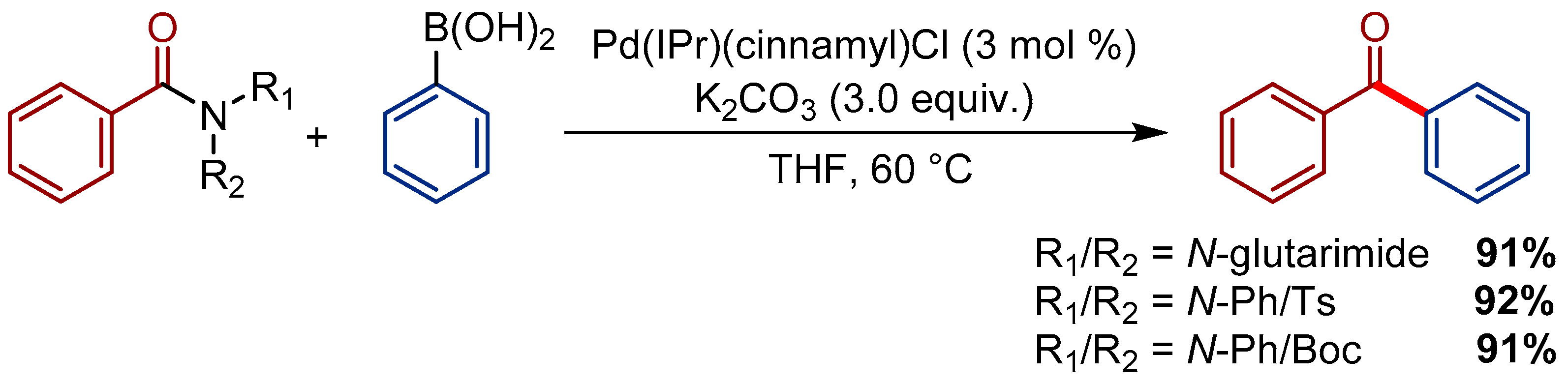
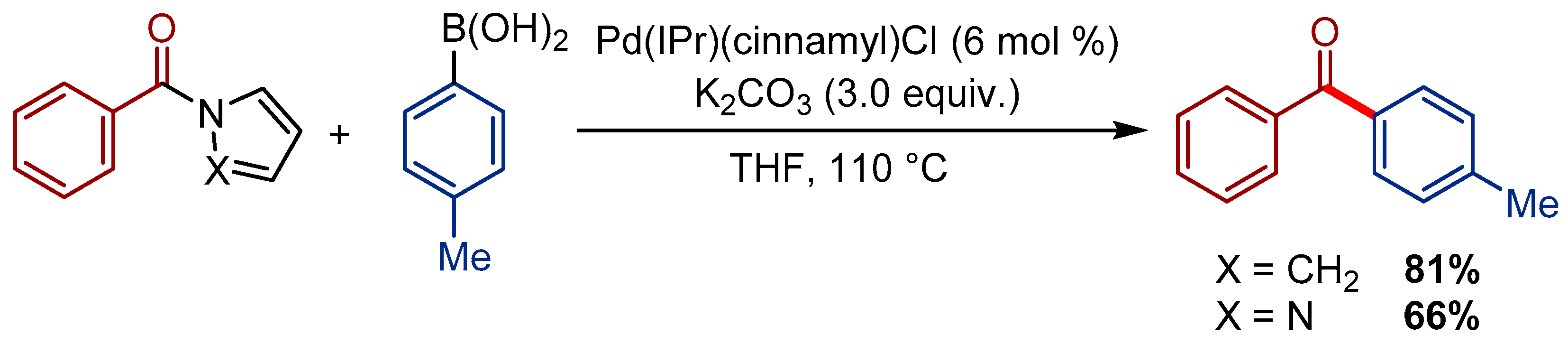

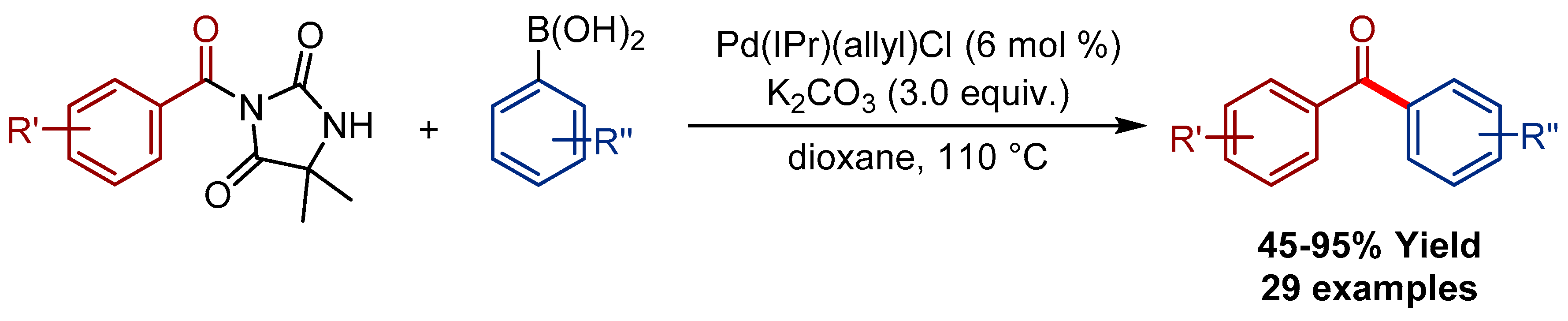
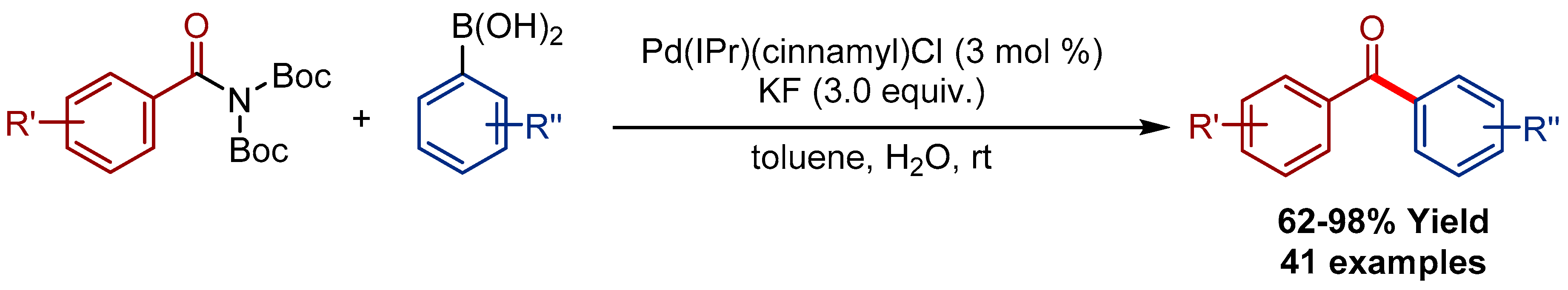
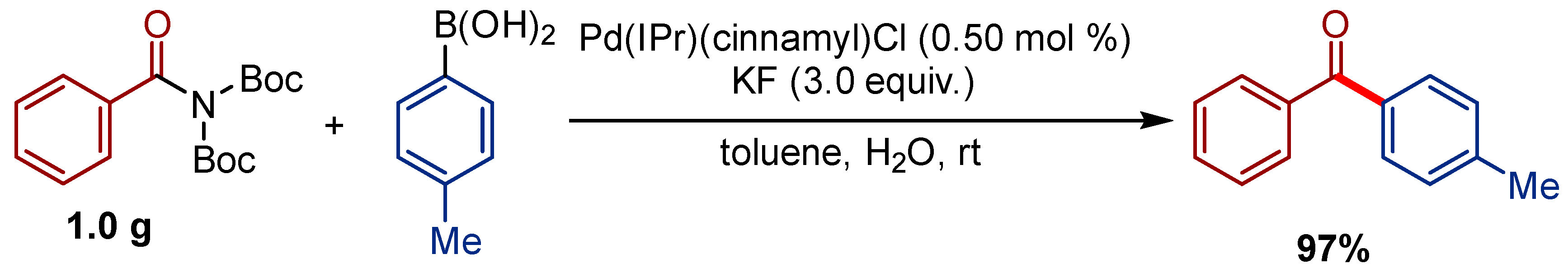

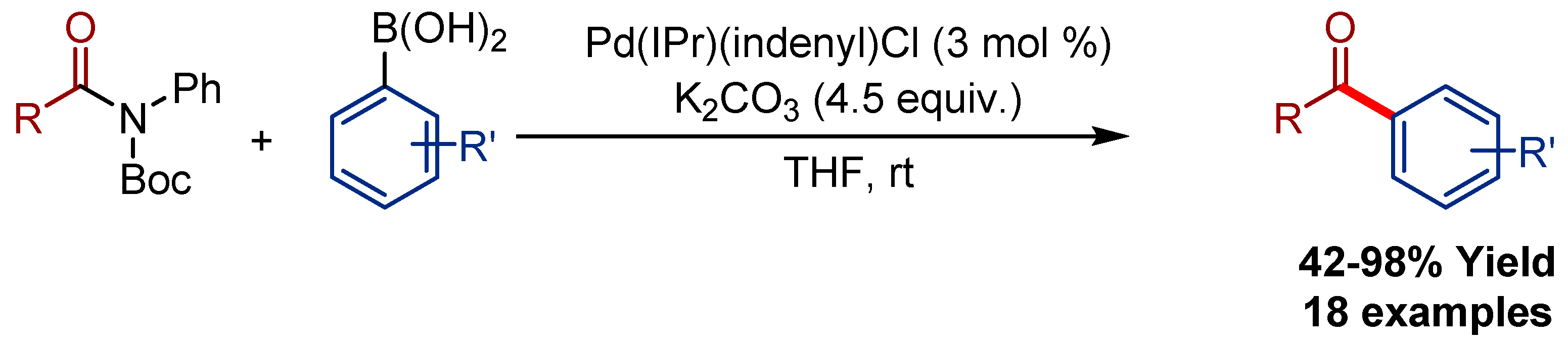
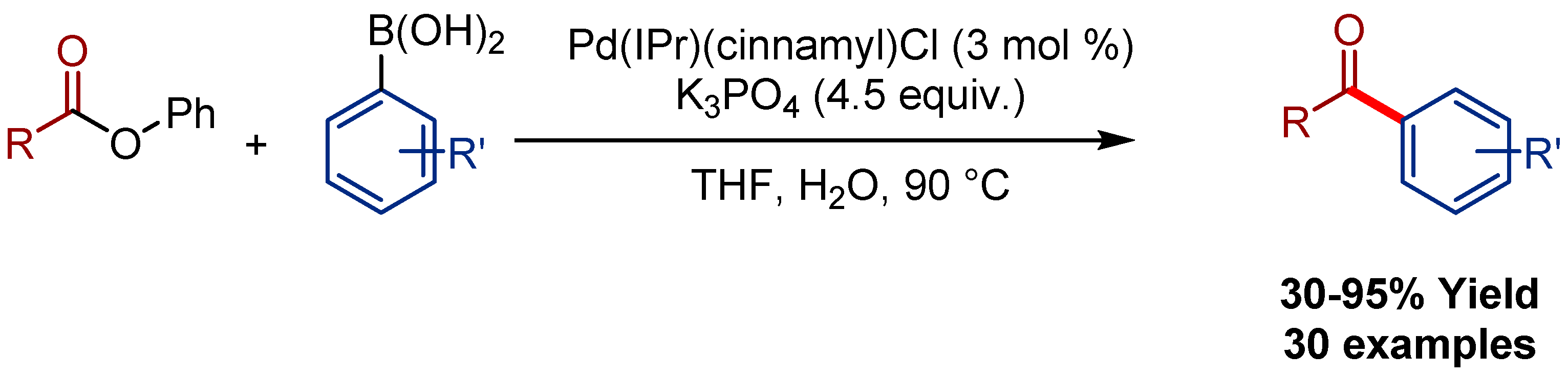
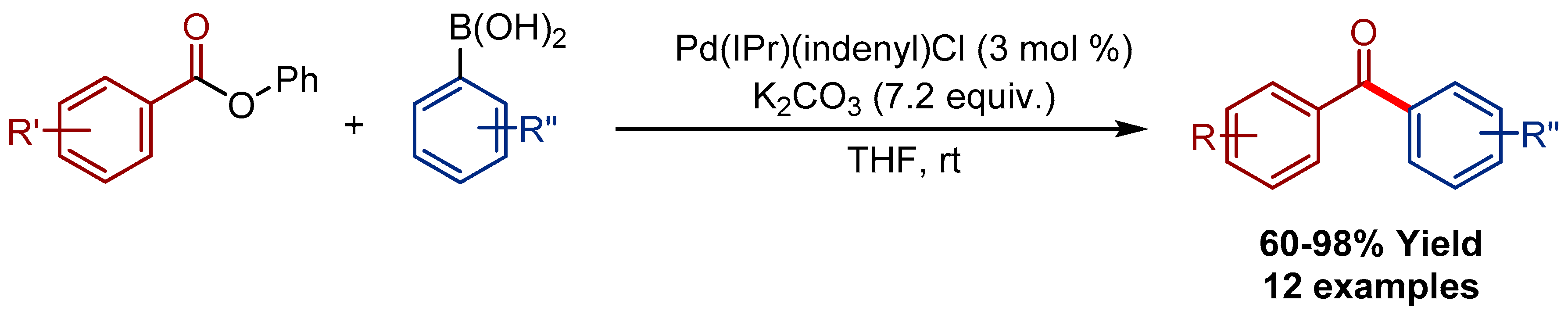

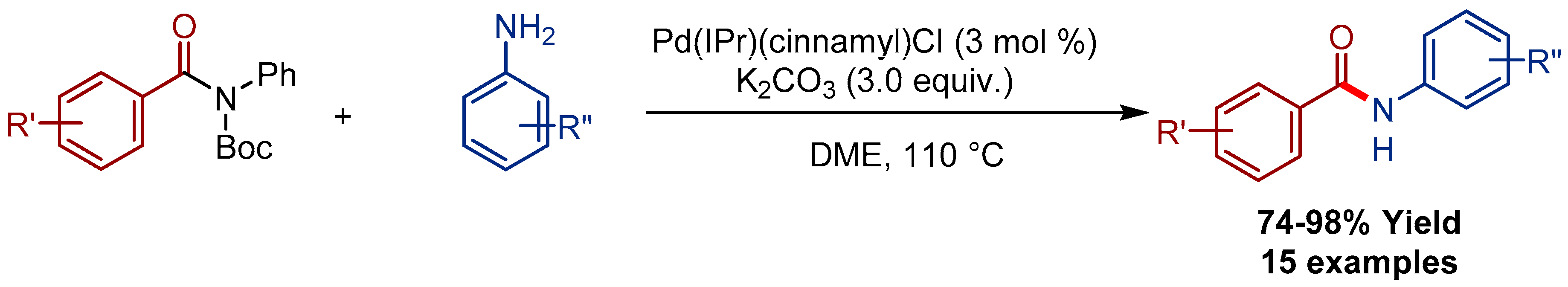

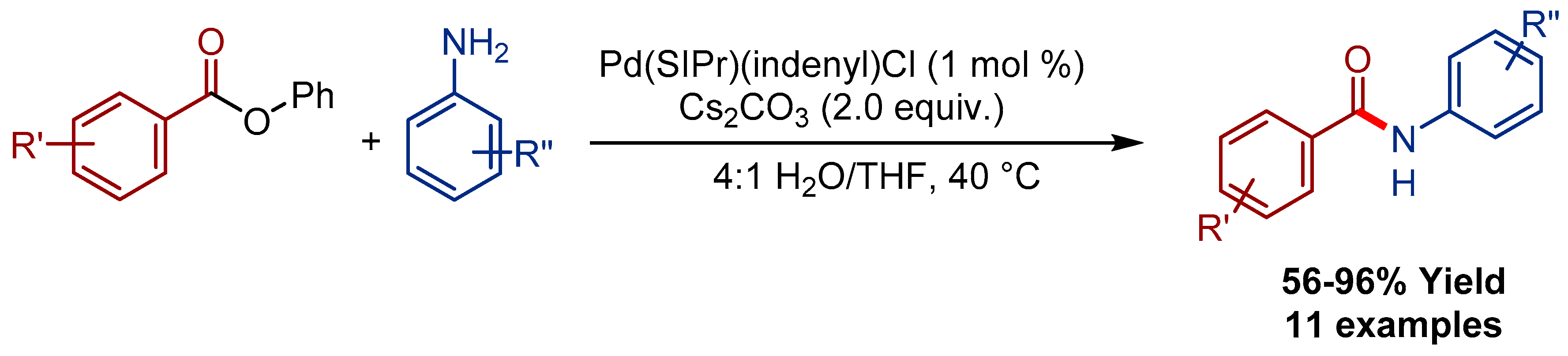
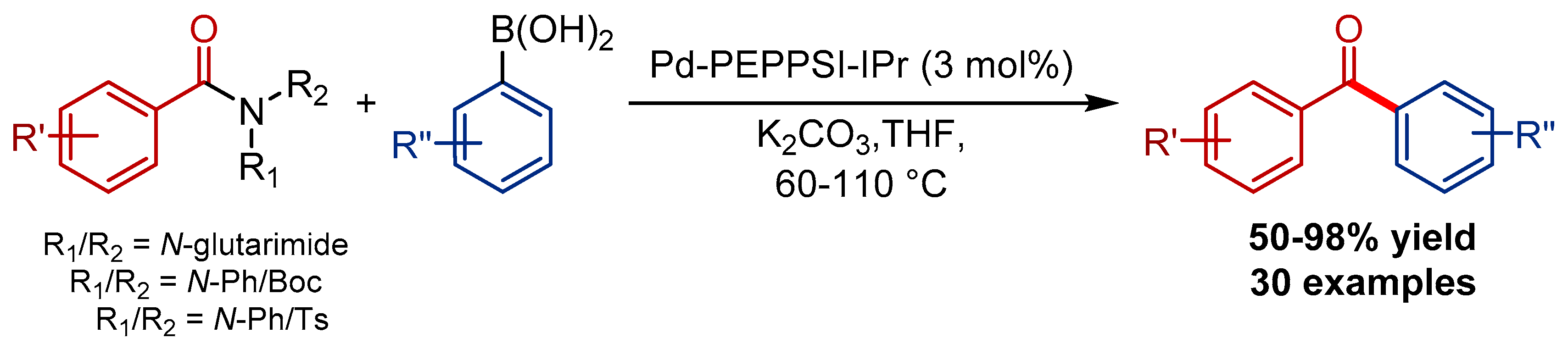


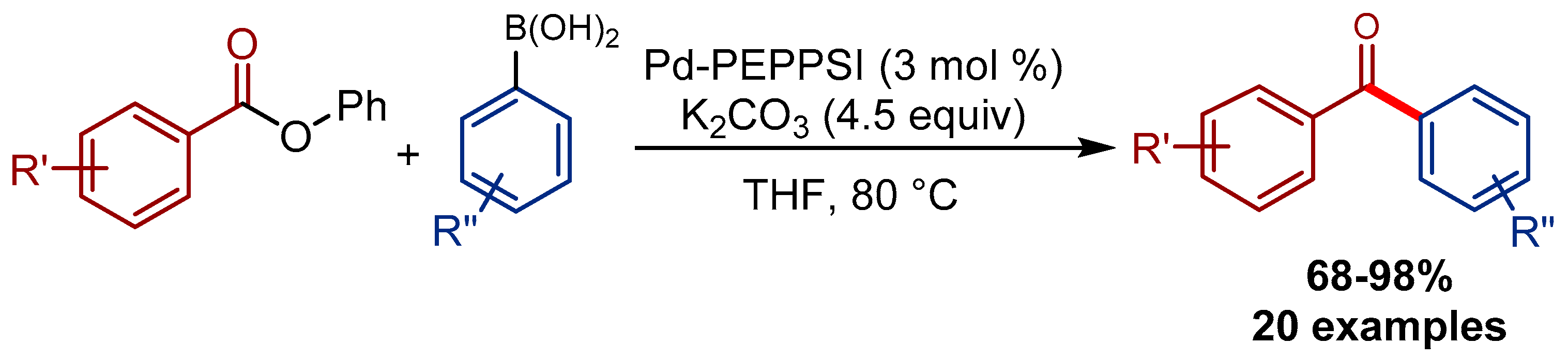





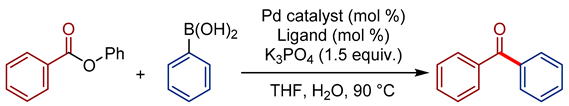
| Entry | Pd Source (mol %) | Ligand (mol %) | Yield (%) |
|---|---|---|---|
| 1 | Pd(OAc)2 (5) | IPr·HCl (10) | 11 |
| 2 | Pd(dba)3 (5) | IPr·HCl (10) | 16 |
| 3 | [Pd(allyl)Cl]2 (5) | IPr·HCl (10) | 19 |
| 4 | [Pd(cinnamyl)Cl]2 (5) | IPr·HCl (10) | 21 |
| 5 | [Pd(cinnamyl)Cl]2 (5) | IPr·HCl (5) | 59 |
| 6 | Pd(IPr)(cinnamyl)Cl (5) | none | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vemula, S.R.; Chhoun, M.R.; Cook, G.R. Well-Defined Pre-Catalysts in Amide and Ester Bond Activation. Molecules 2019, 24, 215. https://doi.org/10.3390/molecules24020215
Vemula SR, Chhoun MR, Cook GR. Well-Defined Pre-Catalysts in Amide and Ester Bond Activation. Molecules. 2019; 24(2):215. https://doi.org/10.3390/molecules24020215
Chicago/Turabian StyleVemula, Sandeep R., Michael R. Chhoun, and Gregory R. Cook. 2019. "Well-Defined Pre-Catalysts in Amide and Ester Bond Activation" Molecules 24, no. 2: 215. https://doi.org/10.3390/molecules24020215






