Tolerance and Recovery of Ultralow-Loaded Platinum Anode Electrodes upon Carbon Monoxide and Hydrogen Sulfide Exposure
Abstract
:1. Introduction
2. Materials and Methods
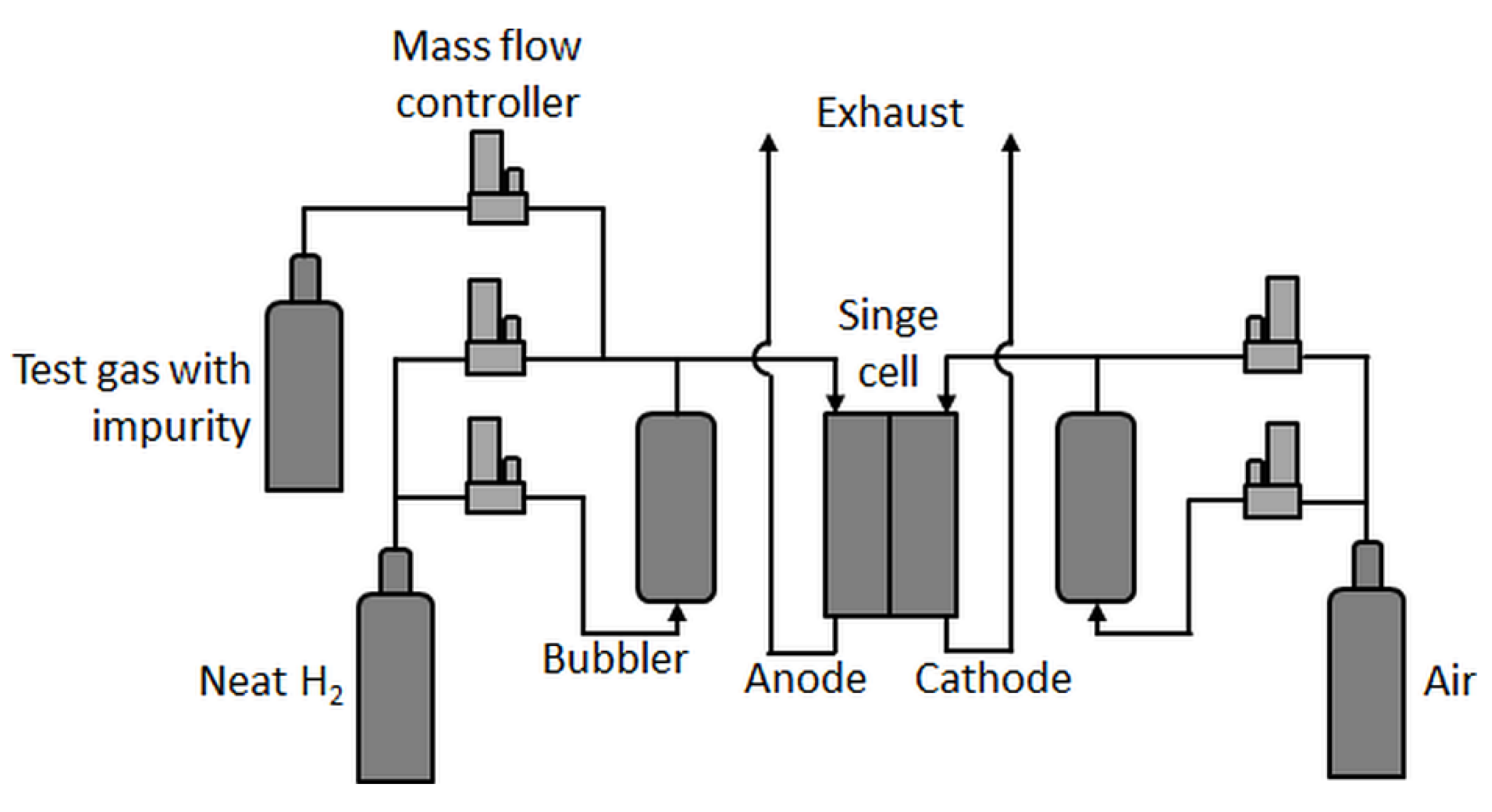
2.1. Test Station and Contaminant Introduction
2.2. Materials
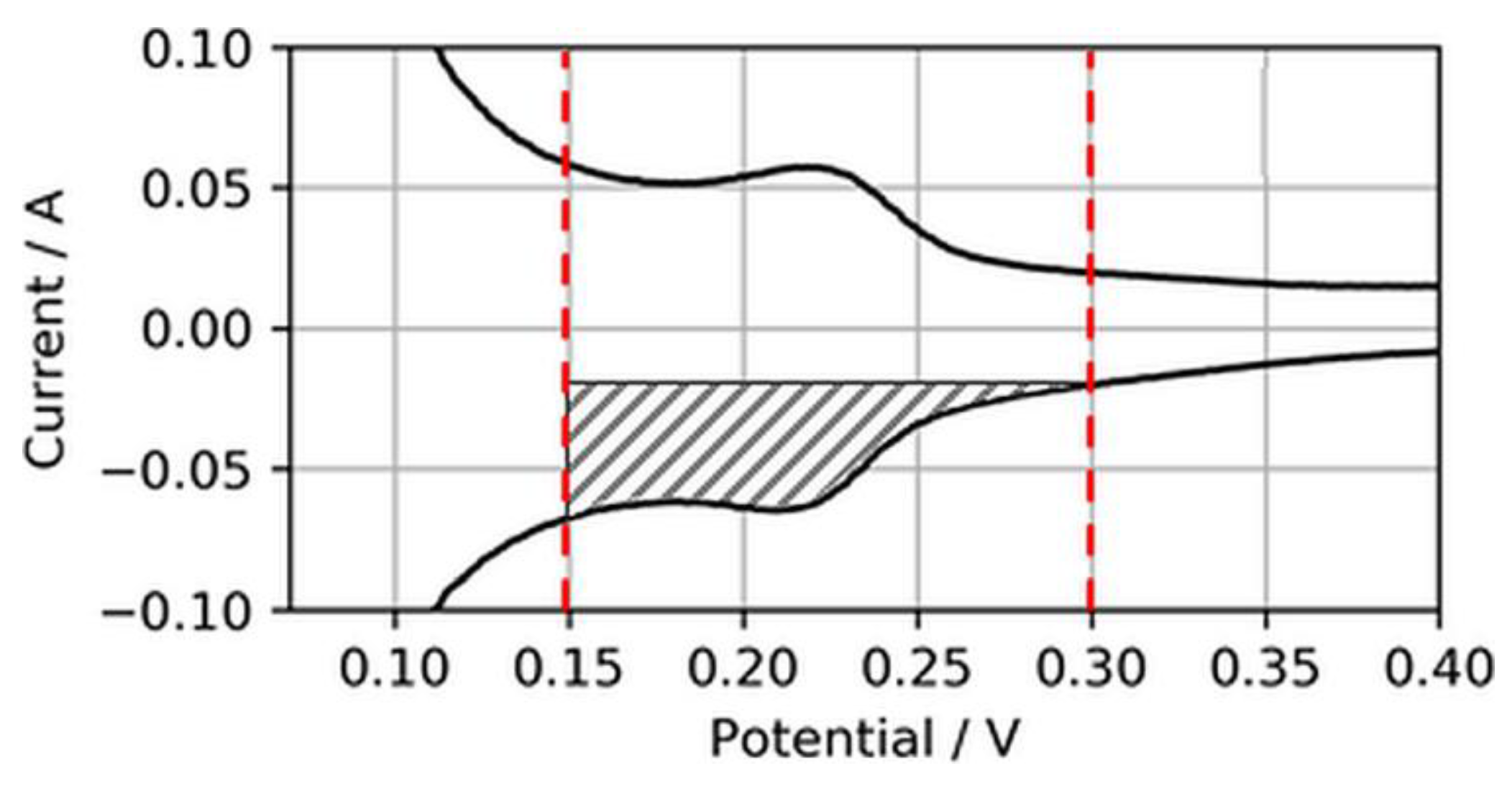
2.3. Testing Procedure and Conditions
3. Results and Discussion
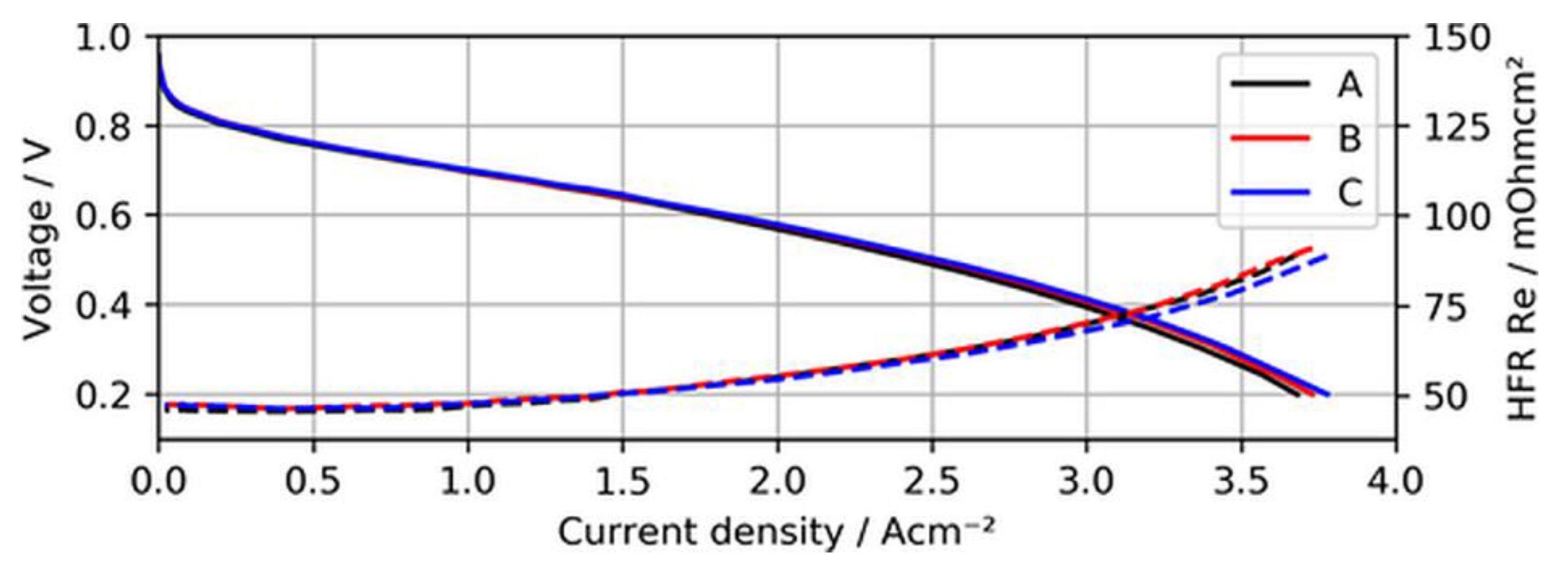
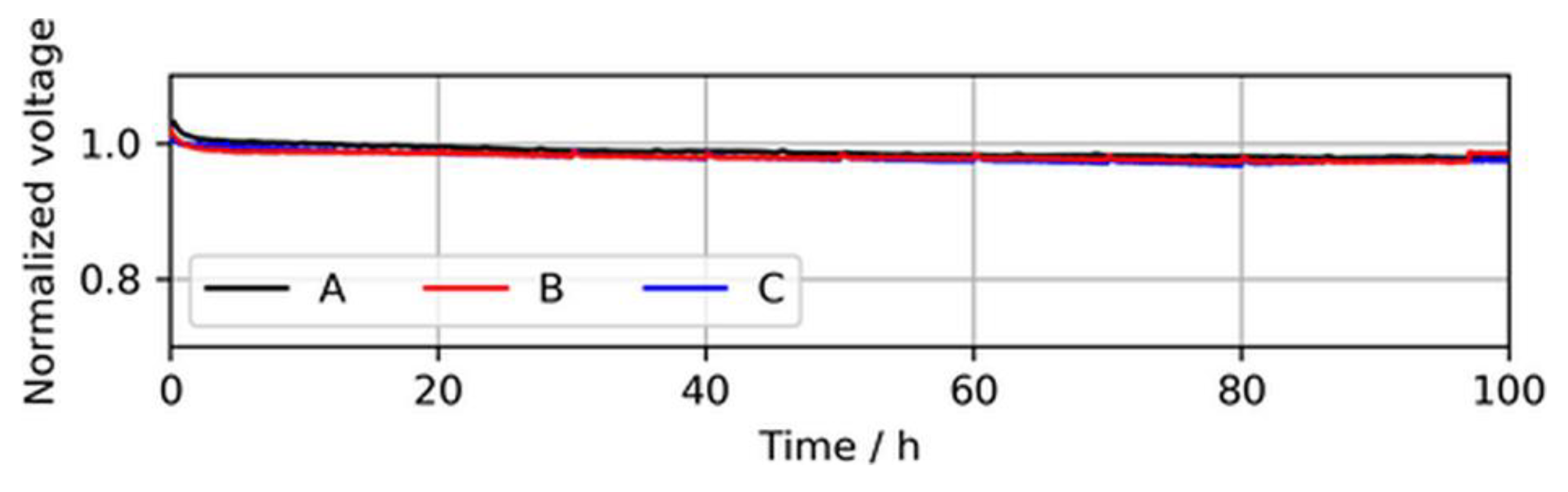
3.1. Performance and Stability of Ultralow-Loaded Anodic CLs
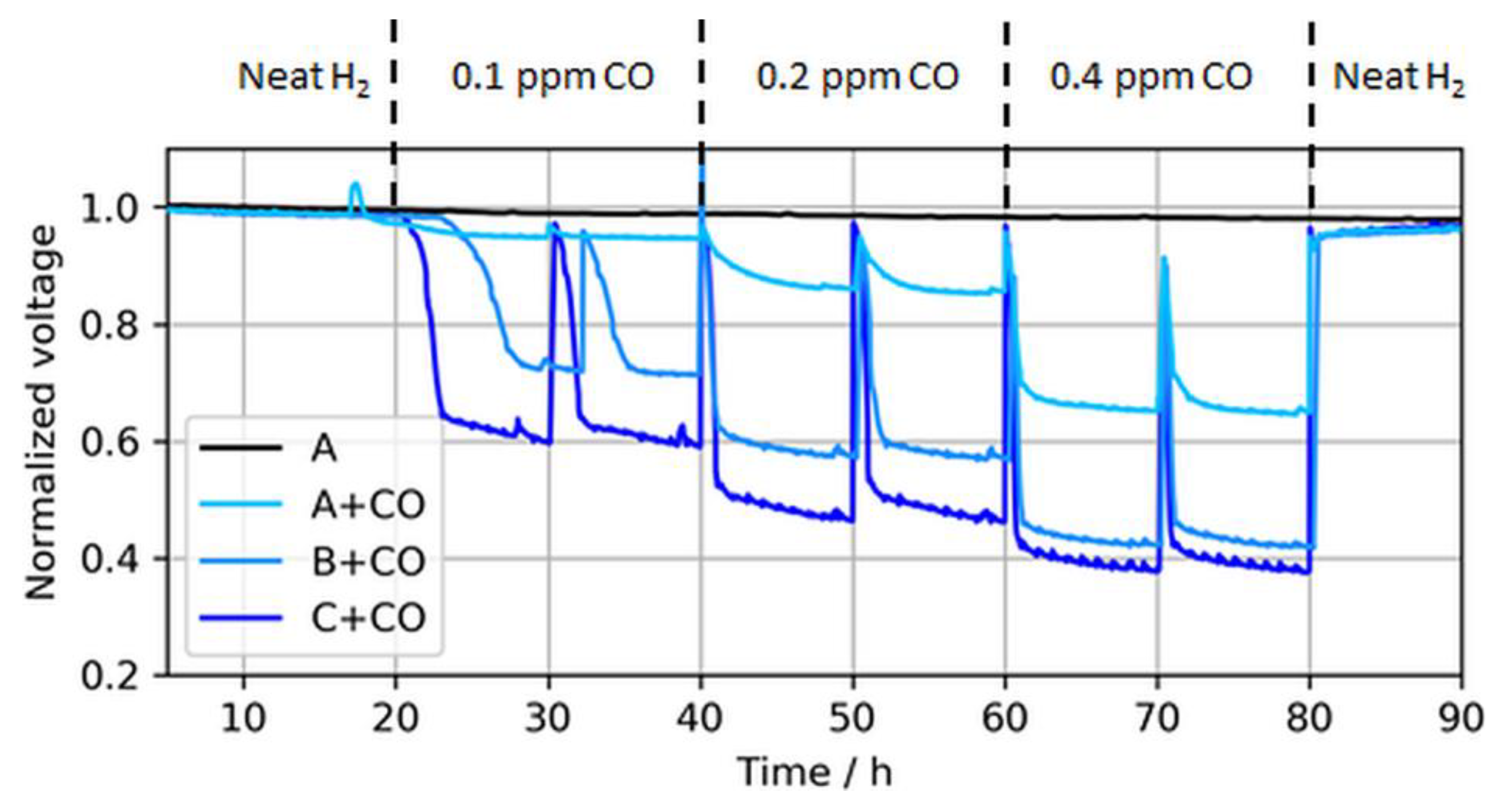
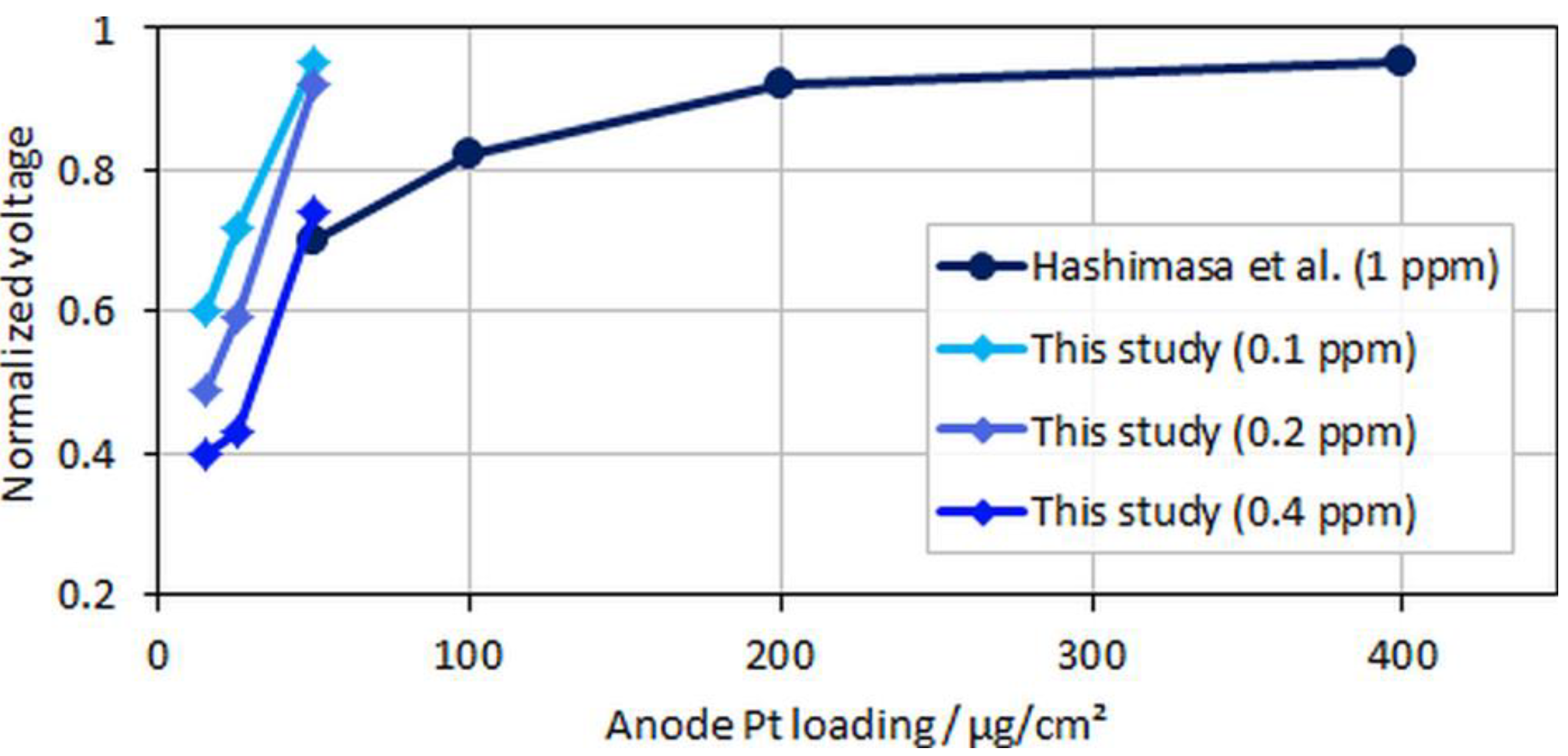
3.2. Effect of CO on Ultralow-Loaded Anode CLs
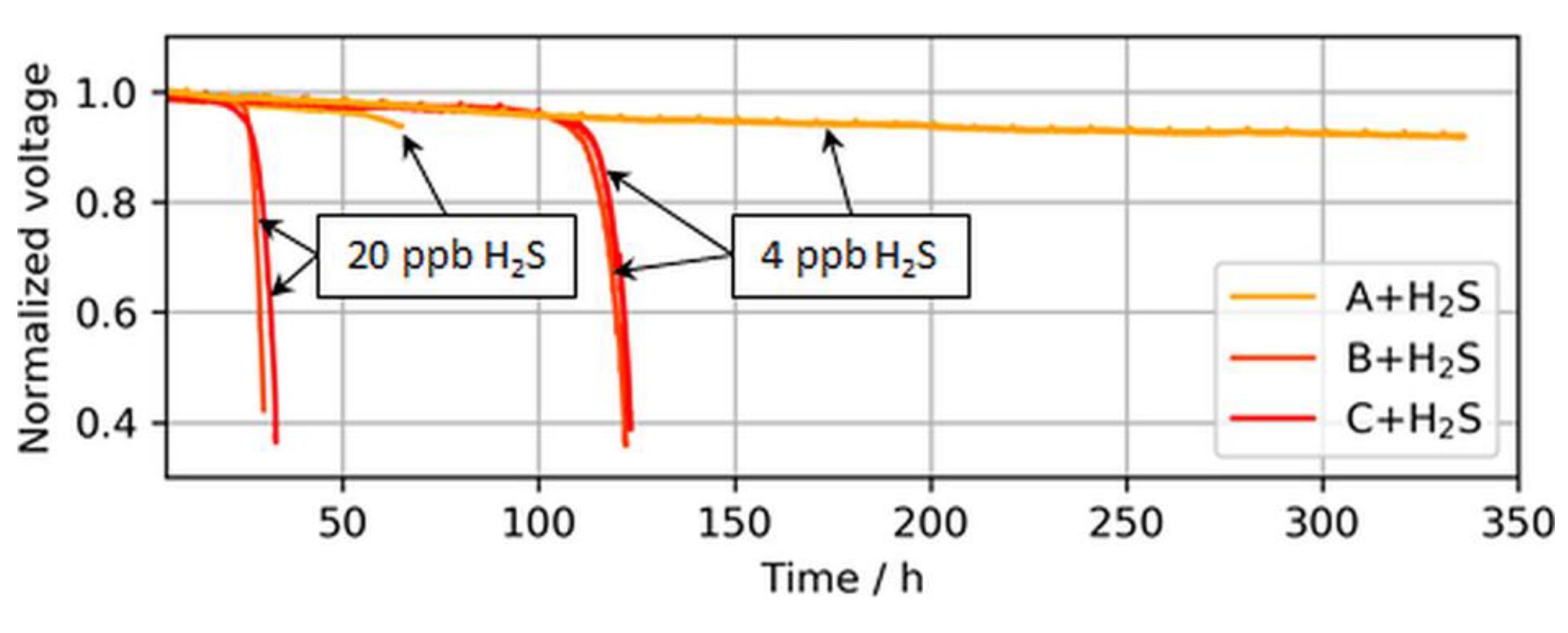
3.3. Effect of H2S on Ultralow-Loaded Anode CLs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.S.; Wang, H.; Shen, J. A Review of PEM Hydrogen Fuel Cell Contamination: Impacts, Mechanisms, and Mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X. Effect of Contaminants on Polymer Electrolyte Membrane Fuel Cells. Prog. Energy Combust. Sci. 2011, 37, 292–329. [Google Scholar] [CrossRef]
- Klages, M.; Tjønnås, J.; Zenith, F.; Halvorsen, I.J.; Scholta, J. Dual Control of Low Concentration CO Poisoning by Anode Air Bleeding of Low Temperature Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2016, 336, 212–223. [Google Scholar] [CrossRef]
- Iezzi, R.C.; Santos, R.D.M.; da Silva, G.C.; Paganin, V.A.; Ticianelly, E.A. CO Tolerance and Stability of Proton Exchange Membrane Fuel Cells with Nafion® and Aquivion® Membranes and Mo-Based Anode Electrocatalysts. Braz. Chem. Soc. 2018, 29, 1094–1104. [Google Scholar] [CrossRef]
- Lee, S.J.; Mukerjee, S.; Ticianelli, E.A.; McBreen, J. Electrocatalysis of CO Tolerance in Hydrogen Oxidation Reaction in PEM Fuel Cells. Electrochim. Acta 1999, 44, 3283–3293. [Google Scholar] [CrossRef]
- Mohtadi, R.; Lee, W.-K.; Cowan, S.; Van Zee, J.W.; Murthy, M. Effects of Hydrogen Sulfide on the Performance of a PEMFC. Electrochem. Solid State Lett. 2003, 6, A272–A274. [Google Scholar] [CrossRef]
- Gubán, D.; Tompos, A.; Bakos, I.; Vass, A.; Pászti, Z.; Szabó, E.G.; Sajó, I.E.; Borbáth, I. Preparation of CO-Tolerant Anode Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 13741–13753. [Google Scholar] [CrossRef]
- Inaba, M.; Sugishita, M.; Wada, J.; Matsuzawa, K.; Yamada, H.; Tasaka, A. Impacts of Air Bleeding on Membrane Degradation in Polymer Electrolyte Fuel Cells. J. Power Sources 2008, 178, 699–705. [Google Scholar] [CrossRef]
- Hengge, K.; Gänsler, T.; Pizzutilo, E.; Heinzl, C.; Beetz, M.; Mayrhofer, K.J.J.; Scheu, C. Accelerated Fuel Cell Tests of Anodic Pt/Ru Catalyst via Identical Location TEM: New Aspects of Degradation Behavior. Int. J. Hydrogen Energy 2017, 42, 25359–25371. [Google Scholar] [CrossRef]
- Li, H.; Gazzarri, J.; Tsay, K.; Wu, S.; Wang, H.; Zhang, J.; Wessel, S.; Abouatallah, R.; Joos, N.; Schrooten, J. PEM Fuel Cell Cathode Contamination in the Presence of Cobalt Ion (Co2+). Electrochim. Acta 2010, 55, 5823–5830. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low- Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Hashimasa, Y.; Matsuda, Y.; Akai, M. Effects of Platinum Loading on PEFC Power Generation Performance Deterioration by Carbon Monoxide in Hydrogen Fuel. ECS Trans. 2010, 26, 131–142. [Google Scholar] [CrossRef]
- Matsuda, Y.; Shimizu, T.; Mitsushima, S. Adsorption Behavior of Low Concentration Carbon Monoxide on Polymer Electrolyte Fuel Cell Anodes for Automotive Applications. J. Power Sources 2016, 318, 1–8. [Google Scholar] [CrossRef]
- Hashimasa, Y.; Matsuda, Y.; Imamura, D.; Motoaki, A. PEFC Power Generation Performance Degradation by Hydrogen Sulfi de and Ammonia—Eff Ects of Lowering Platinum Loading. Electrochemistry 2011, 79, 343–345. [Google Scholar] [CrossRef]
- Kakati, B.K.; Kucernak, A.R.J.; Fahy, K.F. Using Corrosion-like Processes to Remove Poisons from Electrocatalysts: A Viable Strategy to Chemically Regenerate Irreversibly Poisoned Polymer Electrolyte Fuel Cells. Electrochim. Acta 2016, 222, 888–897. [Google Scholar] [CrossRef]
- Lopes, T.; Paganin, V.A.; Gonzalez, E.R. The Effects of Hydrogen Sulfide on the Polymer Electrolyte Membrane Fuel Cell Anode Catalyst: H2S-Pt/C Interaction Products. J. Power Sources 2011, 196, 6256–6263. [Google Scholar] [CrossRef]
- Bonnet, C.; Franck-Lacaze, L.; Ronasi, S.; Besse, S.; Lapicque, F. PEM Fuel Cell Pt Anode Inhibition by Carbon Monoxide: Non-Uniform Behaviour of the Cell Caused by the Finite Hydrogen Excess. Chem. Eng. Sci. 2010, 65, 3050–3058. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Camara, G.A.; Ticianelli, E.A.; Mukerjee, S.; Lee, S.J.; McBreen, J. The CO Poisoning Mechanism of the Hydrogen Oxidation Reaction in Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2002, 149, A748–A753. [Google Scholar] [CrossRef]
- Igarashi, H.; Fujino, T.; Watanabe, M. Hydrogen Electro-Oxidation on Platinum Catalysts in the Presence of Trace Carbon Monoxide. J. Electroanal. Chem. 1995, 391, 119–123. [Google Scholar] [CrossRef]
- Akemann, W.; Friedrich, K.A.; Stimming, U. Potential-Dependence of CO Adlayer Structures on Pt(111) Electrodes in Acid Solution: Evidence for a Site Selective Charge Transfer. J. Chem. Phys. 2000, 113, 6864–6874. [Google Scholar] [CrossRef]
- St-Pierre, J. PEMFC Contamination Model: Competitive Adsorption Followed by an Electrochemical Reaction. J. Electrochem. Soc. 2009, 156, B291–B300. [Google Scholar] [CrossRef]
- St-Pierre, J. Proton Exchange Membrane Fuel Cell Contamination Model: Competitive Adsorption Followed by a Surface Segregated Electrochemical Reaction Leading to an Irreversibly Adsorbed Product. J. Power Sources 2010, 195, 6379–6388. [Google Scholar] [CrossRef]
- Schiller, C.A.; Richter, F.; Gülzow, E.; Wagner, N. Relaxation Impedance as a Model for the Deactivation Mechanism of Fuel Cells Due to Carbon Monoxide Poisoning. Phys. Chem. Chem. Phys. 2001, 3, 2113–2116. [Google Scholar] [CrossRef]
- Wagner, N.; Schulze, M. Change of Electrochemical Impedance Spectra during CO Poisoning of the Pt and Pt--Ru Anodes in a Membrane Fuel Cell (PEFC). Electrochim. Acta 2003, 48, 3899–3907. [Google Scholar] [CrossRef]
- St-Pierre, J.; Wetton, B.; Zhai, Y.; Ge, J. Liquid Water Scavenging of PEMFC Contaminants. J. Electrochem. Soc. 2014, 161, E3357–E3364. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Weidner, J.W. Analysis of Sulfur Poisoning on a PEM Fuel Cell Electrode. Electrochim. Acta 2010, 55, 5683–5694. [Google Scholar] [CrossRef]
- Gould, B.D.; Bender, G.; Bethune, K.; Dorn, S.; Baturina, O.A.; Rocheleau, R.; Swider-Lyons, K.E. Operational Performance Recovery of SO[Sub 2]-Contaminated Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2010, 157, B1569. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Hinkley, J.T.; Donne, S.W.; Lindquist, S.E. The Electrochemical Oxidation of Aqueous Sulfur Dioxide: A Critical Review of Work with Respect to the Hybrid Sulfur Cycle. Electrochim. Acta 2010, 55, 573–591. [Google Scholar] [CrossRef]
- Prass, S.; Hasanpour, S.; Sow, P.K.; Phillion, A.B.; Mérida, W. Microscale X-Ray Tomographic Investigation of the Interfacial Morphology between the Catalyst and Micro Porous Layers in Proton Exchange Membrane Fuel Cells. J. Power Sources 2016, 319, 82–89. [Google Scholar] [CrossRef]
- Vidakovic-Koch, T.; Hanke-Rauschenbach, R.; Gonzales Martinez, I.; Sundmacher, K. Catalyst Layer Modelling. In Handbook of Electrochemical Energy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 259–285. [Google Scholar]
Sample Availability: Not available. |



| Active Cell Area | 20.25 cm2 | |
|---|---|---|
| Catalyst | Anode | Pt/C |
| Cathode | Pt/C | |
| Electrode loading | Anode | 50/25/15 µg/cm2 (named A, B, and C hereafter) |
| Cathode | 400 µg/cm2 | |
| Membrane thickness | ~15 µm | |
| Gas diffusion layer | Freudenberg H23C9 | |
| Cell Temperature | 80 °C |
| Outlet pressure anode/cathode | 1.2/1.2 bara |
| Relative humidity anode/cathode | 90%/75% |
| Current density | 1.0 A/cm2 |
| Stoichiometry anode/cathode | 12/14 |
| Cell Temperature | 80 °C |
| Outlet pressure anode/cathode | 2/2 bara |
| Relative humidity anode/cathode | 95%/75% |
| Gas flow anode/cathode | 3/7 l/min |
| Cell Temperature | 80 °C |
| Outlet pressure anode/cathode | 1.05/1.05 bara |
| Relative humidity anode/cathode | 95%/95% |
| Gas flow anode/cathode | 1.0/0 l/min (1.0 l/min N2 for 12 min prior to CV on cathode) |
| Scan range | 50–700 mV |
| Sweep rate | 100 mV/s |
| MEA Type | ECSA (m2/g Pt) | |||
|---|---|---|---|---|
| BOL | After SD/SU | After H2S | After H2S + SD/SU | |
| A | 20.5 | 20.0 (98%) | 4.4 (22%) | 7.1 (35%) |
| B | 24.4 | 24.2 (99%) | 16.8 (69%) | 20.6 (84%) |
| C | 19.6 | 19.1 (97%) | 14.0 (71%) | 18.4 (94%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prass, S.; Friedrich, K.A.; Zamel, N. Tolerance and Recovery of Ultralow-Loaded Platinum Anode Electrodes upon Carbon Monoxide and Hydrogen Sulfide Exposure. Molecules 2019, 24, 3514. https://doi.org/10.3390/molecules24193514
Prass S, Friedrich KA, Zamel N. Tolerance and Recovery of Ultralow-Loaded Platinum Anode Electrodes upon Carbon Monoxide and Hydrogen Sulfide Exposure. Molecules. 2019; 24(19):3514. https://doi.org/10.3390/molecules24193514
Chicago/Turabian StylePrass, Sebastian, Kaspar Andreas Friedrich, and Nada Zamel. 2019. "Tolerance and Recovery of Ultralow-Loaded Platinum Anode Electrodes upon Carbon Monoxide and Hydrogen Sulfide Exposure" Molecules 24, no. 19: 3514. https://doi.org/10.3390/molecules24193514






